Abstract
We previously engineered Escherichia coli YL104 to efficiently produce succinate from glucose. In this study, we investigated the relationships between the NADH/NAD+ ratio, ATP level, and overall yield of succinate production by using glucose as the carbon source in YL104. First, the use of sole NADH dehydrogenases increased the overall yield of succinate by 7% and substantially decreased the NADH/NAD+ ratio. Second, the soluble fumarate reductase from Saccharomyces cerevisiae was overexpressed to manipulate the anaerobic NADH/NAD+ ratio and ATP level. Third, another strategy for reducing the ATP level was applied by introducing ATP futile cycling for improving succinate production. Finally, a combination of these methods exerted a synergistic effect on improving the overall yield of succinate, which was 39% higher than that of the previously engineered strain YL104. The study results indicated that regulation of the NADH/NAD+ ratio and ATP level is an efficient strategy for succinate production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Succinic acid, a C4-dicarboxylic acid, is a precursor of many important chemicals in the food, chemical, and pharmaceutical industries (Ahn et al. 2016; Olajuyin et al. 2016; Wang et al. 2014). Certainly, the widespread importance of succinate has secured its listing among the top 12 chemical building blocks by the US Department of Energy (Werpy and Petersen 2004). To improve succinate production by using Escherichia coli, researchers have explored various metabolic engineering strategies (Ahn et al. 2016; Forster and Gescher 2014; Kang et al. 2010; Liang et al. 2015) such as activating the glycolytic pathway, overexpressing pyruvate-metabolizing enzymes, eliminating competing pathways, and providing reducing equivalents and energy (Li et al. 2016; Liang and Qi 2014; Meng et al. 2016; Vemuri et al. 2002; Vuoristo et al. 2016).
We previously constructed succinate-producing strains by using a series of genetic modifications, and a high overall volumetric productivity and concentration of succinate were achieved (Li et al. 2013). A whole-phase succinate production strategy was developed in this study. In this method, the engineered strain produced succinate under aerobic, microaerobic, and anaerobic conditions. Compared with this whole-phase fermentation, strain growth uncouples product formation in the typical dual-phase fermentation. However, the overall yield from our constructed engineered strain should be increased further. Recently, we explored the relationships between ATP generation, substrate ratio of xylose to glucose, and succinate production (Zhang et al. 2016). We observed that excess energy exerted a negative effect on succinate production in the engineered E. coli that could not grow anaerobically. Therefore, regulating ATP generation for improving the overall yield of succinate by using glucose as the carbon source in this engineered E. coli is essential.
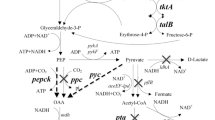
The NADH/NAD+ ratio is also crucial for succinate production (Singh et al. 2009). Under aerobic growth conditions, NAD+ is regenerated through oxidative phosphorylation. Under anaerobic conditions, NAD+ serves as an electron acceptor during substrate degradation, and NADH provides the reducing power for reductive product formation. A balance of NAD+ reduction and NADH oxidation is a prerequisite for anaerobic fermentation (de Graef et al. 1999). In the engineered E. coli, NADH-dependent lactate dehydrogenase and ethanol dehydrogenase were inactivated. Inactivation limits the means by which NAD+ may be regenerated from NADH formed from aerobic to anaerobic growth conditions, thus affecting the redox balance to increase the NADH/NAD+ ratio (Fig. 1a; Li et al. 2013). Therefore, we assumed that the low yield of succinate production was because of the limited NAD+ regeneration rate, which resulted in the increase in the NADH/NAD+ ratio from the aerobic to anaerobic phase; however, NAD+ could be regenerated in the reductive tricarboxylic acid (TCA) cycle.
Central carbon metabolism and electron transport chain in our engineered E. coli. a Gene deletions in strain YL104 are represented by black stars, and gene overexpression is depicted in red. ATP futile cycling reactions are denoted by purple circles. PEP phosphoenolpyruvate. b Red and blue lines represent the aerobic respiratory chain and anaerobic respiratory chain, respectively. Black stars represent the gene mutation in YL104. FRDS1 is soluble fumarate reductase from S. cerevisiae (color figure online)
Another possible cause of the increased NADH/NAD+ ratio at the initial anaerobic condition is the change in NADH dehydrogenases during the aerobic–anaerobic fermentation process. The aerobic respiratory chain of E. coli involves three membrane-bound NADH dehydrogenases (NDH-I, NDH-II, and WrbA) and three ubiquinol oxidases (cytochromes bd-I, bd-II, and bo; Fig. 1b). These enzymes have different efficiencies in coupling electron transfer for the generation of an electrochemical proton gradient (Bekker et al. 2009; Borisov et al. 2011). Under aerobic conditions, E. coli mainly uses NADH dehydrogenases II (ndh), and under anaerobic conditions, it mainly uses NADH dehydrogenases I (nuo). The change in main NADH dehydrogenase in E. coli from the aerobic to anaerobic condition may be time-consuming, and this change also resulted in an elevated NADH/NAD+ ratio. Therefore, we speculated that using one type of NADH dehydrogenase during aerobic and anaerobic conditions could reduce the NADH/NAD+ ratio to increase the overall yield of succinate (Singh et al. 2009; Vemuri et al. 2006).
In this study, by regulating the NADH/NAD+ ratio and ATP generation through various strategies, we explored the relationships between the NADH/NAD+ ratio, ATP level, and overall yield of succinate production with glucose as the carbon source.
Materials and methods
Strains and plasmids
All strains, plasmids, and oligonucleotides used in this study are listed in Table 1 and Table S1. All genetic modifications were made in E. coli MG1655.
The one-step inactivation of the chromosomal gene method was used for single gene mutants (Datsenko and Wanner 2000). The primers ndh-pKD4-F and ndh-pKD4-R and the template plasmids pKD4 for ndh knockouts were used to obtain linear DNA fragments with antibiotic-resistant gene cassettes flanked by FLP recognition target sites and 39-bp homologous arms for the construction of the single gene mutants. The purified DNA was electroporated into E. coli K-12 MG1655 cells harboring the helper plasmid pTKRed expressing Red recombinase enzymes (Kuhlman and Cox 2010). Positive clones were selected using appropriate antibiotics. Subsequently, the helper plasmid and resistance gene were eliminated.
For gene overexpression, pCL1920 was used and digested using HindIII–KpnI to obtain the plasmid skeleton and resistant gene. A DNA fragment with frd sequence (GenBank, KX505965) from Saccharomyces cerevisiae S288c was synthesized with codon optimization by Generay Biotechnology (Shanghai, China). This fragment was also digested using HindIII–KpnI, and the sequence was ligated into the vector pCL1920, thus generating the plasmid pCLfrd. Furthermore, nuoC was amplified from wild-type E. coli MG1655 by using the primers nuoC PF and nuoC PR. The vector skeleton and ppsA were amplified from the pCL1920 plasmid and E. coli MG1655 genome by using the primers pCL1920-F and pCL1920-R and trc-ppsA-F and trc-ppsA-R, respectively. The plasmid pCLnuoC was obtained through Gibson assembly with the pCL1920 skeleton and nuoC fragment. The primers trc-RBS-F and trc-RBS-R from the pTrc99a plasmid were annealed to obtain the trc promoter with RBS. Subsequently, the three fragments, plasmid skeleton, trcRBS, and ppsA, were assembled together through Gibson assembly. In addition, pCLppsA/frd was constructed using Gibson assembly with the pCLppsA skeleton and frd fragment.
Culture medium and growth conditions
Luria–Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl; pH 7.2) was used for all DNA manipulations. During the construction of the single-gene-deficient mutants, cultures were grown aerobically at 30, 37, or 42 °C in Super Optimal Broth medium (20 g/L tryptone, 5 g/L yeast extract, 0.5 g/L NaCl, 2.5 mM KCl, and 10 mM MgCl2). All cultures were incubated at 37 °C, and the pH was measured using a glass electrode and controlled at 6.4–6.8 by using 5 M K2CO3 and 0.5 M NaOH.
For succinate production, a single clone of each single gene mutant and the parent strain were cultured in a 300-mL Erlenmeyer flask containing 50 mL of LB medium at 37 °C and 250 rpm for 12 h. Subsequently, 4 mL (5% v/v) of the overnight culture was transferred to a 500-mL Erlenmeyer flask containing 80 mL AM1 [2.63 g/L (NH4)2HPO4, 0.87 g/L NH4H2PO4, 0.15 g/L KCl, and 20.9 g/L 3-(N-morpholino)propanesulfonic acid (MOPS)] with an additional 1 g/L yeast extract and 30 g/L glucose and shaken at 250 rpm for 21 h. Antibiotics were added to provide the selective pressure during the cultivation, if necessary (ampicillin 100 μg/mL, kanamycin 25 μg/mL, spectinomycin 25 μg/mL, and chloromycetin 17 μg/mL).
During the succinate fermentation in a multiple mini-fermenter system (INFORS HT, Switzerland), the aforementioned seed culture (10% v/v) was transferred into 800 mL AM1 medium (without MOPS) supplemented with 40 g/L glucose in a 1-L fermenter for batch fermentation. The total fermentation time (70 h) included a 28-h aerobic phase (pass into O2) and a 42-h anaerobic phase (pass into CO2). The air flow rate and agitation were maintained at 1 vvm and 350 rpm, respectively. During the fermentation, 0.05 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) was added at the beginning of anaerobic fermentation with strains harboring plasmids during the batch fermentation. Samples were taken at intervals of 6 or 12 h.
Analytical methods
Biomass was detected by measuring the density at 600 nm [1 OD600 ≈ 0.34 g cell dry weight (CDW) L−1] by using a spectrophotometer (Shimadzu, Japan). The organic acid concentrations and the residual glucose concentration were measured using high-performance liquid chromatography (HPLC; Shimadzu, Japan) and an Aminex HPX-87H ion exclusion column (Bio-Rad, USA). H2SO4 solution (5 mM) was applied as a mobile phase at a flow rate of 0.6 mL/min to the column at 65 °C.
The intracellular ATP concentrations were measured using the BacTiter-Glo™ Microbial Cell Viability Assay Kit (Promega, USA), and the intracellular concentrations of NADH and NAD+ were assayed using a cycling method (Leonardo et al. 1996).
Results
Use of sole anaerobic NADH dehydrogenases to increase succinate yield
First, we inactivated the NADH dehydrogenase II encoded by ndh in the previously constructed YL104 (Fig. 1a; Li et al. 2013), thus generating YL104N. In response to this modification, succinate production was similar but the overall yield of succinate in YL104N increased by 7%, reaching 1.05 mol/mol (Table 2). The biomass of YL104N exhibited no substantial change (Table 2), which suggested that the inactivation of NDH-II dehydrogenase did not have a negative effect on the growth of the engineered strains. In addition, we regulated NADH dehydrogenase I by overexpressing an important subunit (encoded by nuoCD) of this enzyme (Singh et al. 2009). However, the titer of succinate decreased, and the overall yield only slightly improved (Table 2). We speculated that excess energy was generated by overexpression of the important subunit of NDH-1 that generates proton gradients across cell membranes (Verkhovskaya et al. 2008). In addition, metabolic burdens resulting from overexpression of enzymes may influence the growth of the engineered strain, leading to decreased succinate production. The aerobic NADH/NAD+ ratio increased in YL104N compared with YL104 (Fig. 2). Moreover, the NADH/NAD+ ratio in YL104N increased less from the aerobic to anaerobic condition compared with that in YL104 (94 and 29% increases in YL104 and YL104N, respectively). These results indicated that the inactivation of main aerobic NADH dehydrogenase (ndh) in YL104 could alleviate the change in the NADH/NAD+ ratio, resulting in a more stable physiological state, which may be beneficial for anaerobic succinate production (Liang et al. 2012).
Modulating the NADH/NAD+ ratio and ATP level through overexpression of soluble fumarate reductases FRDS1 from S. cerevisiae
Fumarate reductases (FRDs), which catalyze the reduction of endogenous or exogenous fumarate into succinate, are key factors in the anaerobic metabolism of many organisms (Camarasa et al. 2007). The E. coli FRD is a respiratory enzyme, covalently linked to flavin cofactors (FAD) and membrane-bound enzyme (located in the inner membrane) structurally similar to succinate dehydrogenases. Being associated with the respiratory chain, they are directly involved in the production of ATP through oxidative phosphorylation (Tielens and Van Hellemond 1998). NADH-dependent FRD in E. coli can catalyze the metabolic flux flow to succinate and lower the NADH/NAD+ ratio. However, this type of FRD may generate more energy in the interface (a particular period in which the fermentation is switched from the aerobic to the anaerobic condition) from the aerobic to the anaerobic phase because of the connection of the electron transfer chain in YL104N (Fig. 1b), which has a negative effect on succinate production (Zhang et al. 2016). However, inactivating the main aerobic NADH dehydrogenase in YL104 can increase the ATP level at the initial anaerobic phase (Calhoun et al. 1993). The FRDS1 encoded by frd from S. cerevisiae is a soluble enzyme that noncovalently binds FADH2 (cytoplasmic isozyme) and irreversibly catalyzes the reduction of fumarate independently of the electron transfer chain (Camarasa et al. 2007; Enomoto et al. 1996; Muratsubaki and Enomoto 1998). Thus, we speculated that overexpressing frd from S. cerevisiae in E. coli may change the anaerobic ATP level and NADH/NAD+ ratio of YL104N at the beginning of the anaerobic phase.
We placed frd under the tac promoter and introduced it into YL104N. The resulting strain YL104NF produced more succinate (28.0 g/L), with a 19% higher yield than that of YL104N (24.4 g/L; Fig. 3a). The overall yield of succinate was also improved in YL104NF (an increase of approximately 13%). The biomass and aerobic glucose consumption of these two strains were similar; however, the anaerobic glucose consumption of YL104NF (9.27 g/L) was faster than that of YL104 (8.71 g/L). This may have been the key factor for the increased succinate production. In addition, after 63 h, YL104NF generated less ATP and a lower NADH/NAD+ ratio during anaerobic fermentation (Fig. 3b). The intracellular ATP level of YL104NF decreased by 10%, and the NADH/NAD+ ratio decreased by 24%. The results indicated that overexpression of soluble FRDS1 could certainly reduce the anaerobic ATP level and NADH/NAD+ ratio. Consequently, both anaerobic succinate production and the total succinate production were improved.
Growth, succinate production, NADH/NAD+ ratio, and ATP level. a Growth, glucose consumption, and succinate production of strains YL104N and YL104NF. Glucose consumption (dark blue lines), succinate production (red lines), and OD600 (dark cyan lines). b Measurements of ATP level and NADH/NAD+ ratio of strains YL104N and YL104NF at 63 h. Standard deviations were calculated from the results of four independent experiments (color figure online)
ATP futile cycling to reduce the ATP level under anaerobic conditions
In the glycolytic pathway, pyruvate kinase catalyzes the formation of pyruvate from phosphoenolpyruvate (PEP) with the production of one ATP molecule. The reverse reaction catalyzed by PEP synthase (encoded by ppsA) from pyruvate to PEP requires ATP as a cofactor; it is converted into AMP. Thus, a cycle in which one ATP molecule is consumed in net is formed (ATP futile cycling; Fig 1a; Hadicke et al. 2015). We introduced this ATP futile cycling into YL104N to improve the overall yield of succinate by reducing the anaerobic ATP level. The resulting strain YL104NP produced more succinate, with a 7% higher yield than that of YL104N (Fig. 4a), and the overall yield of succinate in YL104NP also increased by 17%. Compared with YL104N, biomass, aerobic succinate production, and glucose consumption decreased, but anaerobic succinate production and glucose consumption were faster than that of YL104N. Moreover, the anaerobic succinate titer of YL104NP was 47% higher than that of YL104N (Fig. 4a). These results suggested that a decreased ATP level resulted in increased succinate production and glucose consumption under anaerobic conditions. The decline in biomass and aerobic succinate of YL104NP may result from the leaky expression of ppsA from the trc promoter under aerobic conditions, which consumed the available ATP for growth. Regarding the by-products of YL104NP, formate, acetate, and lactate, the quantities produced were nearly half of those produced with YL104N (Fig. 4b).
Growth, succinate production, and by-products. a Growth, glucose consumption, and succinate production of strains YL104N and YL104NF. Glucose consumption (dark blue lines), succinate production (red lines), and OD600 (dark cyan lines). b By-products of strains YL104N and YL104NP were measured at the end of fermentation (color figure online)
The anaerobic ATP level of YL104NP decreased by 33% compared with that of YL104N at the end of fermentation (Fig. 5a). In addition, the aerobic ATP level in YL104NP decreased compared with YL104N because of the leaky expression of ppsA. Moreover, the anaerobic ATP level in YL104NP decreased more rapidly. The tendency of the regression line (the slope k), which was calculated using the ATP levels throughout the entire fermentation process, is crucial (Stouthamer 1979). In the present study, the slope k 1 of the anaerobic trend line of the ATP curve in YL104N was clearly higher than the slope k 2 of YL104NP (−32.3 and −55.6, respectively), indicating that under the anaerobic condition, YL104NP generated less ATP than YL104N (Fig. 5b; Liu et al. 2013; Zhang et al. 2016).
Coexpression of frd and ppsA to increase succinate yield
Overexpression of frd and ppsA could individually reduce the anaerobic intracellular ATP level and increase the overall succinate yield, respectively. Thus, we examined whether coexpression of frd and ppsA had a synergistic effect on improving the succinate yield and titer. The data illustrated that overexpression of frd and ppsA individually produced succinate with titers 28.19 and 29.05 g/L, 12 and 9% lower than that produced by YL104NPF (titer 31.87 g/L), respectively (Table 3). Compared with strains YL104NF and YL104NP, the biomass and glucose consumption of YL104NPF were slightly decreased, whereas the overall yield of succinate in YL104NPF was 1.36 mol/mol, which was 13 and 9% higher than those of strains YL104NF and YL104NP, respectively. The final yield of strain YL104NPF was 39% higher than that of the initial strain YL104. Our results suggested that coexpression of frd and ppsA exhibited a synergistic effect on improving succinate production and yield.
Discussion
Manipulating redox equivalent and energy balance is crucial for the efficient synthesis of target products, particularly for succinate biosynthesis (Singh et al. 2011; Zhu et al. 2014). In the present study, we applied the soluble FRDs from S. cerevisiae for the first time to modulate the ATP level and NADH/NAD+ ratio to enhance biochemical production.
Most FRDs belong to a multimeric complex associated with the respiratory chain and transfer electrons from quinol to fumarate (Van Hellemond and Tielens 1994). However, soluble monomeric FRDs with FADH2/FMNH2 as an electron donor, not linked to the electron transfer chain, have been identified in several Shewanella species, S. cerevisiae, and the protozoan Trypanosoma brucei (Besteiro et al. 2002; Gordon et al. 1998; Muratsubaki and Enomoto 1998). Overexpression of NADH-dependent FRDs from T. brucei were employed to maintain the cellular redox balance and enhance the cytosolic NAD+ and NADPH pools (Besteiro et al. 2002; Coustou et al. 2005; Salusjarvi et al. 2013). Soluble frds1 from S. cerevisiae were inserted into Aspergillus for reoxidizing NADH to NAD+ under anaerobic conditions, thereby increasing the productivity and yield of citrate on glucose (de Jongh and Nielsen 2008; Enomoto et al. 2002). In the present study, the results revealed that expressing soluble FRDS1 from S. cerevisiae in E. coli could modulate not only the NADH/NAD+ ratio but also the ATP level under anaerobic conditions for increasing succinate production (Cabrera et al. 2011; Hadicke et al. 2015; Liang et al. 2012; Singh et al. 2009; Vemuri et al. 2006; Zhang et al. 2016).
In this study, sole anaerobic NADH dehydrogenase and ATP futile cycling were employed to regulate the NADH/NAD+ ratio and ATP level in the interface from the aerobic to anaerobic phase, respectively.
In previous studies, through respiratory chain engineering, combining various NADH dehydrogenases and terminal oxidases with different efficiencies of proton translocation enabled E. coli flexibility to provide diverse growth conditions to produce fermentation products (Becker et al. 1997; Portnoy et al. 2008). Yokota et al. observed the highest rate of glucose metabolism in the double mutant (NAD-1 and cytochrome bo3; Kihira et al. 2012). In a glucose-limited chemostat, a branched electron transport chain was adopted rather than a linear one, and the growth behavior and acetate formation were analyzed under this condition (Steinsiek et al. 2014). The quinone synthesis pathway (Wu et al. 2015) and NDH-II dehydrogenase (Liu et al. 2014) were manipulated for enhancing lactate and polyhydroxybutyrate production, respectively. Furthermore, in succinate production, various strategies for reducing the NADH/NAD+ ratio have been employed. These strategies include regeneration of NAD+ in the reductive TCA cycle (Goldberg et al. 1983; Liang et al. 2011; Millard et al. 1996; Stols and Donnelly 1997) and provision of additional reducing power (Van der Werf et al. 1997). In the present study, only one type of NADH dehydrogenase of the respiratory chain was adopted, thus increasing the overall yield of succinate and alleviating the change in the NADH/NAD+ ratio during the entire fermentation process.
High ATP levels have been proven to inhibit the activities of key enzymes involved in the central metabolism, thus downregulating the glycolytic pathway and TCA cycle (Kihira et al. 2012; Kim et al. 2008; Weitzman 1981). By using enhanced futile cycling to decrease the ATP supply, production rates and yields of fermentation products were increased, and glucose consumption was elevated under aerobic conditions (Chao and Liao 1994; Patnaik et al. 1992). Recently, the same strategy was also applied for improving the production of lactate under anaerobic conditions (Hadicke et al. 2015). In the present study, we proved that ATP futile cycling is an effective strategy for improving succinate yield.
Finally, our results demonstrated that a combination of these methods exerted a synergistic effect on improving the overall yield of succinate, which was 39% higher than that of the strain constructed in our previous study. Moreover, the results indicate that the regulation of the NADH/NAD+ ratio and ATP level, which is not involved in the succinate biosynthetic pathway, is an efficient strategy for succinate production.
References
Ahn JH, Jang YS, Lee SY (2016) Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol 42:54–66
Becker S, Vlad D, Schuster S, Pfeiffer P, Unden G (1997) Regulatory O2 tensions for the synthesis of fermentation products in Escherichia coli and relation to aerobic respiration. Arch Microbiol 168(4):290–296
Bekker M, de Vries S, Ter Beek A, Hellingwerf K, de Mattos MT (2009) Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase. J Bacteriol 191(17):5510–5517
Besteiro S, Biran M, Biteau N, Coustou V, Baltz T, Canioni P, Bringaud F (2002) Succinate secreted by Trypanosoma brucei is produced by a novel and unique glycosomal enzyme, NADH-dependent fumarate reductase. J Biol Chem 277(41):38001–38012
Borisov VB, Murali R, Verkhovskaya ML, Bloch DA, Han H, Gennis RB, Verkhovsky MI (2011) Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc Natl Acad Sci U S A 108(42):17320–17324
Cabrera R, Baez M, Pereira HM, Caniuguir A, Garratt RC, Babul J (2011) The crystal complex of phosphofructokinase-2 of Escherichia coli with fructose-6-phosphate: kinetic and structural analysis of the allosteric ATP inhibition. J Biol Chem 286(7):5774–5783
Calhoun MW, Oden KL, Gennis RB, de Mattos MJ, Neijssel OM (1993) Energetic efficiency of Escherichia coli: effects of mutations in components of the aerobic respiratory chain. J Bacteriol 175(10):3020–3025
Camarasa C, Faucet V, Dequin S (2007) Role in anaerobiosis of the isoenzymes for Saccharomyces cerevisiae fumarate reductase encoded by OSM1 and FRDS1. Yeast 24(5):391–401
Chao YP, Liao JC (1994) Metabolic responses to substrate futile cycling in Escherichia coli. J Biol Chem 269(7):5122–5126
Coustou V, Besteiro S, Riviere L, Biran M, Biteau N, Franconi JM, Boshart M, Baltz T, Bringaud F (2005) A mitochondrial NADH-dependent fumarate reductase involved in the production of succinate excreted by procyclic Trypanosoma brucei. J Biol Chem 280(17):16559–16570
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. PNAS 97(12):6640–6645
de Graef MR, Alexeeva S, Snoep JL, Teixeira de Mattos MJ (1999) The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J Bacteriol 181(8):2351–2357
de Jongh WA, Nielsen J (2008) Enhanced citrate production through gene insertion in Aspergillus niger. Metab Eng 10(2):87–96
Enomoto K, Arikawa Y, Muratsubaki H (2002) Physiological role of soluble fumarate reductase in redox balancing during anaerobiosis in Saccharomyces cerevisiae. FEMS Microbiol Lett 215(1):103–108
Enomoto K, Ohki R, Muratsubaki H (1996) Cloning and sequencing of the gene encoding the soluble fumarate reductase from Saccharomyces cerevisiae. DNA Res 3(4):263–267
Forster AH, Gescher J (2014) Metabolic engineering of Escherichia coli for production of mixed-acid fermentation end products. Frontiers Biotech Bioeng 2:16
Goldberg I, Lonberg-Holm K, Bagley EA, Stieglitz B (1983) Improved conversion of fumarate to succinate by Escherichia coli strains amplified for fumarate reductase. Appl Environ Microbiol 45(6):1838–1847
Gordon EH, Pealing SL, Chapman SK, Ward FB, Reid GA (1998) Physiological function and regulation of flavocytochrome c3, the soluble fumarate reductase from Shewanella putrefaciens NCIMB 400. Microbiology 144:937–945
Hadicke O, Bettenbrock K, Klamt S (2015) Enforced ATP futile cycling increases specific productivity and yield of anaerobic lactate production in Escherichia coli. Biotechnol Bioeng 112(10):2195–2199
Kang Z, Gao C, Wang Q, Liu H, Qi Q (2010) A novel strategy for succinate and polyhydroxybutyrate co-production in Escherichia coli. Bioresour Technol 101(19):7675–7678
Kihira C, Hayashi Y, Azuma N, Noda S, Maeda S, Fukiya S, Wada M, Matsushita K, Yokota A (2012) Alterations of glucose metabolism in Escherichia coli mutants defective in respiratory-chain enzymes. J Bacteriol 158(4):215–223
Kim Y, Ingram LO, Shanmugam KT (2008) Dihydrolipoamide dehydrogenase mutation alters the NADH sensitivity of pyruvate dehydrogenase complex of Escherichia coli K-12. J Bacteriol 190(11):3851–3858
Kuhlman TE, Cox EC (2010) Site-specific chromosomal integration of large synthetic constructs. Nucleic Acids Res:gkp1193
Leonardo MR, Dailly Y, Clark DP (1996) Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol 178(20):6013–6018
Li Y, Huang B, Wu H, Li Z, Ye Q, Zhang YP (2016) Production of succinate from acetate by metabolically engineered Escherichia coli. ACS Synth Biol 5(11):1299–1307
Li Y, Li M, Zhang X, Yang P, Liang Q, Qi Q (2013) A novel whole-phase succinate fermentation strategy with high volumetric productivity in engineered Escherichia coli. Bioresour Technol 149:333–340
Liang L, Liu R, Wang G, Gou D, Ma J, Chen K, Jiang M, Wei P, Ouyang P (2012) Regulation of NAD(H) pool and NADH/NAD+ ratio by overexpression of nicotinic acid phosphoribosyltransferase for succinic acid production in Escherichia coli NZN111. Enzym Microb Technol 51(5):286–293
Liang LY, Liu RM, Ma JF, Chen KQ, Jiang M, Wei P (2011) Increased production of succinic acid in Escherichia coli by overexpression of malate dehydrogenase. Biotechnol Lett 33(12):2439–2444
Liang Q, Qi Q (2014) From a co-production design to an integrated single-cell biorefinery. Biotechnol Adv 32(7):1328–1335
Liang Q, Zhang F, Li Y, Zhang X, Li J, Yang P, Qi Q (2015) Comparison of individual component deletions in a glucose-specific phosphotransferase system revealed their different applications. Sci Rep 5:13200
Liu Q, Lin Z, Zhang Y, Li Y, Wang Z, Chen T (2014) Improved poly(3-hydroxybutyrate) production in Escherichia coli by inactivation of cytochrome bd-II oxidase or/and NDH-II dehydrogenase in low efficient respiratory chains. J Bacteriol 192:170–176
Liu R, Liang L, Li F, Wu M, Chen K, Ma J, Jiang M, Wei P, Ouyang P (2013) Efficient succinic acid production from lignocellulosic biomass by simultaneous utilization of glucose and xylose in engineered Escherichia coli. Bioresour Technol 149:84–91
Meng J, Wang B, Liu D, Chen T, Wang Z, Zhao X (2016) High-yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum in Escherichia coli. Microb Cell Factories 15(1):141
Millard CS, Chao YP, Liao JC, Donnelly MI (1996) Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl Environ Microbiol 62(5):1808–1810
Muratsubaki H, Enomoto K (1998) One of the fumarate reductase isoenzymes from Saccharomyces cerevisiae is encoded by the OSM1 gene. Arch Biochem Biophys 352(2):175–181
Olajuyin AM, Yang M, Liu Y, Mu T, Tian J, Adaramoye OA, Xing J (2016) Efficient production of succinic acid from Palmaria palmata hydrolysate by metabolically engineered Escherichia coli. Bioresour Technol 214:653–659
Patnaik R, Roof WD, Young RF, Liao JC (1992) Stimulation of glucose catabolism in Escherichia coli by a potential futile cycle. J Bacteriol 174(23):7527–7532
Portnoy VA, Herrgard MJ, Palsson BO (2008) Aerobic fermentation of D-glucose by an evolved cytochrome oxidase-deficient Escherichia coli strain. Appl Environ Microbiol 74(24):7561–7569
Salusjarvi L, Kaunisto S, Holmstrom S, Vehkomaki ML, Koivuranta K, Pitkanen JP, Ruohonen L (2013) Overexpression of NADH-dependent fumarate reductase improves D-xylose fermentation in recombinant Saccharomyces cerevisiae. J Ind Microbiol Biotechnol 40(12):1383–1392
Singh A, Cher Soh K, Hatzimanikatis V, Gill RT (2011) Manipulating redox and ATP balancing for improved production of succinate in E. coli. Metab Eng 13(1):76–81
Singh A, Lynch MD, Gill RT (2009) Genes restoring redox balance in fermentation-deficient E. coli NZN111. Metab Eng 11(6):347–354
Steinsiek S, Stagge S, Bettenbrock K (2014) Analysis of Escherichia coli mutants with a linear respiratory chain. PLoS One 9(1):e87307
Stols L, Donnelly MI (1997) Production of succinic acid through overexpression of NAD(+)-dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol 63(7):2695–2701
Stouthamer A (1979) The search for correlation between theoretical and experimental growth yields. Int Rev Biochem 21(1):1–47
Tielens AG, Van Hellemond JJ (1998) The electron transport chain in anaerobically functioning eukaryotes. Biochim Biophys Acta 1365(1–2):71–78
Van der Werf MJ, Guettler MV, Jain MK, Zeikus JG (1997) Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol 167(6):332–342
Van Hellemond JJ, Tielens AG (1994) Expression and functional properties of fumarate reductase. The Biochem J 304:321–331
Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol 72(5):3653–3661
Vemuri GN, Eiteman MA, Altman E (2002) Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 68(4):1715–1727
Verkhovskaya ML, Belevich N, Euro L, Wikstrom M, Verkhovsky MI (2008) Real-time electron transfer in respiratory complex I. Proc Natl Acad Sci U S A 105(10):3763–3767
Vuoristo KS, Mars AE, Sanders JP, Eggink G, Weusthuis RA (2016) Metabolic engineering of TCA cycle for production of chemicals. Trends Biotechnol 34(3):191–197
Wang C, Yan D, Li Q, Sun W, Xing J (2014) Ionic liquid pretreatment to increase succinic acid production from lignocellulosic biomass. Bioresour Technol 172:283–289
Weitzman PD (1981) Unity and diversity in some bacterial citric acid-cycle enzymes. Adv Microb Physiol 22:185–244
Werpy T, Petersen G (2004) Volume 1: results of screening for potential candidates from sugars and synthetic gas. Oak Ridge, TN, US Department of Energy
Wu H, Tuli L, Bennett GN, San KY (2015) Metabolic transistor strategy for controlling electron transfer chain activity in Escherichia coli. Metab Eng 28:159–168
Zhang F, Li J, Liu H, Liang Q, Qi Q (2016) ATP-based ratio regulation of glucose and xylose improved succinate production. PLoS One 11(6):e0157775
Zhu X, Tan Z, Xu H, Chen J, Tang J, Zhang X (2014) Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli. Metab Eng 24:87–96
Acknowledgements
This work was financially supported by a grant from the National Basic Research Program of China (2012CB725202), a grant from the National Natural Science Foundation of China (31170097, 31370085), and a grant from the Shandong Science and Technology Development Plan (2015GSF121042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
.
ESM 1
(PDF 182 kb)
Rights and permissions
About this article
Cite this article
Li, J., Li, Y., Cui, Z. et al. Enhancement of succinate yield by manipulating NADH/NAD+ ratio and ATP generation. Appl Microbiol Biotechnol 101, 3153–3161 (2017). https://doi.org/10.1007/s00253-017-8127-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8127-6