Abstract
Cyanophycin (CP) can be successfully produced in plants by the ectopic expression of the CphA synthetase from Thermosynechococcus elongatus BP-1 (Berg et al. 2000), yielding up to 6.8 % of dry weight (DW) in tobacco leaf tissue and 7.5 % in potato tubers (Huehns et al. 2008, 2009). Though, high amounts of the polymer lead to phenotypical abnormalities in both crops. The extension of abnormalities and the maximum amount of CP tolerated depend on the compartment that CP production is localized at the tissue/crop in which CP was produced (Huehns et al. 2008, 2009; Neumann et al. 2005). It cannot be ascribed to a depletion of arginine, lysine, or aspartate, the substrates for CP synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
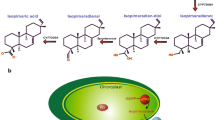
Cyanophycin, also known as cyanophycin granule polypeptide (CGP), is a polymer predominantly composed of arginine-aspartate and lysine-aspartate dipeptides (Fig. 1). It is naturally produced by prokaryotes with the help of a cyanophycin synthetase (CphA) via non-ribosomal protein biosynthesis and accumulates as water-insoluble granules in the cytosol (Simon and Weathers 1976; Ziegler et al. 1998). Being resistant to common proteases, CP serves as storage for nitrogen, carbon, and energy; it is exclusively degrades on demand by so-called cyanophycinases into dipeptides (Law et al. 2009; Sallam and Steinbuchel 2009). CP is of particular interest due to its ability to substitute petroleum-derived polyacrylates in the synthesis of plastics and chemicals (Börnke and Broer 2010; Mooibroek et al. 2007). Furthermore, it might be used for the production of dipeptides. Their high nutritional value makes them highly desirable for human diets and industrial animal feed (Sallam and Steinbuchel 2009, 2010; Santos et al. 2012). Up to now, CP has been predominantly produced in cell cultures (Frommeyer et al. 2014) which are limited in terms of scalability, productivity, and process economics when compared to plants (Merlin et al. 2014).
CP has already been successfully produced in tobacco and potato plants up to 6.8 and 7.5 % DW, respectively, and economic aspects were calculated (Huehns 2010; Huehns et al. 2008, 2009; Neumann et al. 2005). Whereas the synthesis in plastids of tobacco leaves and potato tubers did not cause any phenotypical abnormalities, the presence of less than 1 % DW of CP in the cytosol led to severe disorders. This review outlines the peculiarities and impacts of expression of bacterial cyanophycin synthetases in plants. In particular, it points out possible connections between the features of plant-made CP and its influence on plant physiology and yield.
Characteristics of plant-produced CP
CP synthetase genes from Anabaena variabilis ATCC 29413, Thermococcus elongatus BP-1, and Synechocystis sp. PCC 6803 have been transferred to tobacco (Huehns et al. 2004), but CP can only be produced by the CphA from T. elongatus BP-1. This phenomenon could not be explained until Arai and Kino (2008) demonstrated that CphATe—in contrast to the other two enzymes—does not need a low molecular mass cyanophycin oligodipeptide as primer for CP production (Arai and Kino 2008). The origin of the primer or the factors, synthesizing the CP-primer, respectively, has not yet been identified (Frommeyer et al. 2014). It might be concluded that the necessary primers or additional unknown enzymes do not exist in tobacco. Nevertheless, heterologous expression of the same primer-dependent CP synthetase has been reported in Escherichia coli (Berg et al. 2000; Ziegler et al. 1998) and of others in yeast (Steinle et al. 2008, 2009, 2010) as well as in fungi (Meussen et al. 2012), leading to the production of CP. Hence, primer production seems to be possible not only in bacteria but also in lower eukaryotes.
Plant-produced CP has a limited polydispersity between 20 and 35 kDa (Huehns et al. 2008, 2009; Neumann et al. 2005). This holds true both for cytosolic and plastidic CP, even though plastids are descendants of cyanobacteria. CP derived from various non-cyanobacterial, native, and recombinant hosts displays a similar distribution pattern in polyacrylamide gel electrophoresis (PAGE), whereas polydispersity of CP derived from cyanobacteria ranges from 25 up to 125 kDa (Frommeyer et al. 2014). Hence, the cyanobacterial factors assumed to be responsible for polydispersity (Aboulmagd et al. 2001) might have been lost during endosymbiosis or evolved after the separation of chloroplasts and cyanobacteria.
Insoluble CP is the predominant form in plants ranging from 80 % of total CP in potato to 98 % in tobacco (Table 1). Only when produced in the tobacco cytosol does the average amount of soluble CP rise to 50 % of total CP (Neumann et al. 2005).
The amount of soluble CP correlates with the promotor used for the expression of the CpATe-synthetase. In potato tubers, the enzyme has been expressed with either the constitutive 35S promoter from the cauliflower mosaic virus or the tuber-specific B33-promotor from Solanum tuberosum—both in the cytosol and in plastids (Huehns et al. 2009; Neubauer et al. 2012; Neumann et al. 2005). Driven by the 35S, soluble CP averaged 10 % in the cytosol and plastids, whereas B33 yielded up to 23 % of soluble CP (Table 1).
Comparing tobacco and potato (Table 1), which vary in their content of water-soluble CP as well, it might be concluded that the plant species has a significant influence. This is in line with the fact that in E. coli, CP is predominantly produced as insoluble CP, but is almost exclusively found in soluble form in yeast (Steinle et al. 2008, 2009, 2010). No clear correlation was found between the total amount of CP and the percentage of soluble CP (Table 1). Interestingly, soluble CP does not occur in naturally CP-producing prokaryotes but only in heterologous hosts (Füser and Steinbüchel 2005).
Although in bacteria soluble CP contains at least 17 mol% lysine (Frommeyer and Steinbuchel 2013; Tseng et al. 2012) and it is assumed that the incorporation of lysine might be an important characteristic of soluble CP, Neumann et al. (2005) found only very low amounts of lysine in the soluble CP (molar ratio asp:arg:lys 1:1.05:0.1) isolated from three different tobacco plants.
Both in tobacco and potato, CP dissolved from granular into fibrillary structures during senescence. In tobacco, this phenomenon was observed in older, yellow leaves and paralleled a transformation of chloro- into gerontoplasts. These gerontoplasts were not observed in non-transgenic control plants (Huehns et al. 2008). A similar decomposition of compact CP aggregates was observed in older tubers of potato, accompanied by a disintegration of the amyloplasts (Huehns et al. 2009). Dissolving CP granules have not yet been observed in any other CP-producing organism, and the reason as well as the effect of this process remains unknown. The total amount of CP was not affected by this process (Huehns et al. 2009).
Similarly, the CP content remained unchanged during storage of potato tubers over 32 weeks (Huehns et al. 2009). Hence, CP does not seem to be metabolized by plant proteases. The resistance of CP to degradation could be explained by its unique branched structure (Simon and Weathers 1976). Cyanophycinases, the only enzymes known to be capable for metabolizing CP, do not occur in plants (Law et al. 2009). Taking this into account, the CP content might be higher in older tissues and might be enhanced by prolonging the plant growth period.
Limitations of CP production in plants
Plastidic CP production yielded up to 6.8 % DW in tobacco leaf tissue and 7.5 % DW in potato tubers (Huehns et al. 2008, 2009). However, these yields do not reach the amounts found in other CP-producing native and recombinant hosts. For example in cyanobacteria, CP yields up to 16 % of cell dry matter (CDM) (Allen et al. 1980). Up to 28 % CDM were obtained in recombinant E. coli strains (Elbahloul et al. 2005a) and up to 21 % CDM in recombinant yeast (Steinle et al. 2010). Moreover, the CP composition could be modified and the yield increased by adaptation of cultivation medium and conditions. In E. coli, the CP amount was significantly improved by additionally supplying amino acid-rich substrates to the cultivation medium (Elbahloul et al. 2005a; Frey et al. 2002). In yeast, a similar approach increased the amount of CP up to 1.5-fold. Moreover, the selective supply of either aspartate and arginine or aspartate and lysine correlated with an enhanced incorporation of the arginine or lysine into CP (Steinle et al. 2008). The addition of these amino acids also improved the CP production in transgenic Pseudomonas putida and Ralstonia eutropha (Diniz et al. 2006). In line with this, arginine has been identified as a limiting factor of CP synthesis in native CP-producing prokaryotes (Elbahloul et al. 2005b; Maheswaran et al. 2006). CP production in these organisms has also been boosted by inhibiting biosynthesis with the help of chloramphenicol (Elbahloul et al. 2005b; Simon 1973). The authors argued that protein and CP biosynthesis compete for amino acids and that blocking ribosomes increases the pool for CP production.
The amounts of free aspartate and glutamate, as precursors for arginine, were slightly, but significantly, reduced in transgenic potato tubers with the highest CP content (Huehns and Broer 2009). This might suggest that CP production in plants is also limited by substrate. Yet, increased nitrogen supply did not affect the amount of CP in potato tubers (Huehns et al. 2009) and the addition of casein hydolysate as organic nitrogen source to the transformation media did not change the reduced regeneration frequency observed in leaf disk transformation (Huehns 2010). In addition, neither the coexpression of a glutamine synthetase from E. coli (glnA) or Streptomyces coelicolor (glnII) nor the ornithine acetyltransferase (argJ) from S. coelicolor in CP-producing tobacco increased the amount of CP (Huehns 2010). Hence, it might be hypothesized that in contrast to other hosts, the CP production in plants is limited by substrate availability. Though, it remains unclear whether both approaches increased the level of aspartate and glutamate at all, since the pool of free amino acids was not determined.
On the other hand, CP content in potato tubers could be increased from 0.24 to 2.29 % DW and 0.98 to 7.52 % in the cytosol and plastids, respectively (Table 1), by employing the tuber-specific B33 promotor instead of the constitutive p35S for the expression of the cphA gene (Huehns et al. 2009; Neubauer et al. 2012; Neumann et al. 2005). The transcription efficiency of the transgene might therefore still limit the amount of CP in plants.
Nevertheless, the granule-bound starch synthetase (GBSS) promotor derived from the GBSS from Manihot esculenta did not trigger any CP production in tubers (Huehns 2010). This indicates that further unknown determinants are involved in CP synthesis, which may affect factors such as the activity of the CphA enzyme.
Impact of CP production on plants
Both in tobacco and potato, CP has been produced in the cytosol and plastids, leading to different phenotypes (Fig. 2). Transgenic plants with cytosolic production of CP showed a retarded development, limited growth, and thicker stems and leaves. The leaf tissue was variegated and the grana stacks in the chloroplast were reduced in number and size (Neumann et al. 2005). In the case of tobacco, transformants were generally fertile, but the event with the highest CP content did not produce any transgenic descendants. Potato tubers were smaller and deformed (Fig. 2). In addition, tubers of the potato top producer did not sprout (Neumann et al. 2005). Hence, in the cytosol, CP might interfere with both the metabolism in vegetative tissue and the generative processes that take place during germination and sprouting. This is supported by the fact that the number of transgenic shoots/explant was reduced to 86 % (tobacco) and 70 % (potato) compared to the control explants transformed with the empty vector that contained only the selection marker gene (Neumann et al. 2005). Important processes occurring during the de- and redifferentiation of leaf cells within the transformation process seem to be affected by the presence or production of CP.
Schematic overview of cytosolic (brown) and plastidal (purple) expression of Cyanophycin-synthetase (CphA) in tobacco and potato: nuclear (blue)-encoded CphA from Thermosynecchococcus elongatus BP-1 (CphATe) is expressed by the 35S promotor and terminator from the cauliflower mosaic virus and targeted to plastids (green) by the fusion to the sequence of transit peptide of the integral protein of photosystem II (PsbY). Representative phenotypical disorders are shown at the left side
When CP production was targeted to the plastids, the abnormalities of vegetative tissue were substantially mitigated and plants showed a normal phenotype (Fig. 2) and growth rate, comparable to that of the near-isogenic control (Huehns et al. 2008, 2009). CP yielded up to 1.7 % in the transformants. The fact that one out of four events with that amount displayed an altered phenotype and produced only a small amount of seeds (Huehns et al. 2008) might result from a somaclonal variation due to the transformation or regeneration process.
In contrast to the mitigated abnormalities of vegetative tissue, the impact of CP on regenerative processes during leaf disk transformation was not reduced. The shoot formation was still impaired with 60 % (tobacco) and 59–71 % (potato) compared to the control (Huehns et al. 2008, 2009; Neubauer et al. 2012).
In addition, as described in the earlier chapter, CP seems to induce a premature conversion of chloro- into gerontoplasts in senescing tobacco leaves (Huehns et al. 2008) and to cause the development of brown sunken staining in stored potato tubers (Huehns et al. 2009). Hence, the presence of CP seems to be problematic whenever cells or their structures dissolve, possibly because this might lead to a release of CP from the plastid to the cytosol.
Potential causes for phenotypic disorders associated with CP production in plants
Although the production of CP does not seem to be limited by substrate, high levels of expression of CphATe led to a reduction of free aspartate and glutamate that are necessary for CP synthesis (Huehns and Broer 2009). This might interfere with the production of endogenous proteins, promoting stress symptoms.
More importantly, the amount of soluble CP seems to correlate with the adverse effects (Table 1). In tobacco with cytosolic CP production, where soluble CP reached up to 50 %, disorders were most pronounced (Neumann et al. 2005). Interestingly, the impact of similar amounts of soluble CP differed in the cytosol and plastids, as observed in potato (Table 1). However, the reduced amount of soluble CP in tobacco T1 descendants with cytosolic production did not reduce the abnormalities observed (Neumann et al. 2005). In addition, comparing different events and lines of the same crop producing CP in the same compartment, the strength of the phenotype correlates neither with the amount of CP nor with the ratio of soluble/insoluble CP (Huehns et al. 2009; Neumann et al. 2005). Together with the fact that no abnormalities were reported for any other CP-producing recombinant host, the role of soluble CP remains questionable and is not the only effect influencing the plant fitness. Nevertheless, it cannot be excluded that besides the total amount of CP and localization of CP production, phenotypic disorders might be influenced by the amount of soluble CP.
CP in comparison to other plant-made polymers
Apart from CP, several other polymers were produced in plants by the overexpression of the corresponding genes, such as (1) polyhydroxyalkanoates such as polyhydroxybutyrate (PHB), (2) fibrous proteins like silk, elastin and collagen, as well as (3) polyamino acids like poly-ε-lysine, poly-γ-glutamate, or poly-α-aspartate (Snell et al. 2015; van Beilen and Poirier 2012). The production of CP and PHB is particular since they are both produced by a nuclear-encoded synthetase. In all other cases, the recombinant polymers were directly encoded by the transgene. Therefore, CP is mainly compared to PHB.
The highest CP production detected was 6.8 % DW in tobacco leaf tissue and 7.5 % DW in potato tubers, respectively (Huehns et al. 2008, 2009). With 18.8 % of leaf dry weight, the amount of PHB in tobacco (Bohmert-Tatarev et al. 2011) exceeds the level of CP approximately twofold. In contrast to that, PHB accumulated only up to 0.009 % DW in potato (Bohmert et al. 2002). The endogenous determinants limiting CP and PHB production, respectively, are still unknown, however. Since CP and PHB are produced from different substrates—aspartate/arginine/lysine and acetyl-CoA, respectively—it might be speculated that different amounts of PHB result from a differing substrate availability. Yet, as already mentioned in the earlier chapter, CP production seems to be substantially influenced by other factors, apart from substrate access.
In tobacco, CP and PHB led to similar drastic disorders when the synthetases were constitutively expressed (Bohmert-Tatarev et al. 2011; Bohmert et al. 2002; Huehns et al. 2008; Lössl et al. 2003; Ruiz and Daniell 2005). In the case of PHB, it was argued that the phenotypical stress symptoms results from a depletion of acetyl-CoA as key metabolite and precursor for PHB synthesis (Morandini 2013; van Beilen and Poirier 2012). This has been supported by the study of Xing et al. (2014), in which the negative effects of PHB were reduced by the overexpression of the ATP citrate lyase (ACL), increasing the pool of acetyl-CoA (Xing et al. 2014).
In tobacco, the impact of CP depended on its localization, either in the cytosol or in chloroplasts. A similar correlation has been observed for PHB when produced in both compartments in Arabidopsis thaliana. Yields of cytosolic PHB were quiet low and had deleterious effects on plant growth and fertility. Transferring PHB synthesis to chloroplasts increased its amount 100-fold and mitigated adverse effects (Nawrath et al. 1994; Poirier et al. 1992). In line with the data of Xing et al. (2014), this was explained by the higher metabolic flux of acetyl-CoA in plastids compared to the cytosol.
In contrast to CP, PHB synthesis in potato induced no abnormalities (Bohmert et al. 2002; Huehns et al. 2009). However, PHB levels were low (0.009 % DW) and a detailed analysis of tubers was not done (Bohmert et al. 2002).
Taken together, the adverse effects of PHB on the plant host were similar to that of CP: (1) cytosolic production was detrimental, (2) the disorders could be mitigated by the translocation to the plastids, and (3) high PHB content interfered with seed germination. In the case of PHB, the negative effects correlate with a depletion of the substrate for PHB production acetyl-CoA, which is a key metabolite of plant metabolism (Morandini 2013). Moreover, Nawrath et al. (1994) argued that the mitigation of abnormalities by plastidic PHB production results from the high metabolic flux of acetyl-CoA. In contrast to that, there are several indications that CP is not limited by substrate (Huehns 2010; Huehns et al. 2009).
Conclusions
The composition and yield of CP is influenced by a variety of unknown factors in plants. Yields do not seem to be limited by the substrates. Phenotypical disorders do not correlate with the CP content but possibly with the amount of soluble CP. Further investigations are necessary to clarify its role. Additional research needs to be undertaken to identify the factors that lead to the preferential incorporation of arginine/aspartate dipeptides in plant CP.
References
Aboulmagd E, Voss I, Oppermann-Sanio FB, Steinbüchel A (2001) Heterologous expression of cyanophycin synthetase and cyanophycin synthesis in the industrial relevant bacteria corynebacterium glutamicum and Ralstonia eutropha and in Pseudomonas putida. Biomacromolecules 2(4):1338–1342. doi:10.1021/bm010075a
Allen MM, Hutchison F, Weathers PJ (1980) Cyanophycin granule polypeptide formation and degradation in the cyanobacterium Aphanocapsa 6308. J Bacteriol 141(2):687–693
Arai T, Kino K (2008) A cyanophycin synthetase from Thermosynechococcus elongatus BP-1 catalyzes primer-independent cyanophycin synthesis. Appl Microbiol Biotechnol 81(1):69–78. doi:10.1007/s00253-008-1623-y
Berg H, Ziegler K, Piotukh K, Baier K, Lockau W, Volkmer-Engert R (2000) Biosynthesis of the cyanobacterial reserve polymer multi-L-arginyl-poly-L-aspartic acid (cyanophycin). Eur J Biochem 267(17):5561–5570. doi:10.1046/j.1432-1327.2000.01622.x
Bohmert K, Balbo I, Steinbüchel A, Tischendorf G, Willmitzer L (2002) Constitutive expression of the β-ketothiolase gene in transgenic plants. A major obstacle for obtaining polyhydroxybutyrate-producing plants. Plant Physiol 128(4):1282–1290. doi:10.1104/pp.010615
Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD (2011) High levels of bioplastic Are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol 155(4):1690–1708. doi:10.1104/pp.110.169581
Börnke F, Broer I (2010) Tailoring plant metabolism for the production of novel polymers and platform chemicals. Curr Opin Plant Biol 13(3):353–361. doi:10.1016/j.pbi.2010.01.005
Diniz SC, Voss I, Steinbüchel A (2006) Optimization of cyanophycin production in recombinant strains of Pseudomonas putida and Ralstonia eutropha employing elementary mode analysis and statistical experimental design. Biotechnol Bioeng 93(4):698–717. doi:10.1002/bit.20760
Elbahloul Y, Frey K, Sanders J, Steinbüchel A (2005a) Protamylasse, a residual compound of industrial starch production, provides a suitable medium for large-scale cyanophycin production. Appl Environ Microbiol 71(12):7759–7767. doi:10.1128/AEM.71.12.7759-7767.2005
Elbahloul Y, Krehenbrink M, Reichelt R, Steinbüchel A (2005b) Physiological conditions conducive to high cyanophycin content in biomass of Acinetobacter calcoaceticus strain ADP1. Appl Environ Microbiol 71(2):858–866. doi:10.1128/AEM.71.2.858-866.2005
Frey KM, Oppermann-Sanio FB, Schmidt H, Steinbüchel A (2002) Technical-scale production of cyanophycin with recombinant strains of Escherichia coli. Appl Environ Microbiol 68(7):3377–3384. doi:10.1128/AEM.68.7.3377-3384.2002
Frommeyer M, Steinbuchel A (2013) Increased lysine content is the main characteristic of the soluble form of the polyamide cyanophycin synthesized by recombinant Escherichia coli. Appl Environ Microbiol 79(14):4474–4483. doi:10.1128/AEM.00986-13
Frommeyer M, Wiefel L, Steinbüchel A (2014) Features of the biotechnologically relevant polyamide family “cyanophycins” and their biosynthesis in prokaryotes and eukaryotes. Crit Rev Biotechnol 0(0):1–12 doi: 10.3109/07388551.2014.946467
Füser G, Steinbüchel A (2005) Investigations on the solubility behavior of cyanophycin. Solubility of cyanophycin in solutions of simple inorganic salts. Biomacromolecules 6(3):1367–1374. doi:10.1021/bm049371o
Huehns M (2010) Production of the biodegradable polymer cyanophycin in transgenic Nicotiana tabacum and Solanum tuberosum plants. PhD thesis, University of Rostock
Huehns M, Broer I (2009) Characters of transgenic plants and their application in plant production: biopolymers. In: Kempken F, Jung C (eds) Biotechnology in agriculture and forestry Vol64 Genetic modification of plants—agriculture, horticulture and forestry. Springer Verlag, Heidelberg, pp 237–252
Huehns M, Neumann K, Lockau W, Ziegler K, Pistorius EK, Broer I (2004) Bioplastic in transgenic plants: cyanophycin as a suitable resource for polyaspartate. Paper presented at the Agricultural Biotechnology International Conference, Cologne, Germany
Huehns M, Neumann K, Hausmann T, Ziegler K, Klemke F, Kahmann U, Staiger D, Lockau W, Pistorius EK, Broer I (2008) Plastid targeting strategies for cyanophycin synthetase to achieve high-level polymer accumulation in Nicotiana tabacum. Plant Biotechnol J 6(4):321–336. doi:10.1111/j.1467-7652.2007.00320.x
Huehns M, Neumann K, Hausmann T, Klemke F, Lockau W, Kahmann U, Kopertekh L, Staiger D, Pistorius EK, Reuther J, Waldvogel E, Wohlleben W, Effmert M, Junghans H, Neubauer K, Kragl U, Schmidt K, Schmidtke J, Broer I (2009) Tuber-specific cphA expression to enhance cyanophycin production in potatoes. Plant Biotechnol J 7(9):883–898. doi:10.1111/j.1467-7652.2009.00451.x
Law AM, Lai SWS, Tavares J, Kimber MS (2009) The structural basis of β-peptide-specific cleavage by the serine protease cyanophycinase. J Mol Biol 392(2):393–404. doi:10.1016/j.jmb.2009.07.001
Lössl A, Eibl C, Harloff HJ, Jung C, Koop HU (2003) Polyester synthesis in transplastomic tobacco (Nicotiana tabacum L.): significant contents of polyhydroxybutyrate are associated with growth reduction. Plant Cell Rep 21(9):891–899
Maheswaran M, Ziegler K, Lockau W, Hagemann M, Forchhammer K (2006) P(II)-regulated arginine synthesis controls accumulation of cyanophycin in Synechocystis sp. strain PCC 6803. J Bacteriol 188(7):2730–2734. doi:10.1128/JB.188.7.2730-2734.2006
Merlin M, Gecchele E, Capaldi S, Pezzotti M, Avesani L (2014) Comparative evaluation of recombinant protein production in different biofactories: the green perspective. BioMed Res Int. doi:10.1155/2014/136419
Meussen BJ, Weusthuis RA, Sanders JPM, de Graaff LH (2012) Production of cyanophycin in Rhizopus oryzae through the expression of a cyanophycin synthetase encoding gene. Appl Microbiol Biotechnol 93(3):1167–1174. doi:10.1007/s00253-011-3604-9
Mooibroek H, Oosterhuis N, Giuseppin M, Toonen M, Franssen H, Scott E, Sanders J, Steinbüchel A (2007) Assessment of technological options and economical feasibility for cyanophycin biopolymer and high-value amino acid production. Appl Microbiol Biotechnol 77(2):257–267. doi:10.1007/s00253-007-1178-3
Morandini P (2013) Control limits for accumulation of plant metabolites: brute force is no substitute for understanding. Plant Biotechnol J 11(2):253–267. doi:10.1111/pbi.12035
Nawrath C, Poirier Y, Somerville C (1994) Targeting of the polyhydroxybutyrate biosynthetic pathway to the plastids of Arabidopsis thaliana results in high levels of polymer accumulation. Proc Natl Acad Sci U S A 91(26):12760–12764. doi:10.1073/pnas.91.26.12760
Neubauer K, Hühns M, Hausmann T, Klemke F, Lockau W, Kahmann U, Pistorius EK, Kragl U, Broer I (2012) Isolation of cyanophycin from tobacco and potato plants with constitutive plastidic cphATe gene expression. J Biotechnol 158(1–2):50–58. doi:10.1016/j.jbiotec.2011.12.008
Neumann K, Stephan DP, Ziegler K, Huhns M, Broer I, Lockau W, Pistorius EK (2005) Production of cyanophycin, a suitable source for the biodegradable polymer polyaspartate, in transgenic plants. Plant Biotechnol J 3(2):249–258. doi:10.1111/j.1467-7652.2005.00122.x
Poirier Y, Dennis DE, Klomparens K, Somerville C (1992) Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science 256(5056):520–523
Ruiz ON, Daniell H (2005) Engineering cytoplasmic male sterility via the chloroplast genome by expression of β-ketothiolase. Plant Physiol 138(3):1232–1246. doi:10.1104/pp.104.057729
Sallam A, Steinbuchel A (2009) Cyanophycin-degrading bacteria in digestive tracts of mammals, birds and fish and consequences for possible applications of cyanophycin and its dipeptides in nutrition and therapy. J Appl Microbiol 107(2):474–484. doi:10.1111/j.1365-2672.2009.04221.x
Sallam A, Steinbuchel A (2010) Dipeptides in nutrition and therapy: cyanophycin-derived dipeptides as natural alternatives and their biotechnological production. Appl Microbiol Biotechnol 87(3):815–828. doi:10.1007/s00253-010-2641-0
Santos S, Torcato I, Castanho MARB (2012) Biomedical applications of dipeptides and tripeptides. Pept Sci 98(4):288–293. doi:10.1002/bip.22067
Simon R (1973) The effect of chloramphenicol on the production of cyanophycin granule polypeptide in the blue-green alga Anabaena cylindrica. Archiv Mikrobiol 92(2):115–122. doi:10.1007/BF00425009
Simon RD, Weathers P (1976) Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim Biophys Acta Protein Struct Mol Enzymol 420(1):165–176. doi:10.1016/0005-2795(76)90355-X
Snell KD, Singh V, Brumbley SM (2015) Production of novel biopolymers in plants: recent technological advances and future prospects. Curr Opin Biotechnol 32:68–75. doi:10.1016/j.copbio.2014.11.005
Steinle A, Oppermann-Sanio FB, Reichelt R, Steinbüchel A (2008) Synthesis and accumulation of cyanophycin in transgenic strains of Saccharomyces cerevisiae. Appl Environ Microbiol 74(11):3410–3418. doi:10.1128/AEM.00366-08
Steinle A, Bergander K, Steinbüchel A (2009) Metabolic engineering of Saccharomyces cerevisiae for production of novel cyanophycins with an extended range of constituent amino acids. Appl Environ Microbiol 75(11):3437–3446. doi:10.1128/AEM.00383-09
Steinle A, Witthoff S, Krause JP, Steinbüchel A (2010) Establishment of cyanophycin biosynthesis in Pichia pastoris and optimization by use of engineered cyanophycin synthetases. Appl Environ Microbiol 76(4):1062–1070. doi:10.1128/AEM.01659-09
Tseng W-C, Fang T-Y, Cho C-Y, Chen P-S, Tsai C-S (2012) Assessments of growth conditions on the production of cyanophycin by recombinant Escherichia coli strains expressing cyanophycin synthetase gene. Biotechnol Prog 28(2):358–363. doi:10.1002/btpr.1513
van Beilen JB, Poirier Y (2012) Plants as factories for bioplastics and other novel biomaterials. Plant Biotechnol Agric. 481–494
Xing S, van Deenen N, Magliano P, Frahm L, Forestier E, Nawrath C, Schaller H, Gronover CS, Prüfer D, Poirier Y (2014) ATP citrate lyase activity is post-translationally regulated by sink strength and impacts the wax, cutin and rubber biosynthetic pathways. Plant J 79(2):270–284. doi:10.1111/tpj.12559
Ziegler K, Diener A, Herpin C, Richter R, Deutzmann R, Lockau W (1998) Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartate (cyanophycin). Eur J Biochem 254(1):154–159. doi:10.1046/j.1432-1327.1998.2540154.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Nausch, H., Huckauf, J. & Broer, I. Peculiarities and impacts of expression of bacterial cyanophycin synthetases in plants. Appl Microbiol Biotechnol 100, 1559–1565 (2016). https://doi.org/10.1007/s00253-015-7212-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7212-y