Abstract
Increasing the arginine (Arg) content in plants used as feed or food is of interest, since the supplementation of food with conditionally essential Arg has been shown to have nutritional benefits. An increase was achieved by the expression of the Arg-rich bacterial storage component, cyanophycin (CGP), in the chloroplast of transgenic plants. CGP is stable in plants and its degradation into β-aspartic acid (Asp)-Arg dipeptides, is solely catalyzed by bacterial cyanophycinases (CGPase). Dipeptides can be absorbed by animals even more efficiently than free amino acids (Matthews and Adibi 1976; Wenzel et al. 2001). The simultaneous production of CGP and CGPase in plants could be a source of β-Asp-Arg dipeptides if CGP degradation can be prevented in planta or if dipeptides are stable in the plants. We have shown for the first time that it is possible to co-express CGP and CGPase in the same plant without substrate degradation in planta by transient expression of the cyanobacterial CGPase CPHB (either in the plastid or cytosol), and the non-cyanobacterial CGPase CPHE (cytosol) in CGP-producing Nicotiana tabacum plants. We compared their ability to degrade CGP in planta and in crude plant extracts. No CGP degradation appeared prior to cell homogenization independent of the CGPase produced. In crude plant extracts, only cytosolic CPHE led to a fast degradation of CGP. CPHE also showed higher stability and in vitro activity compared to both CPHB variants. This work is the next step to increase Arg in forage plants using a stable, Arg-rich storage protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arginine (Arg) is important for animal nutrition and has shown beneficial effects on growth, health, reproduction and meat quality (Ma et al. 2015; Wang et al. 2015; Wu et al. 2014) while no adverse effects of long-term Arg supplementation were found in pigs, sheep and rats (Hu et al. 2015; Wu et al. 2007). Since Arg also plays an important role in human bio-vital processes, it is used in medicine and as an additive in the food industry (Sallam and Steinbuchel 2010). Arg is commonly produced by fermentation (Utagawa 2004) and is supplemented as a free amino acid. Enhancing the Arg content in forage crops could lead to a cheaper, easier and sufficient Arg supply for livestock. To our best knowledge, no successful breeding attempt to increase the content of free Arg in plants has been described so far. One reason for this failure might be the feedback inhibition of Arg synthesis (Sancho-Vaello et al. 2009; Winter et al. 2015). In order to prevent this, newly synthesized Arg needs to be bound, keeping the free Arg content at the endogenous level.
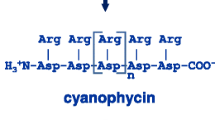
Storage can be achieved by incorporating Arg in the cyanobacterial storage polypeptide cyanophycin (multi-L-arginyl-poly-l-aspartic acid, CGP). CGP is a nitrogen, carbon and energy storage protein, which is synthesized by most cyanobacteria and also several non-photosynthetic bacteria (Allen et al. 1984; Simon 1987; Simon and Weathers 1976; Ziegler et al. 2002). It is created via non-ribosomal biosynthesis by the enzyme cyanophycin synthetase (cphA) (Ziegler et al. 1998) and consists of an l-aspartic acid (Asp) backbone with linked L-Arg residues (Simon and Weathers 1976). CGP proved to be stable and resistant to common eukaryotic and prokaryotic proteases (Simon and Weathers 1976), and its degradation is restricted to cyanophycinases (CGPases), which are produced by prokaryotes and occur in two classes: (1) Intracellular, called CPHB, mainly produced by cyanobacteria (Allen et al. 1984; Gupta and Carr 1981; Richter et al. 1999), and (2) extracellular CPHE, produced by non-cyanobacterial prokaryotes (Obst et al. 2002). CPHB and CPHE catalyze the degradation of CGP into β-Asp-Arg dipeptides (Gupta and Carr 1981; Obst et al. 2002; Richter et al. 1999). In human trials, these dipeptides had an improved nutritional effect compared to free AA, since they are taken up more efficiently (Matthews and Adibi 1976; Wenzel et al. 2001).
The chloroplast-targeted expression of the cphA coding region from Thermosynechococcus elongatus BP-1 in tobacco and potato led to the stable production of CGP in plants and an increase in the amount of total Arg (Hühns et al. 2008, 2009; Nausch et al. 2016; Neumann et al. 2005). Although CPHE-producing bacteria were found in the colon of mammals (Sallam and Steinbuchel 2009a), feeding of CGP should not result in an increase in Arg in the blood, since Arg uptake is mainly restricted to the small intestine (Bröer 2008). This has been confirmed in a previous study where we showed that CGP can only be degraded by mice when degrading enzymes have been added to the feed (Ponndorf et al. 2016). Thus the co-expression of CGPase and CGP might enable the release of β-Asp-Arg dipeptides in the gut, making the addition of the enzyme unnecessary.
As already shown for bacteria (Richter et al. 1999; Sallam et al. 2009; Sallam and Steinbuchel 2009b, 2010), CPHB and CPHE were successfully produced in Nicotiana benthamiana. In case of CPHB, it was necessary to improve translation and stability for successful enzyme accumulation in plants, while CPHE was produced in high amounts in a stable manner without any adaptations. In addition, CPHE demonstrated a higher CGP degrading activity. Nevertheless, when added to crude N. benthamiana extracts, both CPHB and CPHE were able to degrade purified CGP at room temperature (RT) (Nausch and Broer 2016; Ponndorf et al. 2016).
In this study we analyzed the co-expression of different CGPases in CGP-producing Nicotiana tabacum var. Badischer Geudertheimer plants (BG) (Nausch et al. 2016) to allow the release of dipeptides during digestion of the feed.
Materials and methods
Transient expression in Nicotiana tabacum var. badischer geudertheimer (BG) and sample preparation
We used the constructs S-cphB-s and gfp-cphB-s for the production of S-CPHB and GFP::CPHB (Ponndorf et al. 2016) and cphE241syn (pcphE-s) for the production of CPHE (Nausch and Broer 2016), respectively. Clones of one parental BG plant were used for transient expression [Event 176 (Nausch et al. 2016)]. Four-week-old clones were transferred from tissue culture, containing Murashige–Skoog medium, to peat soil and grown under greenhouse conditions for another 2 weeks before vacuum infiltration as described by Ponndorf et al. (2016). The vectors were transferred into the Agrobacterium tumefaciens strain ICF320, cultivated in LB with 50 µg × ml−1 rifampicin and kanamycin, each. Cell cultures were centrifuged and diluted in infiltration buffer (100 mM MES (pH 5.5), 10 mM MgCl2, 0.02% Silwet Gold). BG plants were submerged into the infiltration buffer and vacuum was applied (50 mbar, 5 min), using a freeze drier. Infiltrated plants were kept in the dark for one night. After 10 days post infiltration (dpi) 3–5 leaves per plant were harvested and cut vertically. One half was frozen immediately without homogenization to analyze the in planta state, while the other half was homogenized using a PT2100-Homogenizer (Kinematica AG, Littau-Lucerne, Switzerland) (30.000 rpm; 30–45 s) and incubated overnight at 22–24 °C.
Quantification of CGP
CGP was quantified as described by Nausch et al. (2016). Freeze dried leaf material (30–35 mg) was homogenized with ceramic pills using a Precellys 24 homogenisator (VWR International GmbH, Erlangen, Germany) and incubated in 1 mL 50 mM Tris (pH 8) for 30 min. After centrifugation, the pellet was resuspended in 1 mL of 0.1 M HCl and incubated for 1 h at room temperature. After another centrifugation step, 800 mL of the supernatant was used for CGP analysis. For that 1–10 µL of sample were diluted with 0.1 M HCl to a final volume of 800 and 200 mL of 5 × RotiQuant Bradford reagent (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) was added. Samples were measured at 595 nm. A calibration curve was prepared with purified CGP from potato tubers, as described in 2.3. OD values of leaf samples from non-transgenic plants that were infiltrated with the corresponding Agrobacterium strain were subtracted from OD values of samples of infiltrated transgenic plants. Complete Protease Inhibitor Cocktail Tablets EASYpacks (Roche) were added to the 50 mM Tris buffer according to the manufacturer’s advice. Additionally, we added 2 mM Pefabloc® (Sigma-Aldrich), 1 µg ml−1Aprotinin and 1 mM PMSF (Sigma-Aldrich) to prevent unwanted CGP degradation during protein extraction.
Isolation of CGP and CGPase
Isolation of CGP from Solanum tuberosum tubers was conducted as described by Neubauer et al. (2012). Ground potatoes were stirred in 1% (w/v) NaHSO3 for 1 h and the homogenate decanted through a sieve (≤0.5 mm pore diameter). The aqueous CGP-containing flow-through was passed again through a sieve (70 µm pore diameter) and the residue treated with 0.1 M HCl to solubilize CGP. CGP was separated from insoluble starch via centrifugation (4466×g, 15 min) and CGP precipitated in the supernatant by adjusting to pH 5 with NaOH.
CGPase from E. coli and N. benthamiana were isolated as described by Ponndorf et al. (2016) for CPHB and by Nausch and Broer (2016) for CphE. Since all CGPases contain a His-Tag, they were purified via Ni–NTA purification. The ProBond™ Purification System (Thermo Fisher Scientific) and a Glass Econo-Column® (BioRad, Hercules, USA), that were packed with the nickel resin was used. Crude E. coli or leaf extracts were centrifuged at (16,260×g, 4 °C) and after an initial equilibration of the resin with NPI buffer [50 mM NaH2PO4 (pH 8), 300 mM NaCl] containing 10 mM Imidazol, the supernatant was loaded onto the column. After two washing steps with buffer, containing 20 and 40 mM imidiazol, the His-tagged target protein was eluted with NPI buffer and 300 mM imidazol.
The eluted proteins were desalted via Sephadex G25 M Desalting Columns (Column PD-10; GE Healthcare Europe GmbH; Freiburg, Germany) and desalting buffer [20 mM Tris (pH 8) and 1 mM DTT].
In planta, semi in vivo and in vitro activity assays
The CGPase activity assay in planta was carried out as described above. The activity in vitro and in crude extracts was determined as described by Nausch and Broer (2016). Purified CGP (200 μg) was diluted either in PBS or in 1000 μg of crude TSP extracts of BG and 500 ng or 5 μg of purified CGPase were added respectively, diluted with PBS to a final volume of 5 ml and incubated at 22–24 °C. At 15 min time points, one tenth of the original reactions was collected and the reaction stopped by TCA precipitation. Pellets were resuspended in 25 μl SDS-PAGE loading buffer, separated in a 12% SDS-PAGE and subsequently Coomassie stained.
SDS PAGE and western blot
Samples were resuspended in loading buffer, (10% glycerin, 150 mM Tris (pH 6.8), 3% SDS, 1% β-mercaptoethanol, and 2.5% bromophenol blue) and denaturated at 95 °C for 5 min prior to separation in a 12% SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane, using a BioRad Trans-Blot semi-dry transfer cell. Two mA/cm2 were applied for 1 h using 50 mM Tris, 40 mM glycine, 0.01% SDS, and 20% methanol as the transfer buffer (pH 8.5). CPHB variants were detected via a self-made rabbit anti CPHB antibody (Ponndorf et al. 2016) and a goat anti-rabbit, POD-conjugated antibody (Dianova, Hamburg, Germany). For the detection of GFP, a rabbit anti-GFP antibody (SySy GmbH, Göttingen, Germany) and the same secondary antibody were used as previously described. CPHE was detected as described by Nausch and Broer (2016) via its His-Tag using a mouse anti-His and a donkey anti-mouse, POD conjugated antibody (both Dianova). Proteins were detected via chemiluminescence using a Kodak Biomax light X-ray film (VWR; Darmstadt, Germany).
Calculation and prediction of protein properties
Molecular weight of proteins was predicted using the sequence manipulation suite homepage (Stothard 2000).
Statistical evaluation
Exploratory data analysis, the comparison of means and creation of box plots was carried out using IBM SPSS Statistics 22. Tests were chosen depending on the data properties. Normal distribution was tested using the Shapiro–Wilk test with p > 0.05 defined as normally distributed. Homogeneity of variation was tested using the Levene statistic with p > 0.05 defined as homogenous. Depending on these requirements and the respective dataset the corresponding statistical tests were chosen.
Results
The MagnICON® transient expression system (Marillonnet et al. 2005) was used to co-express CGPase in transgenic N. tabacum var. BG plants (event BG 176) producing CGP in the plastid (Nausch et al. 2016). We analyzed three CGPase variants, which had been successfully produced in plants before and were confirmed to be active in plant material. The intercellular CGPase CPHB from Thermosynechococcus elongatus BP-1 was targeted to the chloroplast by the fusion to the transit peptide of the small subunit of RuBisCO (S-CPHB) or stabilized in the cytosol by the fusion to the green fluorescence protein (GFP) (GFP::CPHB) (Ponndorf et al. 2016). The intercellular CGPase CPHE from Pseudomonas alcaligenes (Nausch and Broer 2016; Sallam et al. 2011) was expressed in the cytosol.
Infiltrated clones of BG 176 were harvested at 10 dpi, and the CGP content was determined in freeze-dried material. Leaves were harvested at 10 dpi to allow even small amounts of CGPase to degrade CGP in the chloroplast (S-CPHB), or in the case that the separation might not be complete (GFP::CPHB, CPHE). The CGP content varied between independent experimental replicates (data not shown), but these differences did not occur between plants of the same replicate.
CPHE shows higher activity compared to S-CPHB under in vitro conditions
As shown previously in N. benthamiana, CPHB variants and CPHE were active in vitro, and S-CPHB and GFP::CPHB did not differ in their activity in N. benthamiana (Nausch and Broer 2016; Ponndorf et al. 2016). Therefore, purified S-CPHB (5 µg) and CPHE (0.5 µg) isolated from N. benthamiana were incubated with purified CGP (20 µg) either under in vitro conditions or in crude BG extracts, containing 100 µg of total soluble protein (TSP, Fig. 1). The reaction was stopped every 15 min and analyzed with SDS-PAGE by Coomassie staining. Experiments were conducted at least twice. Within the time investigated, the activity of CPHE in BG crude extracts was much higher than S-CPHB. Although the amount of S-CPHB added to the samples was 10 times higher than CPHE, its degradation of the same amount of CGP was approximately 2 times slower (Fig. 1).
Comparison of enzyme activity of S-CPHB and CPHE a in vitro (CPHE already shown by Nausch and Broer 2016). b Degradation of 20 µg isolated CGP in 100 µg total soluble protein (TSP) isolated from N. tabacum Badischer Geudertheimer M = PageRuler™ Plus Prestained Protein ladder; -CGPase = control without the addition of S-CPHB and CPHE respectively
Additionally, when the amount of S-CPHB was reduced to 2.5 µg per sample, no visible decrease in CGP content occurred over the time investigated (data not shown). In contrast, only 100 ng of CPHE per sample are sufficient for a detectable CGP degradation (Nausch and Broer 2016).
Expression of chloroplast-targeted CGPase does not result in CGP degradation in planta
In order to analyze whether the storage of Arg in β-Asp-Arg dipeptides is superior to CGP storage in the chloroplasts, CGPases should be transported to the chloroplast where CGP is present. Since CPHE could not be targeted to the plastids of N. benthamiana (Nausch and Broer 2016), we only investigated the effect of chloroplast targeting for S-CPHB in BG. At 10 dpi comparable amounts of CGP were analyzed in uninfiltrated plants [median value, 6.7 µg mg−1 dry weight (dw)], the empty vector control (10.0 µg mg−1 dw) and in plants transfected with the S-CPHB expressing vector (8.0 µg mg−1dw); (Fig. 2). The observed differences were not significant (Dunnet-T3 test). The presence of S-CPHB was verified by western blot (Fig. 3). The two bands represent the expected size of the monomer [29 kilo Dalton (kDa)] and dimer (ca 70 kDa) of the mature protein. The expected size of the unprocessed monomer including the signal peptide is about 35 kDa. Stronger signal intensity was observed for S-CPHB compared to GFP::CPHB in the anti-CPHB western blot, but was not detectable at all when using anti-His antibodies, probably due to the lower sensitivity of this antibody. Because only the mature CPHB was detected in the western blot, it is likely that the enzyme enters the chloroplast. However, this expression did not decrease the amount of CGP in the plastids. After homogenization and incubation of the plant material for 24 h, no S-CPHB signals could be detected (Fig. 3) indicating instability of the enzyme after cell homogenization.
In planta and crude plant extract activity assay of different cyanophycinase (CGPase) variants at two different experimental time points (I and II). Infiltrated N. tabacum Badischer Geudertheimer leaves were harvested at 10 days post-infiltration (dpi), and vertically cut into halves. One half was frozen immediately without homogenization (T0). The other half was homogenized and incubated for 24 h at room temperature (T24). The material was freeze dried and CGP content was measured. Circles in the box plot show outliers; CGP, cyanophycin; dw, dry weight; NIC, N. tabacum Badischer Geudertheimer-176 near isogenic control; GFP, empty vector control: pICH18711 expressing the green fluorescent protein (Marillonnet et al. 2005). Values with different letters (a, b) significantly differ between groups (Dunnet T3, p < 0.01)
Western blot analysis of 100 µg total soluble protein (TSP) isolated from N. tabacum Badischer Geudertheimer leaf material. Leaves were cut vertically. One half was frozen immediately (T0) the other half was homogenized and incubated for 24 h (T24). GFP, expressed by vector pICH18711 (Marillonnet et al. 2005) was used as control. kDa, kilodalton; P1, positive control GFP::CPHB isolated from E.coli; P2, positive control CPHB isolated from E.coli; P3, positive control: CPHE isolated from E.coli; anti-his western: 5 ng of P1, P2 and P3; anti-CPHB western: 1 ng of P1 and P2, anti-GFP western: 1 ng of P1
Transient expression of CPHE in CGP producing N. tabacum BG plants is sufficient to completely degrade CGP in homogenized leaf tissue
The spatial separation of CGP and CGPase should prevent degradation of CGP in the chloroplast in planta and might enable the controlled degradation of CGP after cell homogenization. Therefore, BG 176 clones were infiltrated with vectors gfp-cphB-s and cphE241syn encoding GFP::CPHB and CPHE to allow cytosolic enrichment of CGPase. Leaf material was harvested at 10dpi, homogenized and incubated for 24 h at RT. The CGP content was measured directly after harvest and after cell homogenization and incubation. The expression of cytosolic GFP::CPHB did not lead to a measurable degradation of CGP in comparison to the GFP control, neither before nor after cell homogenization (Fig. 2). The expression of CPHE in the cytosol did not result in CGP degradation in intact leaves, but a significant decrease (p = 0.00, Dunnet T3 Test, α = 0.01) was measured in homogenized tissue. While the mean CGP content averaged 21 µg CGP mg dw−1 directly after homogenization, only 1 µg CGP mg dw−1 was present after 24 h (Fig. 2).
In order to investigate whether the absence of CGP degradation for most constructs was caused by the absence of the CGPase or its inactivity, western blots were conducted (Fig. 3). Anti-CPHB and anti-GFP western blots seem to be more sensitive compared anti-His western blots, since 5 times more purified protein was necessary to give the same signal intensity in anti-His blots compared to anti-CPHB and anti-GFP blots.
As shown in Fig. 3, GFP::CPHB was detected in samples directly after harvest (T0), while a decrease in protein was observed after cell homogenization and incubation (T24). Using anti-GFP antibodies, a protein corresponding to the size of the GFP protein (29 kDa) was detected. Although GFP and CPHB monomers have an equal size we assume this band is specific for GFP, because no CPHB signals were detected in the anti-CPHB western blots for the same sample
In contrast to S-CPHB and GFP::CPHB, CPHE was clearly detectable in T0 as well as T24 samples.
Discussion
Here we could show for the first time that sufficient storage of arginine and timely delivery of β-Asp Arg dipeptides is possible when CGP and CPHE are coexpressed in a commercial tobacco cultivar but produced in separate compartments. The commercial usage of CGP producing plants to supplement Arg in feed depends on the storage of high amounts of CGP or β-Asp-Arg dipeptides in the plant and its controlled and complete degradation to β-Asp-Arg dipeptides in the extract. The fact that the separation of CGP (chloroplast) and CGPase (cytosol) is sufficient to prevent premature degradation and that the co-expression of CPHE in the CGP-producing commercial N. tabacum variety BG leads to complete CGP degradation 24 h after homogenization supports the assumption that the valorization of feed is possible without additional effort. Considering that the degradation of CGP in the gut after co-delivery of isolated CGP and CGPase is possible, and that β-Asp-Arg dipeptide uptake has been proven (Ponndorf et al. 2016), this result represents another crucial step for the enrichment of β-Asp-Arg dipeptides in feed.
Degradation of CGP could not be observed after targeting CPHB to the chloroplast. In contrast to CPHE, where only unprocessed protein was found when targeted to plastids (Nausch and Broer 2016), in this study the presence of processed CPHB after infiltration of BG indicated an import of at least parts of the enzyme (Abad et al. 1989; Lamppa and Abad 1987; Richter and Lamppa 1998; Robinson and Ellis 1984). In a previous study, we demonstrated that different CPHB variants are unstable outside of the chloroplast (Ponndorf et al. 2016). Here we show in addition that CGP degradation depends on the amount of CGPase, hence the most obvious cause for non-degradation of CGP in the chloroplast is low CPHB activity or low protein content, which is in line with the results obtained for the cytosol.
All cytosolic CGPase variants could be detected but, as expected, no CGP degradation was shown without cell homogenization. As desired, the spatial separation of CGP and CGPase into different cell compartments prevents the degradation of CGP. The protection of recombinant proteins from cytosolic proteases via localization in different plant compartments has often been described as reviewed by Pillay et al. (2014). Hence storage and accumulation of CGP in chloroplasts is possible in parallel to the accumulation of the degrading enzyme in the cytosol.
After cell homogenization, nearly the complete pool of CGP was degraded in plants expressing CPHE after 24 h. This is accompanied by stable and high expression of CPHE similar to that described by Nausch and Broer (2016). In contrast to this, GFP::CPHB did not cause a measurable degradation of CGP and proved to be instable in crude plant extract. This instability seems to be due to a degradation of CPHB, since only the GFP domain of the fusion protein was still detectable, likely due to its high stability in plant cells (Sheen et al. 1995). This instability of GFP::CPHB was not observed in previous studies in N. benthamiana (Ponndorf et al. 2016), probably due to the substantially higher expression levels of the MagnICON vectors in N. benthamiana (Nausch et al. 2012a).
In addition to the protein stability, CPHE is more active compared to both S-CPHB and GPF::CPHB and therefore more suitable for the expression in CGP-producing plants. The data presented here show for the first time that it is possible to express sufficient amounts of active CPHE in plants to degrade the complete pool of CGP present in the same plant.
However, the high amounts of CPHE observed after transient expression will probably not be achieved in stably transformed plants (Gleba et al. 2005; Nausch et al. 2012b). Therefore, it remains to be seen whether stably transformed plants can produce sufficient amounts of CGPase to degrade CGP into β-Asp-Arg dipeptides. Stable expression might also demand another expression system such as seeds. Huckauf et al. (personal communication) could demonstrate that the expression of the viral antigen VP60 in pea seeds was significantly higher compared to transient expression in N. benthamiana. Due to their high nutritional value, peas would be a perfect feed additive. Stable accumulation of CGP has already been shown by Baars et al. (in preparation) in this plant species. Hence the coproduction of CGP and CPHE in pea seeds might be a promising strategy to enhance the Arg content in food and feed. Nevertheless, at least in mice, Arg derived from β-Asp-Arg dipeptides were not bioavailable (Ponndorf et al. 2016).
However positive effects of β-Asp-Arg dipeptide supplementation on fish body mass have been described in aquaculture (Dr. Martin Krehenbrink, personal communication). Hence β-Asp-Arg dipeptides might also be bioavailable for other species, and it remains to be ascertained whether this holds true for other mammals. The coexpression of a suitable isoaspartyl dipeptidase might also increase bioavailability.
References
Abad MS, Clark SE, Lamppa GK (1989) Properties of a chloroplast enzyme that cleaves the chlorophyll a/B binding-protein precursor—optimization of an organelle-free reaction. Plant Physiol 90:117–124. doi:10.1104/Pp.90.1.117
Allen MM, Morris R, Zimmerman W (1984) Cyanophycin granule polypeptide protease in a unicellular cyanobacterium. Arch Microbiol 138:119–123
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286
Gleba Y, Klimyuk V, Marillonnet S (2005) Magnifection—a new platform for expressing recombinant vaccines in plants. Vaccine 23:2042–2048. doi:10.1016/j.vaccine.2005.01.006
Gupta M, Carr NG (1981) Enzyme-activities related to cyanophycin metabolism in heterocysts and vegetative cells of Anabaena spp. J Gen Microbiol 125:17–23
Hu SD, Li XL, Rezaei R, Meininger CJ, McNeal CJ, Wu GY (2015) Safety of long-term dietary supplementation with largening in pigs. Amino Acids 47:925–936. doi:10.1007/s00726-015-1921-5
Hühns M et al (2008) Plastid targeting strategies for cyanophycin synthetase to achieve high-level polymer accumulation in Nicotiana tabacum. Plant Biotechnol J 6:321–336. doi:10.1111/j.1467-7652.2007.00320.x
Hühns M et al (2009) Tuber-specific cphA expression to enhance cyanophycin production in potatoes. Plant Biotechnol J 7:883–898. doi:10.1111/j.1467-7652.2009.00451.x
Lamppa GK, Abad MS (1987) Processing of a wheat light-harvesting chlorophyll a/B protein-precursor by a soluble enzyme from higher-plant chloroplasts. J Cell Biol 105:2641–2648. doi:10.1083/jcb.105.6.2641
Ma X, Zheng C, Hu Y, Wang L, Yang X, Jiang Z (2015) Dietary l-arginine supplementation affects the skeletal longissimus muscle proteome in finishing pigs. PLoS ONE 10:1–16
Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Systemic agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23:718–723. doi:10.1038/Nbt1094
Matthews DM, Adibi SA (1976) Peptide absorption. Gastroenterology 71:151–161
Nausch H, Broer I (2016) Cyanophycinase CphE from P. alcaligenes produced in different compartments of N. benthamiana degrades high amounts of cyanophycin in plant extracts. Appl Microbiol Biotechnol. doi:10.1007/s00253-016-8020-8
Nausch H, Mikschofsky H, Koslowski R, Meyer U, Broer I, Huckauf J (2012a) High-level transient expression of ER-targeted human interleukin 6 in Nicotiana benthamiana. PLoS ONE. doi:10.1371/journal.pone.0048938
Nausch H, Mischofsky H, Koslowski R, Meyer U, Broer I, Huckauf J (2012b) Expression and subcellular targeting of human complement factor C5a in Nicotiana species. PLoS ONE. doi:10.1371/journal.pone.0053023
Nausch H et al (2016) Tobacco as platform for a commercial production of cyanophycin. New Biotechnol. doi:10.1016/j.nbt.2016.08.001
Neubauer K et al (2012) Isolation of cyanophycin from tobacco and potato plants with constitutive plastidic cphA(Te) gene expression. J Biotechnol 158:50–58. doi:10.1016/j.jbiotec.2011.12.008
Neumann K, Stephan DP, Ziegler K, Huhns M, Broer I, Lockau W, Pistorius EK (2005) Production of cyanophycin, a suitable source for the biodegradable polymer polyaspartate, in transgenic plants. Plant Biotechnol J 3:249–258. doi:10.1111/j.1467-7652.2005.00122.x
Obst M, Oppermann-Sanio FB, Luftmann H, Steinbuchel A (2002) Isolation of cyanophycin-degrading bacteria, cloning and characterization of an extracellular cyanophycinase gene (cphE) from Pseudomonas anguilliseptica strain BI—the cphE gene from P. anguilliseptica BI encodes a cyanophycin-hydrolyzing enzyme. J Biol Chem 277:25096–25105. doi:10.1074/jbc.M112267200
Pillay P, Schluter U, van Wyk S, Kunert KJ, Vorster BJ (2014) Proteolysis of recombinant proteins in bioengineered plant cells. Bioengineered 5:15–20. doi:10.4161/Bioe.25158
Ponndorf D et al (2016) Stable production of cyanophycinase in Nicotiana benthamiana and its functionality to hydrolyse cyanophycin in the murine intestine. Plant Biotechnol J. doi:10.1111/pbi.12658
Richter S, Lamppa GK (1998) A chloroplast processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci USA 95:7463–7468
Richter R, Hejazi M, Kraft R, Ziegler K, Lockau W (1999) Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-L-arginyl-poly-l-aspartic acid (cyanophycin)—molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur J Biochem 263:163–169. doi:10.1046/j.1432-1327.1999.00479.x
Robinson C, Ellis RJ (1984) Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem 142:337–342
Sallam A, Steinbuchel A (2009a) Cyanophycin-degrading bacteria in digestive tracts of mammals, birds and fish and consequences for possible applications of cyanophycin and its dipeptides in nutrition and therapy. J Appl Microbiol 107:474–484. doi:10.1111/j.1365-2672.2009.04221.x
Sallam A, Steinbuchel A (2009b) Process for the preparation of dipeptides from cyanophycin emplyoing the isolated Pseudomonas alcaligenes DIP1 CGPase CphEaI. European Patent Application, Publication No. 2 133 419 A1 (16.12.2009)
Sallam A, Steinbuchel A (2010) Dipeptides in nutrition and therapy: cyanophycin-derived dipeptides as natural alternatives and their biotechnological production. Appl Microbiol Biotechnol 87:815–828. doi:10.1007/s00253-010-2641-0
Sallam A, Kast A, Przybilla S, Meiswinkel T, Steinbuchel A (2009) Biotechnological process for production of beta-dipeptides from cyanophycin on a technical scale and its optimization. Appl Environ Microbiol 75:29–38. doi:10.1128/AEM.01344-08
Sallam A, Kalkandzhiev D, Steinbuchel A (2011) Production optimization of cyanophycinase ChpEal from Pseudomonas alcaligenes DIP1. AMB Express 1:38. doi:10.1186/2191-0855-1-38
Sancho-Vaello E, Fernandez-Murga ML, Rubio V (2009) Mechanism of arginine regulation of acetylglutamate synthase, the first enzyme of arginine synthesis. FEBS Lett 583:202–206. doi:10.1016/j.febslet.2008.12.001
Sheen J, Hwang SB, Niwa Y, Kobayashi H, Galbraith DW (1995) Green-fluorescent protein as a new vital marker in plant-cells. Plant J 8:777–784. doi:10.1046/j.1365-313X.1995.08050777.x
Simon RD (1987) Inclusion bodies in the cyanobacteria: cyanophycin, polyphosphate, polyhedra bodies. In: Fay P, Van Baalen C (eds) The cyanobacteria. Elsevier, Amsterdam, pp 192–222
Simon RD, Weathers P (1976) Determination of structure of novel polypeptide containing aspartic-acid and arginine which is found in cyanobacteria. Biochem Biophys Acta 420:165–176
Stothard P (2000) The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28:1102
Utagawa T (2004) Production of arginine by fermentation. J Nutr 134:2854s–2857s
Wang B et al (2015) Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem 167:91–99. doi:10.1016/j.foodchem.2014.06.091
Wenzel U, Meissner B, Doring F, Daniel H (2001) PEPT1-mediated uptake of dipeptides enhances the intestinal absorption of amino acids via transport system b(0, +). J Cell Physiol 186:251–259. doi:10.1002/1097-4652(200102)186:2<251:AID-JCP1027>3.0.CO;2-F
Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants. Front Plant Sci. doi:10.3389/Fpls.2015.00534
Wu G et al (2007) Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 137:1673S–1680S
Wu GY, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL (2014) Acid nutrition in animals: protein synthesis and beyond amino. Annu Rev Anim Biosci 2:387–417. doi:10.1146/annurev-animal-022513-114113
Ziegler K, Diener A, Herpin C, Richter R, Deutzmann R, Lockau W (1998) Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-L-arginyl-poly-l-aspartate (cyanophycin). Eur J Biochem 254:154–159
Ziegler K, Deutzmann R, Lockau W (2002) Cyanophycin synthetase-like enzymes of non-cyanobacterial eubacteria: characterization of the polymer produced by a recombinant synthetase of Desulfitobacterium hafniense Zeitschrift Fur Naturforschung section C-a. J Biosci 57:522–529
Acknowledgements
We would like to express our very great appreciations to Alex Rajewski for proof-reading and the helpful suggestions and Dr. Martin Krehenbrink, Cysal GmbH, for sharing their aquaculture results.
Funding
This study was funded by the University of Rostock.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ponndorf, D., Broer, I. & Nausch, H. Expression of CphB- and CphE-type cyanophycinases in cyanophycin-producing tobacco and comparison of their ability to degrade cyanophycin in plant and plant extracts. Transgenic Res 26, 491–499 (2017). https://doi.org/10.1007/s11248-017-0019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-017-0019-0