Abstract
The fermentation process of l-lactic acid is well known. Little importance was attached to d-lactic acid, but in the past 10 years, d-lactic acid gained significantly in importance. d-Lactic acid is an interesting precursor for manufacturing heat-resistant polylactic acid (PLA) bioplastics which can be widely used, for example as packaging material, coatings, for textiles or in the automotive industry.
This review provides a comprehensive overview of the most recent developments, including a spectrum of studied microorganisms and their capabilities for the production of d-lactic acid. Additionally, the technological achievements in biotechnological d-lactic acid production including fermentation techniques like fed batch, simultaneous saccharification, and fermentation and continuous techniques are presented. Attention is also turned to suitable alternative substrates and their applicability in fermentation processes. Furthermore, advantages and disadvantages of product recovery and purification are discussed. Economic aspects of PLA are pointed out, and the present industrial producers of lactic acid are briefly introduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The depleting petrochemical resources and the increasing consumption of oil-based plastics are a growing problem in our society. Thus, it is very important to develop new bio-based products and to establish alternative production processes in the industry. One of the most promising bio-based polymers is polylactic acid (PLA) which is already widely used as a packaging material.
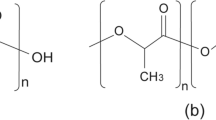
Several forms of PLA can be synthesized by varying the ratio between the l- and d-isomer of lactic acid. Polymers made of enantiomerically pure l- or d-lactic acid are known as PLLA and PDLA, both with a melting point of 170–180 °C. The polymer achieved from the racemic mixture of lactic acid results in amorphous materials.
Enantiomerically pure PLAs, PLLA, and PDLA form a stereocomplex (scPLA) that has a melting temperature of 220–230 °C (Fig. 1, Table 1) (Ikada et al. 1987; Tsuji 2005). The reason for the improved properties of the isotactic stereocomplex PLA (scPLA) is the dense packing of the polymeric crystalline regions. The increased stability can be explained by the sum of intermolecular hydrogen bridge bonds between the methyl group and the carbonyl group of the opposite homochiral polymer chains (Zhang et al. 2005). Besides its superior thermal stability, this stereocomplex has further advantages regarding mechanical performance and hydrolysis resistance (Tsuji 2005). Due to the increased heat resistance, scPLA has the potential to replace petroleum-based packaging materials and increases the application possibilities of PLA for the electronic sector and the automotive industry (Auras et al. 2005; Garlotta 2001). The current price of PLA is 2.0–2.2 $/kg. In order to compete with petrochemical polymers, research is necessary to open up new opportunities to produce PLA more cheaply. Especially, the cost of substrate and fermentation media must be reduced (Datta et al. 1995; E4tech 2015).
The chemical synthesis of lactic acid is based on petrochemical resources such as acetaldehyde and hydrocyanic acid and yields only in a racemic mixture of lactic acid. For this reason, 70–90 % of the yearly worldwide production of lactic acid, which amounts up to 300,000 tons, is gained by fermentation (National STEM Centre 2011; Datta et al. 1995; Ghaffar et al. 2014; Wee et al. 2006).
So far, the industrial production by fermentation is limited to l-lactic acid due to its application in the food industry and the predominant existence of l-lactic acid-producing bacteria. This fermentation process has previously been reviewed (Abdel-Rahman et al. 2013; Datta and Henry 2006; Ghaffar et al. 2014; Hofvendahl and Hahn-Hagerdal 2000; John et al. 2007; Okano et al. 2010; Vijayakumar et al. 2008; Wee et al. 2006). In contrast, little importance was attached to d-lactic acid, but in the past 10 years, d-lactic acid gained significantly in importance. Accordingly, a stable and highly productive fermentation process for d-lactic acid is required, and microorganisms producing enantiomerically pure d-lactic acid with a high selectivity are of great interest. A pilot scale demonstration of d-lactic acid fermentation (6 ton vessel) is described by Liu et al. using a metabolically engineered Escherichia coli strain (Liu et al. 2014), but only few wild-type strains, such as several Sporolactobacillus spp. and Lactobacillus spp., are able to produce d-lactic acid of a suitable purity and efficiency (Tashiro et al. 2011). Because the polymer properties are influenced by the enantiomerical purity of d-lactic acid, high enantiomerical purities of more than 99 % are preferred.
This review provides an overview of d-lactic acid-producing microorganisms, suitable fermentation strategies, and the use of agricultural by-products as alternative substrates. Furthermore, the product purification and recovery are presented and insights into economical and industrial aspects are delivered.
d-Lactic acid producing microorganisms
Microorganisms used for the efficient biotechnological production of d-lactic acid have to meet several requirements. In terms of industrial applications, it is necessary that d-lactic acid be produced from low-cost resources with a high yield and a high enantiomeric purity in a short fermentation time. Several factors like medium composition, growth conditions, pH, and operation mode influence the enantiomeric purity (Hofvendahl and Hahn-Hagerdal 2000).
The lactic acid fermentation is part of the primary metabolism and takes place with different intensity in bacteria, yeast, algae as well as in cyanobacteria.
The most used lactic acid-producing bacteria are from the genus Lactobacillus, E. coli und Corynebacterium glutamicum. E. coli and C. glutamicum form more organic acid by-products; therefore, only genetically modified strains are used for lactic acid production (Chang et al. 1999; Wieschalka et al. 2013). The same applies to the lactic acid production with yeast (Adachi et al. 1998). Photosynthetic microorganisms like algae and cyanobacteria produce glucose or starch from CO2, which can be further converted to lactic acid by some strains. Nannochlorum sp.26A4 produced d-lactic acid with high enantiomerical purity and a yield of 70 % under dark anaerobic conditions (Hirayama and Ueda 2004). Although the substrate costs are eliminated, algae are rarely used in industrial processes because of the high costs caused by the need for supplemental lighting supply and the purchase of photobioreactors (Chen et al. 2011).
An overview is presented of known d-lactic acid-producing bacteria (d-LAB) and yeasts which are investigated for further biotechnological production of enantiomerically pure d-lactic acid. The bacteria can be distinguished into wild-type strains, mutants derived by random mutagenesis, and genetically engineered strains.
Wild-type strains and strains generated by random mutagenesis
The most studied wild-type d-LAB (or conservative mutants) pertains to the genus Lactobacillus along with studies focusing on Sporolactobacilli (Table 2). Both genera have a facultative anaerobic metabolism, are Gram-positive, catalase-negative, and generally recognized as safe (GRAS status). Sporolactobacilli are known as mesophilic d-LAB which grow in a temperature range of 20–45 °C whereas some Lactobacillus delbrueckii strains prefer temperatures between 45 and 47 °C for proliferation (Demirci and Pometto 1992; Hofvendahl and Hahn-Hagerdal 2000). Mesophilic growth temperatures increase the risk of contaminations and conflict with high optimum temperatures of most enzymes used in simultaneous saccharification and fermentation (SSF) techniques (see below).
Lactobacilli are finding broad applications in food industry and biotechnology because of their metabolic versatility, for example the utilization of various sugars such as disaccharides and pentoses as well as hexoses (Giraffa et al. 2010).
Additionally, LAB can be classified as homofermentative and heterofermentative due to their ability to metabolize sugars by different pathways (Kandler 1983).
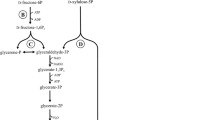
To generate energy in the form of ATP, homofermentative LAB metabolize hexoses like glucose via the Embden-Meyerhof-Parnas (EMP) pathway (Meyerhof 1948) and pentoses via the pentose phosphate (PP) pathway (Horecker et al. 1954). The aldolase which is a key enzyme of homofermentative lactic acid fermentation divides the intermediately formed fructose-1,6-diphosphate into glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (Buyze et al. 1957). This is isomerized to GAP and finally converted to pyruvate in several steps (Fig.2 Glycolysis). Pyruvate can be subsequently converted into d-lactic acid by a d-lactate dehydrogenase (d-LDH) with a theoretical yield of 1.0 g/g hexose (2 mol/mol) (Wang et al. 2015a). Metabolizing pentoses, 3 mol of pentose generate 1 mol GAP and 2 mol fructose-6-phosphate in the PP-pathway. Fructose-6-phosphate is converted to pyruvate in several steps and lactic acid with a theoretical yield of 1.0 g/g pentose (1.67 mol/mol) (Elsden and Peel 1958).
Heterofermentative LAB uses the phosphoketolase pathway to convert pentoses and hexoses to xylulose-5-phosphate which is divided via the key enzyme phosphoketolase into GAP and acetyl phosphate (Fukui et al. 1957; Elsden and Peel 1958). Hereby, one molecule of d-lactic acid, acetate, or ethanol and carbon dioxide are formed (Fig. 2 Phosphoketolase pathway) (Basso et al. 2014; Zaunmuller et al. 2006). The theoretical yield of heterofermentative lactic acid production is 0.5 g/g glucose (1.0 mol/mol) and 0.6 g/g pentose (1.0 mol/mol).
The reaction of pyruvate to l-lactic acid is catalyzed by l(+)-lactate dehydrogenase (EC1.1.1.27), while the reaction of pyruvate to d-lactic acid is catalyzed by d(−)-lactate dehydrogenase (EC1.1.1.28). Most strains possess both lactate dehydrogenases, but their activity and therefore the resulting enantiomeric ratio varies between strains (Garvie 1980). According to the strain, the enantiomeric ratio can be influenced through fermentation conditions, such as pH, temperature, NaCl concentration, nutrient concentration, or gas supply (Bobillo and Marshall 1991; Yoo et al. 1996).
Compared to the phosphoketolase pathway, the glycolysis is more effective with regard to lactic acid yield as well as sugar utilization (Wang et al. 2015a). Most lactic acid bacteria metabolize hexoses homofermentatively and pentoses heterofermentatively. Abdel-Rahman et al. (2011b) depict a wild-type strain producing homofermentative l-lactic acid from xylose. Also a few metabolically engineered strains are able to convert pentoses using the homofermentative lactic acid production (Okano et al. 2009a; Okano et al. 2009b; Tsuge et al. 2014; Wang et al. 2011b; Wang et al. 2015a), which is favorable for the utilization of lignocellulose-containing biomass.
Table 2 gives an overview of the most promising results in d-lactic acid production by wild-type strains and strains generated by random mutagenesis. Wang et al. (2011a) produced in a fed batch mode 207 g/L d-lactic acid with a purity of 99.3 % with the wild-type strain Sporolactobacillus inulinus CASD using glucose as carbon source and peanut meal as nitrogen source.
Li et al. (2016) produced enantiomerically pure d-lactic acid from xylose combining two native bacterial microorganisms. During the first cultivation step Weissella sp.S26 produces 13.7 g/L lactic acid including 13.2 g/L d-lactic acid and 8.5 g/L acetic acid using 2 % xylose as sole carbon source. In the second cultivation step, the sterilized broth was inoculated with Bacillus sp.ADS3. Bacillus sp.ADS3 is able to digest acetic acid and lactic acid. This strain preferred the digestion of acetic acid over lactic acid and l-lactic acid over d-lactic acid. Therefore, the acetic acid was completely removed from the fermentation broth with an increased enantiomerical purity of d-lactic acid (98.5 %).
Genetically engineered strains
d-Lactic acid production by genetically engineered microorganisms is generally based on three major strategies:
-
1.
The heterologous expression of d-LDH genes from d-lactic acid-producing strains in microorganisms with favorable abilities like minimal nutritional requirements or homofermentative pentose conversion. There are several attempts using the rapid growing bacteria E. coli and C. glutamicum for d-lactic acid production on mineral salt media (Lu et al. 2016; Zhou et al. 2016; Okino et al. 2008).
-
2.
The deletion of competing pathways results in higher yields and productivities. A widely used modification is the deletion of l-LDH to improve the enantiomeric purity of d-lactic acid and the interruption of pathways leading to other mixed acid fermentation products (Jia et al. 2011; Yi et al. 2016). Saccharomyces cerevisiae produces large amounts of ethanol and other mixed acid fermentation products. Due to its ability to grow under acidic conditions, the amount of neutralizing agent and purification costs for d-lactic acid can be reduced. An efficient d-lactic acid production can be achieved by disrupting pathways leading to by-products (Baek et al. 2016).
-
3.
The enablement of pentose conversion via the homofermentative pentose phosphate pathway for the efficient production of d-lactic acid from pentoses and lignocellulosic biomass as substrates. Zhang et al. (2016a) modified l-LDH deficient L. plantarum for d-lactic acid production from corn stover and sorghum stalks by inserting xylose assimilating genes encoding xylose isomerase and xylulokinase.
Table 3 gives an overview of the most promising results in d-lactic acid production by genetically modified organisms (GMOs).
Tsuge et al. (2015) produced 195 g/L d-lactic acid within 80 h with C. glutamicum holding a d-LDH from L. delbrueckii. Overexpression of glycolytic genes, in particular of phosphofructokinase, resulted in increased d-lactic acid production. In addition to l-LDH knock-out and d-LDH insertion, Assavasirijinda et al. (2016) disrupted the exopolysaccharide biosynthesis to obtain an increased d-lactic acid titer of 142 g/L by fermentation of an alkaliphilic Bacillus strain with NaOH as neutralizer instead of CaCO3 to prevent the accumulation of CaSO4.
Feng et al. (2014) genetically manipulated Klebsiella pneumoniae to produce d-lactic acid using glycerol as substrate. Overexpression of lactate dehydrogenase LdhA and knockout of genes associated with 1,3-PDO synthesis significantly improved the d-lactic acid production to 142.1 g/L and a productivity of 2.96 g/(L h). Inserting d-LDH in E. coli, alternative substrates such as crude glycerol can be converted to d-lactic acid (Ganesh et al. 2012; Mazumdar et al. 2010; Wang et al. 2015b).
Li et al. (2015) induced d-lactic acid production in the cyanobacterium Synechococcus elongatus PCC7942 by insertion of a d-LDH from Lactobacillus bulgaricus, enabling the usage of NADPH instead of NADH as a cofactor for LDH and introduction of a lactic acid transporter. However, this attempt resulted in low d-lactic acid production of 1.31 g/L with a productivity of 221 mg/L per day.
The drawback to using genetically engineered strains for industrial purposes in the European Union is in the regulation of genetically modified food and feed. In general, the fermentation waste can be used to produce animal feed which can be sold as well as the product, but the sale of animal feed within the EU is strictly regulated if the fermentation waste contains genetically modified biomass (European Parliament and the Council 2003). For this reason, there would be additional costs for waste disposal which could make the production process economically inefficient.
Fermentation strategies to optimize d-lactic acid production
The fermentation strategy to achieve optimal fermentation conditions depends on the used microorganism and substrate. The following section discusses strategies suitable for homofermentative d-lactic acid producing bacteria including batch, fed batch, continuous methods, and immobilized cells.
Batch and fed-batch fermentation
Batch fermentation strategies are less susceptible to contaminations in comparison to fed batch fermentations and high lactic acid concentration with appropriate yield can be attained (Hofvendahl and Hahn-Hagerdal 2000; Sun et al. 2015; Xu et al. 2010). A drawback of this method is the occurring substrate and product inhibition so that batch experiments generally suffer from low productivities.
In order to increase the productivity and to decrease the fermentation times, fed batch strategies are more favorable in comparison to batch methods. Advantages of this method are that substrate concentrations are kept on a subcritical level which reduces inhibition effects and minimizes the lag-phase of microbial growth (Abdel-Rahman et al. 2011a). Consequently, the sugar consumption and the productivity of the biocatalyst can be increased. By applying a two-step fed batch strategy on the fermentation of Sporolactobacillus laevolacticus DSM442, Li et al. (2013) demonstrated that keeping the glucose concentration (60 g/L) on a subcritical level results in a high productivity of 4.13 g/(L h) with a final concentration of 144.4 g/L d-lactic acid after 35 h (Table 4).
Continuous fermentation and cell immobilization
Continuous techniques are useful to reduce process costs by delivering the needed nutrients for fermentation with a constant flow to keep them optimally concentrated. More importantly, this method is reported to gain significantly higher productivities compared to other fermentation strategies for d-lactic acid production (Table 4) (Mimitsuka et al. 2012; Tashiro et al. 2011). Critical factors using continuous methods are the efflux of non-utilized substrate, cell loss, and a decreased lactic acid concentration by an increasing dilution rate (Abdel-Rahman et al. 2013; Zhang et al. 2011).
A combination of continuous fermentation with cell recycling or cell immobilization is an efficient method to gain high cell densities and high productivities. By separating the product from the fermentation broth or diluting it with fresh medium, product inhibition effects can be avoided. Tashiro et al. (2011) applied a continuous, cell-retaining method using L. delbrueckii ssp. lactis QU 41 for fermentation. The MRS medium was supplemented with 20 g/L glucose and refreshed with dilution rates of 0.56 and 0.87 per hour, which resulted in a productivity of 18 g/(L h) with a low residual glucose concentration. Unfortunately, only a short fermentation time of 35 h was applied so that the durability of this continuous process was not proven (Tashiro et al. 2011). A second study concerning continuous fermentation by retaining cells is published by Mimitsuka et al. (2012) using S. laevolacticus JCM 2513. This study focused on the reduction of nutrients for the fermentation and the examination of the long-term stability of this process over 800 h. By using a membrane-integrated fermentation reactor and a reduced amount of yeast extract, an average productivity of 11.2 g/(L h) could be achieved.
Zhao et al. (2014) recently reported results applying fed batch fermentation using a multi-pulse feeding strategy (with hydrolyzed corn flour as substrate) in a fibrous bed reactor (FBB) in combination with immobilized cells. A final titer of 218.8 g/L d-lactic acid can be achieved within 132 h.
Fermentation of alternative substrates
Glucose is a widely used substrate in fermentation processes, but refined sugars are expensive and the raw material costs have a significant influence on the product price (Tejayadi and Cheryan 1995). For this reason, the utilization of alternative substrates is desired. Alternative substrates are polysaccharides, such as starch or cellulose, as well as agricultural raw materials and side products which contain a high amount of polysaccharides or monomeric sugars. Furthermore, the utilized substrate should not conflict with the food industry. Table 5 shows many attempts cited in literature to replace refined glucose by alternative substrates.
Due to the fact that the d-lactic acid-producing strains do not provide the enzymes to directly convert such alternative substrates, high molecular carbohydrates have to be disintegrated to fermentable monomer sugars either by enzymatic or chemical hydrolysis. Whereas chemical pretreatment/hydrolysis can exclusively be carried out prior to fermentation, the enzymatic digest can be carried out either prior to the fermentation or during fermentation. For this purpose, enzymes such as amylases, pullulanase, cellulase, and β-glucosidase can be used, depending on the substrate (Fukushima et al. 2004; Nakano et al. 2012; Nguyen et al. 2012; Singhvi et al. 2010).
A separate hydrolysis and fermentation (SHF) strategy is time consuming due to two step processing and the product formation, in particular intermediately formed cellobiose, leads to product inhibition of the enzymes (Lu et al. 2009; Singhvi et al. 2010). Consequently, higher amounts of enzyme are needed to achieve an appropriate yield of hydrolyzed sugar which makes this method more cost-intensive. Yanez et al. (2005) figured out that the efficiency of the enzymatic hydrolysis can be further increased when the substrate is pre-treated with NaOH, but another more established strategy is to avoid the product inhibition of enzymes in a simultaneous saccharification and fermentation (SSF). In this process, the fermentable sugars are gradually hydrolyzed to substrate level and simultaneously converted into lactic acid by the bacteria. Furthermore, by applying SSF, the bacteria benefit from an initial low substrate concentration which avoids substrate inhibition and the productivity can be enhanced. A disadvantage of this method is the fact that the optimal conditions of the enzymatic hydrolysis (pH value and temperature) can differ from the optimal fermentation conditions, which lead to a lower efficiency (Hofvendahl and Hahn-Hagerdal 2000).
Table 5 highlights the most important d-lactic acid fermentations based on alternative carbon sources. These works show large differences in their final concentrations caused by the different bacterial strains, the utilized substrate and nutrient source, the initial sugar amounts, and the fermentation techniques.
Zhao et al. (2014) demonstrated that high d-lactic acid concentrations of 145.8 g/L in a batch fermentation with immobilized cells and 218 g/L in a fed-batch fermentation (Table 4) can be obtained by fermenting corn flour with Sporolactobacillus inulinus Y2–8. Nguyen et al. (2013a) conducted a batch SSF process with raw sweet potato as carbon source with Lactobacillus coryniformis. L. coryniformis produced homofermentatively a final titer of 186.4 g/L d-lactic acid, with a productivity of 3.11 g/(L h) and a yield of 0.85 (g/g). However, an increasing concentration of yeast extract and peptone led to an increased d-lactic acid production and yield through the SSF process. The supplementation with 7 g/L yeast extract and 3 g/L peptone showed the optimized values for the SSF process.
The d-lactic acid yields vary. Nguyen et al. (2012) reached a low yield of 0.46 g/g, while Calabia and Tokiwa (2007) reached a much higher yield of 0.95 g/g, but these results are only conditionally comparable due to their differing reference of initial sugar amount and the initial substrate amount, which differs in its sugar content. Concerning the productivities, Kangming (2012) and Nakano et al. (2012) achieved distinguished results with more than 3.5 g/(L h). The highest productivity of 5.25 g/(L h) and a final titer of 189 g/L d-lactic acid was attained by Reddy Tadi et al. (2015) in the batch fermentation of unrefined palmyra palm jaggery and whey protein hydrolyzate as nutrient source with S. inulinus.
Besides the carbon source, lactic acid bacteria grow in nutrient-rich media so that the addition of supplines is necessary. However, yeast extract is very expensive and Tejayadi and Cheryan (1995) estimated it to be 38 % of total production cost.
Yeast extract contains a lot of necessary nutrients, such as free amino acids and peptides, B vitamins, trace elements, and nucleotides which can also be supplied by protein-rich agricultural raw materials as alternative nutrient source. Fermentations using such alternative nutrient sources are shown in Table 5, sections B and C. In Table 5, section C, both the carbon and the nutrient sources are replaced. So far, the best result was achieved by Wang et al. (2011a) in an enzymatic hydrolysis and simultaneous fermentation (similar to the SSF), yielding in a high-end concentration of 207 g/L d-lactic acid with a productivity of 3.8 g/(L h). Li et al. (2013) carried out an analog experiment with S. laevolacticus and cottonseed as alternative nutrient source. Okino et al. (2008) used a metabolically engineered C. glutamicum strain, which was able to grow on mineral salt medium. Kangming (2012) achieved similar results with an E. coli strain.
In most cases, the use of yeast extract substitutes has a negative impact on the d-lactic acid production. The high content of B vitamins in yeast extracts is often named as a reason for this, but such assumptions are difficult to make because the nutritional requirements are specific for each strain (Curk et al. 1993; Nancib et al. 2005).
Product recovery and purification
The fermentative production of d-lactic acid requires efficient product purification methods. Depending on the purification method, the utilization of renewable raw materials can entail higher challenges than pure substrates.
Product recovery and purification are said to amount to up to 50 % of the production costs, and thus, they are crucial to the quality and the price of the product (Eyal and Bressler 1993). The purification processes for racemic or l-lactic acid has already been reviewed by Wasewar, López-Garzón and Straathof and do not differ from the purification of d-lactic acid (Lopez-Garzon and Straathof 2014; Wasewar 2005).
The method most commonly used in industrial processes is the precipitation of calcium lactate that can be induced by the application of calcium carbonate or calcium hydroxide for pH control during the fermentation. Calcium lactate is separated from the broth by filtration and dissolved again as lactic acid adding sulfuric acid, whereby calcium sulfate precipitates. After filtration of calcium sulfate, the filtrate is purified using activated carbon, separated, and concentrated. The obtained lactic acid is esterified with methanol, resulting in methyl lactate, which can be easily purified by distillation. Methyl lactate is finally hydrolyzed to lactic acid and methanol by the addition of water (Groot et al. 2010; Wasewar 2005). These reaction steps are shown in Fig. 3. Although large amounts of CaSO4 are formed as by-products, this recovery method is still applied in industry.
Alternatively, in situ product removal methods can be applied. Table 6 shows a summary of the most important continuous purification techniques in comparison to the precipitation of lactic acid.
Further, but less common, work-up methods are the separation by supported liquid membranes, emulsion liquid membranes, aqueous two-phase systems, and reverse osmosis (Wasewar 2005).
Economic aspects
The total polymer consumption of western Europe is about 50 million tonnes per year, and technically 85 % of the these polymers could be substituted by biobased plastics (Shen et al. 2009). According to the projections of the European Bioplastics Association, there is ongoing rapid market growth concerning bio-based plastics by increasing the global production capacity from 1.7 million tonnes in 2014 to approximately 7.85 million tonnes in 2019. An illustration of the material share (in %) of the bioplastic production of 2014 is given in Fig.4. PLA has one of the largest shares of 12.2 % of the total biopolymer production capacities after Bio-PET 30 (35.4 %) and bio-based polyethylene (Bio-PE 11.8 %). Moreover, the PLA market is still expected to grow fourfold between 2013 and 2020 due to its variety of applications. PLA has extensive applications as a biopolymer in the textile and automotive industries, but it can especially be used for daily use packing material (European Bioplastics, Institute for Bioplastics and Biocomposites, nova-Institute (2014), (Cellulac 2013; European-Bioplastics 2015). NatureWorks is the largest producer of bio-based lactides, with high-purity, polymer-grade lactide rich in meso-lactide (NatureWorks 2016). Today, the world’s largest lactic acid company and first industrial producer of d-lactic acid is Corbion Purac. With the fermentative technology from Myriant, Corbion Purac has been producing d-lactic acid for bioplastic applications since 2008 (Myriant-Corporation 2011).
Global production capacities of bioplastics 2014 (by material type) (European-Bioplastics 2015). 1 Biobased content amounts to 30 %, 2 contains durable starch blends, bio-polycarbonate, bio-thermoplastic elastomers, bio-polyurethane (except thermosets), 3 blend components incl. in main materials, 4 contains fossil-based polybutyrate adipate terephthalate, polybutylene succinate, polycaprolactone, 5 incl. newlight technologies (CO2-based), 6 compostable hydrated cellulose foils, 7 biodegradable cellulose ester (Bio-PET bio-based polyethylene terephthalate, Bio-PE bio-based polyethylene, PTT polytrimethylene erephthalate, Bio-PA bio-based polyamide, PLA polylactic acid, PHA polyhydroxyalkanoates)
In May 2014, Corbion Purac started developing heat-resistant PLA in a partnership with FKuR Kunststoff GmbH (Corbion-Purac 2014). The Company Cellulac possesses the technology to produce commercial quantities of lactic acid, l(+) and d(−), and lactate esters from a range of agricultural residues and food by-products. Cellulac optimized the usage of lignocellulosic materials for lactic acid production (Cellulac 2016). An industrial level of continuous production of enantiomerically pure d-lactic acid, suitable for conversion to bioplastics, from deproteinized lactose whey was shown in 2014 (Cellulac 2014). Another leading supplier in lactic acid and d-lactic acid products is Galactic, which founded, together with Total Petrochemicals, the joint venture Futerro in 2007 to develop innovative technologies for PLA production. In 2010, they inaugurated a PLA pilot plant in Belgium which was also the first in Europe (Futerro 2010). Furthermore, Galactic supplies lactic acid products for food, feed, cosmetics, pharmaceutical products, and neuraceutical industry.
The selling price of lactic acid/PLA is extremely variable and depends on the degree of purity. For example, Cellulac sells lactic acid for bioplastic applications in a price range of US$1300–5000 per metric ton and the prices for PLA as substitute for petrochemical derived polymers range between US$2300 and 6000 per metric ton (Cellulac 2013).
Concluding remarks
d-Lactic acid plays an important role in the establishment of PLA as a substitute for our daily used petrochemical plastics. Even though, to date, there are only pilot scale industrial production processes, the market for d-lactic acid is estimated to grow significantly in the next years. The implementation of an industrial production is simplified because the production steps are similar to l-lactic acid production. However, the selection of highly productive d-lactic acid producing bacterial strains and the decrease of production cost by the fermentation of agricultural side products is still a focus for further research. However, some cost-associated difficulties have to be overcome to establish an economical process for the industrial production of d-lactic acid. The final cost of producing PLA depends on the efficiency of the initial fermentation process (Petersen et al. 1999). This includes the nutrients for the fermentation process, such as the carbon and nitrogen source, which are major cost factors and have to be substituted by inexpensive raw materials. Another high cost factor is the purification of lactic acid by precipitation with calcium carbonate (CaCO3) or calcium hydroxide (Ca(OH)2) as neutralizing agents. The generated calcium lactate requires several purification steps, large amount of sulfuric acid is needed, and subsequent gypsum is produced as by-product.
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2011a) Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156(4):286–301. doi:10.1016/j.jbiotec.2011.06.017
Abdel-Rahman MA, Tashiro Y, Zendo T, Hanada K, Shibata K, Sonomoto K (2011b) Efficient homofermentative L-(+)-lactic acid production from xylose by a novel lactic acid bacterium, Enterococcus mundtii QU 25. Appl Environ Microb 77(5):1892–1895. doi:10.1128/Aem.02076-10
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31(6):877–902. doi:10.1016/j.biotechadv.2013.04.002
Adachi E, Torigoe M, Sugiyama M, Nikawa J-I, Shimizu K (1998) Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. J Ferment Bioeng 86(3):284–289. doi:10.1016/S0922-338X(98)80131-1
Assavasirijinda N, Ge DY, Yu B, Xue YF, Ma YH (2016) Efficient fermentative production of polymer-grade D-lactate by an engineered alkaliphilic Bacillus sp strain under non-sterile conditions. Microb Cell Factories 15(3). doi:10.1186/s12934-015-0408-0
Auras RA, Singh SP, Singh JJ (2005) Evaluation of oriented poly(lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag Technol Sci 18(4):207–216. doi:10.1002/pts.692
Baek SH, Kwon EY, Kim YH, Hahn JS (2016) Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyces cerevisiae. Appl Microbiol Biot 100(6):2737–2748. doi:10.1007/s00253-015-7174-0
Bai ZZ, Gao Z, Sun JF, Wu B, He BF (2016) D-lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresour Technol 207:346–352. doi:10.1016/j.biortech.2016.02.007
Basso TO, Gomes FS, Lopes ML, de Amorim HV, Eggleston G, Basso LC (2014) Homo- and heterofermentative Lactobacilli differently affect sugarcane-based fuel ethanol fermentation. Anton Leeuw Int J G 105(1):169–177. doi:10.1007/s10482-013-0063-6
Bobillo M, Marshall VM (1991) Effect of salt and culture aeration on lactate and acetate production by Lactobacillus plantarum. Food Microbiol 8:153–160
Buyze G, Van den Hamer CJA, De Haan PG (1957) Correlation between hexose-monophosphate shunt, glycolytic system and fermentation-type in Lactobacilli. Antonie Van Leeuwenhoek 23(1):345–350. doi:10.1007/bf02545886
Buzatu P, Zsirai T, Aerts P, Judd SJ (2012) Permeability and clogging in an immersed hollow fibre membrane bioreactor. J Membr Sci 421:342–348. doi:10.1016/j.memsci.2012.07.039
Calabia BP, Tokiwa Y (2007) Production of D-lactic acid from sugarcane molasses, sugarcane juice and sugar beet juice by Lactobacillus delbrueckii. Biotechnol Lett 29(9):1329–1332. doi:10.1007/s10529-007-9408-4
Cellulac (2013) Cellulac–Corporate Presentation. http://cellulac.co.uk/en/wp-content/uploads/2013/02/Cellulac-Presentation.pdf. Accessed 15 January 2015
Cellulac (2014) Lactic acid from lactose whey in world first continuous production runs. http://cellulac.co.uk/en/etiam-cursus-leo-vel-metus/lactic-acid-from-lactose-whey-in-world-first-continuous-production-runs/Accessed 7 June 2016
Cellulac (2016) Cellulac - Biochemicals. http://cellulac.co.uk/en/technology/Accessed 6 June 2016
Chae HS, Lee SH, Lee JH, Park SJ, Lee PC (2013) Use of a novel Escherichia coli-Leuconostoc shuttle vector for metabolic engineering of Leuconostoc citreum to overproduce D-lactate. Appl Environ Microb 79(5):1428–1435. doi:10.1128/Aem.03291-12
Chang DE, Jung HC, Rhee JS, Pan JG (1999) Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microb 65(4):1384–1389
Chen CC, Ju LK (2002) Coupled lactic acid fermentation and adsorption. Appl Microbiol Biot 59(2–3):170–174. doi:10.1007/s00253-002-1016-6
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81. doi:10.1016/j.biortech.2010.06.159
Coelho LF, de Lima CJB, Bernardo MP, Contiero J (2011) D(−)-lactic acid production by Leuconostoc mesenteroides B512 using different carbon and nitrogen sources. Appl Biochem Biotech 164(7):1160–1171. doi:10.1007/s12010-011-9202-6
Corbion-Purac (2014) FKuR and Corbion Purac in partnership to develop heat resistant PLA compounds.http://www.corbion.com/media/press-releases?newsId=1847680. Accessed 15 January 2015
Curk MC, Peladan F, Hubert JC (1993) Characterization of Lactobacilli isolated in breweries. Lait 73(2):215–231
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies - a review. J Chem Technol Biot 81(7):1119–1129. doi:10.1002/Jctb.1486
Datta R, Tsai SP, Bonsignore P, Moon SH, Frank JR (1995) Technological and economic-potential of poly(lactic acid) and lactic-acid derivatives. FEMS Microbiol Rev 16(2–3):221–231
Demirci A, Pometto AL (1992) Enhanced production of D(−)-lactic acid by mutants of Lactobacillus delbrueckii ATCC 9649. J Ind Microbiol 11(1):23–28. doi:10.1007/Bf01583728
E4tech R-C, WUR (2015) From the Sugar Platform to biofuels and biochemicals. Final report for the European Commission. contract No. ENER/C2/423–2012/SI2.673791, https://ec.europa.eu/energy/sites/ener/files/documents/EC%20Sugar%20Platform%20final%20report.pdf. Accessed 29. Apr. 2016
Elsden SR, Peel JL (1958) Metabolism of carbohydrates and related compounds. Annu Rev Microbiol 12(1):145–202. doi:10.1146/annurev.mi.12.100158.001045
European Parliament and the Council (2003) Regulation (EC) No 1829/2003 on genetically modified food and feed. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003R1829&from=en. Accessed 16 January 2015
European-Bioplastics (2015) Institute for Bioplastics and Biocomposites, nova-Institute. www.bio-based.eu/markets and www.downloads.ifbb-hannover.de
Eyal AM, Bressler E (1993) Industrial separation of carboxylic and amino-acids by liquid membranes - applicability, process considerations, and potential advantages. Biotechnol Bioeng 41(3):287–295. doi:10.1002/bit.260410302
Feng XJ, Ding YM, Xian M, Xu X, Zhang RB, Zhao G (2014) Production of optically pure D-lactate from glycerol by engineered Klebsiella pneumoniae strain. Bioresour Technol 172:269–275. doi:10.1016/j.biortech.2014.09.074
Fukui S, Oi A, Obayashi A, Kitahara K (1957) Studies on the pentose metabolism by microorganism 1. A new type-lactic acid fermention of pentoses by lactic acid bacteria. J Gen Appl Microbiol 3(4):258–268. doi:10.2323/jgam.3.258
Fukushima K, Sogo K, Miura S, Kimura Y (2004) Production of D-lactic acid by bacterial fermentation of rice starch. Macromol Biosci 4(11):1021–1027. doi:10.1002/mabi.200400080
Futerro (2010) Futerro inaugurates bioplastics pilot unit (PLA), a first in Europe. Futerro News. http://www.futerro.com/documents/futerropressrelease20100416.pdf. Accessed 15 January 2015
Ganesh I, Ravikumar S, Hong SH (2012) Metabolically engineered Escherichia coli as a tool for the production of bioenergy and biochemicals from glycerol. Biotechnol Bioprocess Eng 17(4):671–678. doi:10.1007/s12257-011-0446-3
Gao QA, Liu FB, Zhang TC, Zhang JA, Jia SR, Yu CY, Jiang KY, Gao NF (2010) The role of lactic acid adsorption by ion exchange chromatography. PLoS One 5(11). doi:10.1371/journal.pone.0013948
Garlotta D (2001) A literature review of poly(lactic acid). J Polym Environ 9(2):63–84 . doi:10.1023/A:1020200822435doi: Unsp 1566-2543/01/0400-0063/0
Garvie EI (1980) Bacterial lactate dehydrogenases. Microbiol Rev 44(1):106–139
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: a brief review on production to purification. J Radiat Res Appl Sci 7(2):222–229. doi:10.1016/j.jrras.2014.03.002
Giraffa G, Chanishvili N, Widyastuti Y (2010) Importance of Lactobacilli in food and feed biotechnology. Res Microbiol 161(6):480–487. doi:10.1016/j.resmic.2010.03.001
Groot W, van Krieken J, Sliekersl O, de Vos S (2010) Production and purification of lactic acid and lactide poly(lactic acid). Wiley, Inc., pp 1–18
Habova V, Melzoch K, Rychtera M, Sekavova B (2004) Electrodialysis as a useful technique for lactic acid separation from a model solution and a fermentation broth. Desalination 162(1–3):361–372. doi:10.1016/S0011-9164(04)00070-0
Hama S, Mizuno S, Kihara M, Tanaka T, Ogino C, Noda H, Kondo A (2015) Production of D-lactic acid from hardwood pulp by mechanical milling followed by simultaneous saccharification and fermentation using metabolically engineered Lactobacillus plantarum. Bioresour Technol 187:167–172. doi:10.1016/j.biortech.2015.03.106
Heriban V, Škára J, Šturdík E, Ilavský J (1993) Isolation of free lactic acid using electrodialysis. Biotechnol Tech 7(1):63–68. doi:10.1007/bf00151092
Hirayama S, Ueda R (2004) Production of optically pure D-lactic acid by Nannochlorum sp. 26 A4. Appl Biochem Biotechnol 119(1):71–78
Hofvendahl K, Hahn-Hagerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Tech 26(2–4):87–107. doi:10.1016/S0141-0229(99)00155-6
Hongo M, Nomura Y, Iwahara M (1986) Novel method of lactic-acid production by electrodialysis fermentation. Appl Environ Microb 52(2):314–319
Horecker BL, Gibbs M, Klenow H, Smyrniotis PZ (1954) The mechanism of pentose phosphate conversion to hexose monophosphate. I. With a liver enzyme preparation. J Biol Chem 207(1):393–403
Huang HJ, Yang ST, Ramey DE (2004) A hollow-fiber membrane extraction process for recovery and separation of lactic acid from aqueous solution. Appl Biochem Biotech 113:671–688
Ikada Y, Jamshidi K, Tsuji H, Hyon SH (1987) Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 20(4):904–906
Ishida N, Suzuki T, Tokuhiro K, Nagamori E, Onishi T, Saitoh S, Kitamoto K, Takahashi H (2006) D-lactic acid production by metabolically engineered Saccharomyces cerevisiae. J Biosci Bioeng 101(2):172–177. doi:10.1263/Jbb.101.172
Iyer PV, Lee YY (1999) Simultaneous saccharification and extractive fermentation of lignocellulosic materials into lactic acid in a two-zone fermenter-extractor system. Appl Biochem Biotech 77-9:409–419
Jia XQ, Liu P, Li S, Li SS, Wen JP (2011) D-lactic acid production by a genetically engineered strain Corynebacterium glutamicum. World J Microb Biot 27(9):2117–2124. doi:10.1007/s11274-011-0675-9
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biot 74(3):524–534. doi:10.1007/s00253-006-0779-6
Kandler O (1983) Carbohydrate-metabolism in lactic-acid bacteria. A Van Leeuw J Microb 49(3):209–224. doi:10.1007/Bf00399499
Kangming T (2012) High-efficiency conversion of glycerol to D-lactic acid with metabolically engineered Escherichia coli. Afr J Biotechnol 11(21). doi:10.5897/ajb11.3464
Keil KHD, Greiner UD, Engelhardt FD, Kühlein KD, Hess G, Keller RD, Schlingmann MD (1985) Isolierung von enzymatisch erzeugten Carbonsäuren. EP 0135728:A1
Kertes AS, King CJ (1986) Extraction chemistry of fermentation product carboxylic-acids. Biotechnol Bioeng 28(2):269–282. doi:10.1002/bit.260280217
Li Y, Wang L, Ju J, Yu B, Ma Y (2013) Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM442 with agricultural waste cottonseed as the sole nitrogen source. Bioresour Technol 142(0):186–191. doi:10.1016/j.biortech.2013.04.124
Li C, Tao F, Ni J, Wang Y, Yao F, Xu P (2015) Enhancing the light-driven production of D-lactate by engineering Cyanobacterium using a combinational strategy. Sci Rep-Uk 5. doi:10.1038/srep09777
Li QX, Bin Hudari MS, Wu JC (2016) Production of optically pure D-lactic acid by the combined use of Weissella sp S26 and Bacillus sp ADS3. Appl Biochem Biotech 178(2):285–293. doi:10.1007/s12010-015-1871-0
Liu Y, Gao W, Zhao X, Wang JH, Garza E, Manow R, Zhou SD (2014) Pilot scale demonstration of D-lactic acid fermentation facilitated by Ca(OH)(2) using a metabolically engineered Escherichia coli. Bioresour Technol 169:559–565. doi:10.1016/j.biortech.2014.06.056
Lopez-Garzon CS, Straathof AJJ (2014) Recovery of carboxylic acids produced by fermentation. Biotechnol Adv 32(5):873–904. doi:10.1016/j.biotechadv.2014.04.002
Lu ZD, Lu MB, He F, Yu LJ (2009) An economical approach for D-lactic acid production utilizing unpolished rice from aging paddy as major nutrient source. Bioresour Technol 100(6):2026–2031. doi:10.1016/j.biortech.2008.10.015
Lu HY, Zhao X, Wang YZ, Ding XR, Wang JH, Garza E, Manow R, Iverson A, Zhou SD (2016) Enhancement of D-lactic acid production from a mixed glucose and xylose substrate by the Escherichia coli strain JH15 devoid of the glucose effect. BMC Biotechnol 16. doi:10.1186/s12896-016-0248-y
Mazumdar S, Clomburg JM, Gonzalez R (2010) Escherichia coli Strains engineered for homofermentative production of D-lactic acid from glycerol. Appl Environ Microb 76(13):4327–4336. doi:10.1128/Aem.00664-10
Meyerhof O (1948) New investigations on enzymatic glycolysis and phosphorylation. Experientia 4(5):169–176. doi:10.1007/bf02153873
Mimitsuka T, Na K, Morita K, Sawai H, Minegishi S, Henmi M, Yamada K, Shimizu S, Yonehara T (2012) A membrane-integrated fermentation reactor system: its effects in reducing the amount of sub-raw materials for D-lactic acid continuous fermentation by Sporolactobacillus laevolacticus. Biosci Biotech Bioch 76(1):67–72. doi:10.1271/bbb.110499
Myriant-Corporation (2011) Myriant produces succinic acid and lactic acid from non-food cellulosic feedstocks. http://www.myriant.com/media/press-releases/myriant-produces-succinic-acid-and-lactic-acid-from-non-food-cellulosic-feedstocks.cfm. Accessed 15 January 2015
Nakano S, Ugwu CU, Tokiwa Y (2012) Efficient production of D-(−)-lactic acid from broken rice by Lactobacillus delbrueckii using Ca(OH)2 as a neutralizing agent. Bioresour Technol 104:791–794. doi:10.1016/j.biortech.2011.10.017
Nancib A, Nancib N, Meziane-Cherif D, Boubendir A, Fick M, Boudrant J (2005) Joint effect of nitrogen sources and B vitamin supplementation of date juice on lactic acid production by Lactobacillus casei subsp rhamnosus. Bioresour Technol 96(1):63–67. doi:10.1016/j.biortech.2003.09.018
National STEM Centre (2011) Lactic acid. National Non-Food Crops Centre. http://www.nationalstemcentre.org.uk/dl/ce274003bba8e131e0b7f4d040691f2b6156fa57/14765-lactic_acid.pdf. Accessed 16 January 2015
NatureWorks (2016) NatureWorks introduces its next generation polymer grade lactide. http://www.natureworksllc.com/News-and-Events/Press-Releases/2013/10-24-13-next-generation-polymer-grade-ingeo-lactide. Accessed 1 July 2016
Nguyen CM, Kim JS, Song JK, Choi GJ, Choi YH, Jang KS, Kim JC (2012) D-lactic acid production from dry biomass of Hydrodictyon reticulatum by simultaneous saccharification and co-fermentation using Lactobacillus coryniformis subsp torquens. Biotechnol Lett 34(12):2235–2240. doi:10.1007/s10529-012-1023-3
Nguyen CM, Choi GJ, Choi YH, Jang KS, Kim JC (2013a) D- and L-lactic acid production from fresh sweet potato through simultaneous saccharification and fermentation. Biochem Eng J 81:40–46. doi:10.1016/j.bej.2013.10.003
Nguyen CM, Kim JS, Nguyen TN, Kim SK, Choi GJ, Choi YH, Jang KS, Kim JC (2013b) Production of L- and D-lactic acid from waste Curcuma longa biomass through simultaneous saccharification and cofermentation. Bioresour Technol 146:35–43. doi:10.1016/j.biortech.2013.07.035
Okano K, Yoshida S, Tanaka T, Ogino C, Fukuda H, Kondo A (2009a) Homo-D-lactic acid fermentation from arabinose by redirection of the phosphoketolase pathway to the pentose phosphate pathway in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microb 75(15):5175–5178. doi:10.1128/Aem.00573-09
Okano K, Yoshida S, Yamada R, Tanaka T, Ogino C, Fukuda H, Kondo A (2009b) Improved production of homo-D-lactic acid via xylose fermentation by introduction of xylose assimilation genes and redirection of the phosphoketolase pathway to the pentose phosphate pathway in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microb 75(24):7858–7861. doi:10.1128/Aem.01692-09
Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biot 85(3):413–423. doi:10.1007/s00253-009-2280-5
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008) Production of D-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biot 78(3):449–454. doi:10.1007/s00253-007-1336-7
Ou MS, Awasthi D, Nieves I, Wang L, Erickson J, Vermerris W, Ingram LO, Shanmugam KT (2016) Sweet sorghum juice and bagasse as feedstocks for the production of optically pure lactic acid by native and engineered Bacillus coagulans strains. Bioenerg Res 9(1):123–131. doi:10.1007/s12155-015-9670-6
Petersen K, Nielsen PV, Bertelsen G, Lawther M, Olsen MB, Nilsson NH, Mortensen G (1999) Potential of biobased materials for food packaging. Trends Food Sci Tech 10(2):52–68. doi:10.1016/S0924-2244(99)00019-9
Reddy Tadi SR, EVRA, Limaye AM, Sivaprakasam S (2015) Enhanced production of optically pure D (−) lactic acid from nutritionally rich Borassus flabellifer sugar and whey protein hydrolysate based fermentation medium. Biotechnol Appl Biochem doi. doi:10.1002/bab.1470
Sangproo M, Polyiam P, Jantama SS, Kanchanatawee S, Jantama K (2012) Metabolic engineering of Klebsiella oxytoca M5a1 to produce optically pure D-lactate in mineral salts medium. Bioresour Technol 119:191–198. doi:10.1016/j.biortech.2012.05.114
Shen L, Haufe J, Patel MK (2009) Product overview and market projection of emerging bio-based plastics. http://www.uu.nl/SiteCollectionImages/IMEW/Copernicus/Reports/PROBIP2009 Final June 2009 revised in November 09.pdf. Accessed 16 January 2015
Singhvi M, Joshi D, Adsul M, Varma A, Gokhale D (2010) D-(−)-lactic acid production from cellobiose and cellulose by Lactobacillus lactis mutant RM2-24. Green Chem 12(6):1106–1109. doi:10.1039/b925975a
Sun J, Wang Y, Wu B, Bai Z, He B (2015) Enhanced production of D-lactic acid by Sporolactobacillus sp.Y2–8 mutant generated by atmospheric and room temperature plasma. Biotechnol Appl Bioc 62(2):287–292. doi:10.1002/bab.1267
Tanaka T, Hoshina M, Tanabe S, Sakai K, Ohtsubo S, Taniguchi M (2006) Production of D-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour Technol 97(2):211–217. doi:10.1016/j.biortech.2005.02.025
Tashiro Y, Kaneko W, Sun YQ, Shibata K, Inokuma K, Zendo T, Sonomoto K (2011) Continuous D-lactic acid production by a novel thermotolerant Lactobacillus delbrueckii subsp lactis QU 41. Appl Microbiol Biot 89(6):1741–1750. doi:10.1007/s00253-010-3011-7
Tejayadi S, Cheryan M (1995) Lactic-acid from cheese whey permeate—productivity and economics of a continuous membrane bioreactor. Appl Microbiol Biot 43(2):242–248
Tsuge Y, Kawaguchi H, Sasaki K, Tanaka T, Kondo A (2014) Two-step production of D-lactate from mixed sugars by growing and resting cells of metabolically engineered Lactobacillus plantarum. Appl Microbiol Biot 98(11):4911–4918. doi:10.1007/s00253-014-5594-x
Tsuge Y, Yamamoto S, Kato N, Suda M, Vertes AA, Yukawa H, Inui M (2015) Overexpression of the phosphofructokinase encoding gene is crucial for achieving high production of D-lactate in Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biot 99(11):4679–4689. doi:10.1007/s00253-015-6546-9
Tsuji H (2005) Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci 5(7):569–597. doi:10.1002/mabi.200500062
Vijayakumar J, Aravindan R, Viruthagiri T (2008) Recent trends in the production, purification and application of lactic acid. Chem Biochem Eng Q 22(2):245–264
Wang LM, Zhao B, Li FS, Xu K, Ma CQ, Tao F, Li QG, Xu P (2011a) Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl Microbiol Biot 89(4):1009–1017. doi:10.1007/s00253-010-2904-9
Wang QZ, Ingram LO, Shanmugam KT (2011b) Evolution of D-lactate dehydrogenase activity from glycerol dehydrogenase and its utility for D-lactate production from lignocellulose. P Natl Acad Sci USA 108(47):18920–18925. doi:10.1073/pnas.1111085108
Wang YZ, Tian T, Zhao JF, Wang JH, Yan T, Xu LY, Liu Z, Garza E, Iverson A, Manow R, Finan C, Zhou SD (2012) Homofermentative production of D-lactic acid from sucrose by a metabolically engineered Escherichia coli. Biotechnol Lett 34(11):2069–2075. doi:10.1007/s10529-012-1003-7
Wang Y, Tashiro Y, Sonomoto K (2015a) Fermentative production of lactic acid from renewable materials: recent achievements, prospects, and limits. J Biosci Bioeng 119(1):10–18. doi:10.1016/j.jbiosc.2014.06.003
Wang ZW, Saini M, Lin LJ, Chiang CJ, Chao YP (2015b) Systematic engineering of Escherichia coli for D-lactate production from crude glycerol. J Agr Food Chem 63(43):9583–9589. doi:10.1021/acs.jafc.5b04162
Wasewar KL (2005) Separation of lactic acid: recent advances. Chem Biochem Eng Q 19(2):159–172
Wee YJ, Kim JN, Ryu HW (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotech 44(2):163–172
Wieschalka S, Blombach B, Bott M, Eikmanns BJ (2013) Bio-based production of organic acids with Corynebacterium glutamicum. Microb Biotechnol 6(2):87–102. doi:10.1111/1751-7915.12013
Xu TT, Bai ZZ, Wang LJ, He BF (2010) Breeding of d(−)-lactic acid high producing strain by low-energy ion implantation and preliminary analysis of related metabolism. Appl Biochem Biotech 160(2):314–321. doi:10.1007/s12010-008-8274-4
Yanez R, Moldes AB, Alonso JL, Parajo JC (2003) Production of D(−)-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp torquens. Biotechnol Lett 25(14):1161–1164
Yanez R, Alonso JL, Parajo JC (2005) D-lactic acid production from waste cardboard. J Chem Technol Biot 80(1):76–84. doi:10.1002/jctb.1160
Yi X, Zhang P, Sun JE, Tu Y, Gao QQ, Zhang J, Bao J (2016) Engineering wild-type robust Pediococcus acidilactici strain for high titer L- and D-lactic acid production from corn Stover feedstock. J Biotechnol 217:112–121. doi:10.1016/j.jbiotec.2015.11.014
Yoo I-K, Chang H-N, Lee E-G, Chang Y-K, Moon S-H (1996) Effect of pH on the production of lactic acid and secondary products in batch cultures of Lactobacillus casei. J Microbiol Biotechnol 6(6):482–486
Yu B, Su F, Wang LM, Xu K, Zhao B, Xu P (2011) Draft genome sequence of Sporolactobacillus inulinus strain CASD, an efficient D-lactic acid-producing bacterium with high-concentration lactate tolerance capability. J Bacteriol 193(20):5864–5865. doi:10.1128/Jb.05934-11
Zaunmuller T, Eichert M, Richter H, Unden G (2006) Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. Appl Microbiol Biot 72(3):421–429. doi:10.1007/s00253-006-0514-3
Zhang YX, Vadlani PV (2013) D-lactic acid biosynthesis from biomass-derived sugars via Lactobacillus delbrueckii fermentation. Bioproc Biosyst Eng 36(12):1897–1904. doi:10.1007/s00449-013-0965-8
Zhang JM, Sato H, Tsuji H, Noda I, Ozaki Y (2005) Infrared spectroscopic study of CH3 center dot center dot center dot O = C interaction during poly(L-lactide)/poly(D-lactide) stereocomplex formation. Macromolecules 38(5):1822–1828. doi:10.1021/ma047872w
Zhang Y, Cong W, Shi SY (2011) Repeated fed-batch lactic acid production in a packed bed-stirred fermentor system using a pH feedback feeding method. Bioproc Biosyst Eng 34(1):67–73. doi:10.1007/s00449-010-0447-1
Zhang YX, Kumar A, Hardwidge PR, Tanaka T, Kondo A, Vadlani PV (2016a) D-lactic acid production from renewable lignocellulosic biomass via genetically modified Lactobacillus plantarum. Biotechnol Prog 32(2):271–278. doi:10.1002/btpr.2212
Zhang YX, Vadlani PV, Kumar A, Hardwidge PR, Govind R, Tanaka T, Kondo A (2016b) Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl Microbiol Biot 100(1):279–288. doi:10.1007/s00253-015-7016-0
Zhao T, Liu D, Ren HF, Shi XC, Zhao N, Chen Y, Ying HJ (2014) D-lactic acid production by Sporolactobacillus inulinus Y2-8 immobilized in fibrous bed bioreactor using corn flour hydrolyzate. J Microbiol Biotechn 24(12):1664–1672. doi:10.4014/jmb.1406.06043
Zhou L, Niu DD, Tian KM, Chen XZ, Prior BA, Shen W, Shi GY, Singh S, Wang ZX (2012) Genetically switched D-lactate production in Escherichia coli. Metab Eng 14(5):560–568. doi:10.1016/j.ymben.2012.05.004
Zhou L, Cui WJ, Liu ZM, Zhou ZM (2016) Metabolic engineering strategies for D-lactate over production in Escherichia coli. J Chem Technol Biot 91(3):576–584. doi:10.1002/jctb.4856
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Klotz, S., Kaufmann, N., Kuenz, A. et al. Biotechnological production of enantiomerically pure d-lactic acid. Appl Microbiol Biotechnol 100, 9423–9437 (2016). https://doi.org/10.1007/s00253-016-7843-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7843-7