Abstract
Silica fertilization and nano-MnO2 amendment are reported as useful approaches in lowering the accumulation of arsenic in rice grains, but the effects of silica fertilization or nano-MnO2 amendment on microbial community in the paddy soils containing high concentration of arsenic are still unknown. In order to elucidate this question, the structures and composition of microbial community in the paddy soils, in response to silica fertilization and nano-MnO2 amendment, were investigated using pyrosequencing technique. The results indicated that Proteobacteria, Chloroflexi, and Acidobacteria were the main dominating phyla in these paddy soils. A decrease in the relative abundance of Chloroflexi and Cyanobacteria, but an increase in the relative abundance of Acidobacteria was observed after silica fertilization and nano-MnO2 amendment. The changes of Acidobacteria, Chloroflexi, and Cyanobacteria were strongly correlated with pH and the concentration of bioavailable arsenic in the paddy soils. The α-diversity of bacteria in the paddy soils increased in response to silica fertilization at low amendment level, but decreased under silica or nano-MnO2 amendment at high amendment level. Results of β-diversity analysis indicated that the microbial communities in the control treatment shared more similarity with that of those received low level of nano-MnO2 amendment, and the two silica fertilization treatments also shared more similarity with each other.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic pollution has been recognized as a major public health issue in some parts of the world, especially in Asia (Mukherjee et al. 2006). Long-term arsenic exposure could damage internal organs and induce several cancers, e.g., laryngeal cancer, bladder cancer, lung cancer, and skin cancer (McClintock et al. 2012; Smith et al. 2012). Rice is a very important staple food grain in Southeast Asian countries, including China, India, Bangladesh, and the Philippines. Arsenic in soils can be assimilated from root systems and then accumulated in rice, and arsenic in rice is regarded as a disaster for these countries (Azizur Rahman et al. 2008; Zhu et al. 2008). As for China and some other countries, another serious problem is the limitation of quality arable land without high level of pollutants considering the high population. For example, China has <9 % of the world’s cultivated land to feed 22 % of the global population (Chen 2007). In addition, the limited quantities of arable lands continue to decrease due to urbanization and development (Tan et al. 2005; Chen 2007). Food security and crisis could be aggravated if these countries give up arsenic-contaminated arable lands for food production completely. In order to safely utilize arsenic-contaminated agricultural land for food production, various approaches were proposed to relieve the toxicity of arsenic in soils against rice and lower the accumulation of arsenic from soils into rice grains, e.g., water regime (Xu et al. 2008; Li et al. 2009), silica fertilization (Li et al. 2009), and nano-MnO2 amendment (Zhou et al. 2015).
Microorganisms are ubiquitous in soils and responsible for nutrient cycling. Microbial communities are enormously diverse in paddy soils, and some of them can affect the growth of paddy rice seedlings or arsenic accumulation in rice grains (Pandey et al. 2013; Wang et al. 2009). The structure of microbial community depends on biotic and abiotic factors taking place in soils. Previous studies showed that arsenic contamination affected the composition of microbial community in paddy soils (Somenahally et al. 2011). However, the effects of remedial approaches towards arsenic contamination and pollution on bacterial community remain unknown. Recently, silica fertilization (Li et al. 2009) and nano-MnO2 amendment (Zhou et al. 2015) are reported as effective approaches to decrease the accumulation potential of arsenic into rice, particularly the grains. However, the effects of silica fertilization or nano-MnO2 amendment on the structure of microbial community in paddy soils are poorly understood.
The majority of the soil microflora is difficult to culture using traditional isolation and culturing techniques (Amann et al. 1995; Nocker et al. 2007). It is estimated that only a very small fraction of the indigenous microbial community in soils (0.01–10 %) could be cultured in artificial medium under laboratory conditions (Amann et al. 1995; Richaume et al. 1993). Thus, metagenomics and DNA sequencing-based methods are useful in the study of microbial ecology because they are culture-independent. PCR-based next-generation DNA-sequencing technologies, e.g., 454 pyrosequencing and Illumina pyrosequencing, are proven as useful tools in revealing the microbial ecology and are intensively used in the studying of microbial ecology in various niches from surface to subsurface (Wang et al. 2014; Feng et al. 2015; Yan et al. 2015; Yang et al. 2014). In order to elucidate the effects of silica fertilization and nano-MnO2 amendment on the structures and composition of bacterial community in paddy soils, a field experiment was carried out, and Illumina pyrosequencing method was used to investigate the effects of these remediation methods on the structures and composition of microbial community in paddy soils.
Materials and methods
Field experiment and sampling of non-rhizosphere paddy soil
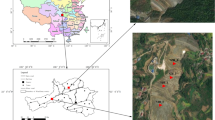
Field experiment was carried out in 2013 at an arsenic-contaminated paddy field (N 25° 36′ 01.11″; E 113° 00′ 42.68″) in the country of Dengjiatang, Cunzhou District, Hunan Province, China. The total arsenic content in the paddy soil was 72.71 mg/kg on average. Slag based silica fertilizer (SiO2) and nano-MnO2 were amended to the top surface of paddy soils at a level of 1.8 and 18 kg/m2, respectively, on May 31, 2013. Control was made without silica or nano-MnO2 addition. Thus, there were five different treatments in this study: control, two silica fertilization treatments (1.8 kg/m2, Si-L; 18 kg/m2, Si-H), and two nano-MnO2 amendment treatments (1.8 kg/m2, Mn-L; 18 kg/m2, Mn-H). Each treatment contained three replicates. Surface paddy soils (0–20 cm) were tilled after silica and nano-MnO2 amendments and then flooded and incubated for 7 days for preparation of rice seedings. Rice seeding transplant was carried out on June 6, 2013. The fields were maintained flooded during the whole duration of the experiment. Non-rhizosphere paddy soil samples were taken on July 29, 2013 at rice tillering stage. Paddy soils (0-10 cm) were collected at three different locations for each treatment using a 4.5-cm-diameter soil sampling auger, put into sterile plastic bags, and transferred on ice back to the laboratory. They were stored at −80 °C.
Soil physicochemical analysis
The contents of total organic carbon (TOC), total phosphorus (TP), total nitrogen (TN), pH, Eh, and bioavailable arsenic of the paddy soil samples were determined. The contents of TOC in paddy soil samples were measured using the total organic carbon analyzer (TOC, Elementar vario TOC, Germany). Total nitrogen was analyzed with Kjeldahl method. Soil total phosphorus content was determined by alkaline digestion followed by molybdate colorimetric measurement (Murphy and Riley 1962). Soil pH and Eh were determined using a pH-Eh meter (Leici, Shanghai, China). The bioavailable arsenic content was assayed by extracting with 0.1 M of HCl according to the method described by Thomas (2006) and determining with atomic fluorescence spectrometry (AFS-920, Titan Instruments, Beijing, China).
DNA extraction, PCR amplification, and pyrosequencing
The soil samples from three replicates of the same treatment were mixed together and homogenized thoroughly. Microbial community DNA from paddy soil samples was extracted using SoilMaster DNA Extraction kit (Epicentre Biotechnologies, Madison, WI) according to manufacturer’s instructions. The 16S universal primers 515F (GTGCCAGCMGCCGCGG) and 907R (CCGTCAATTCMTTTRAGTTT) were used to amplify the V4-V5 region of the 16S ribosomal RNA (rRNA) gene (515–907). The oligonucleotide sequence barcode fused to the forward primer. The PCR reaction mixture (20 μL volume) contained: 4 μL of fivefold FastPfu reaction buffer (TransGen Biotech, Beijing, China), 2 μL of dNTP mixture (2.5 mM), 0.4 μL of each primer (5 μmol/L), 0.4 μL of FastPfu polymerase, 10 ng of the template DNA, and ddH2O to make up the volume. The PCR thermal cycling scheme was set as follows: initial denaturation at 95 °C for 5 min, 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s followed by a final extension period at 72 °C for 5 min. PCR amplification was performed on an ABI GeneAmp PCR System 9700 (Applied Biosystems, CA, USA). PCR products were examined on a 2 % (w/v) agarose gel and further purified using AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). Purified amplicons were quantified using QuantiFluor™-ST (Promega, USA) and paired-end sequenced on an Illumina MiSeq platform at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) according to the standard protocols.
Bioinformatics and statistical analysis
Raw pyrosequencing data were demultiplexed and quality-filtered using Trimmomatic according to the method described by Lohse et al. (2012). Overlapping reads were merged into single long reads using FLASH (Magoc and Salzberg 2011). The qualified sequences were clustered into operational taxonomic units (OTUs) with 97 % similarity cutoff using Usearch (version 7.1 http://qiime.org/). The phylogenetic affiliation of each 16S rDNA sequence was analyzed using RDP Classifier (version 2.2 http://sourceforge.net/projects/rdp-classifier/) with a confidence threshold of 0.7. The reference database was Silva (Release115 http://www.arb-silva.de). Rarefaction curve and alpha diversity including Chao1 estimator, ACE estimator, Shannon index, and Simpson index were analyzed using Mothur (version v.1.30.1 http://www.mothur.org/wiki/Schloss_SOP#Alpha_diversity) (Schloss et al. 2011). Venn diagram and heatmap figures were performed using R package (http://www.Rproject.org/). Principal component analysis (PCoA) and redundancy analysis (RDA) were performed using Canoco (version 4.5, Microcomputer Power, Ithaca, NY, USA). The ANOVA analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, USA).
Sequencing data deposition
The raw data for this study were deposited in the SRA of NCBI (SRP059905).
Results
Soil physicochemical characteristics
The effects of silica fertilization and nano-MnO2 amendment on physicochemical characteristics of the paddy soil samples in this study are presented in Table 1. Silica fertilization or nano-MnO2 amendment had no significant effect on the contents of TN in the paddy soils. The content of TP was not influenced by silica fertilization, but was significantly lower than the control under nano-MnO2 amendment at the level of 18 kg/m2. The contents of TOC were lower than the control in the treatments of silica fertilization or nano-MnO2 amendment. The pH of the soils that received high level of silica or nano-MnO2 amendment was significantly lower than the control treatment. Both silica fertilization and nano-MnO2 amendment effectively lowered the bioavailable arsenic concentration in these paddy soil samples, and the content of bioavailable arsenic decreased with the increase of silica or nano-MnO2 amendment levels.
General characteristics of the pyrosequencing-derived data
For the five paddy soil treatments, a total of 194,594 valid sequences were obtained after quality screening. The number of sequences per sample ranged from 21,682 to 49,586 with an average of 38,919. The length of these valid sequences was mainly about 300–400 bp, accounting for 98.82 % of the total valid sequences. The average length of these valid sequences was 396.43 bp. Rarefaction analysis indicated that sequence obtained from the five soil samples reached near saturation at a genetic distance of 3 % (Fig. 1).
Bacterial richness and diversity
The valid sequences obtained for bacteria could be assigned to 5259 OTUs using Usearch (version 7.1 http://qiime.org/). These OTUs could be clustered into 44 phyla. The main phyla in these soil samples were Proteobacteria (36.95–38.66 %), Chloroflexi (8.64–15.34 %), and Acidobacteria (10.88–19.53 %) (Fig. 2). Other highly represented phyla included Nitrospirae (5.42–9.14 %), Planctomycetes (6.37–7.66 %), Cyanobacteria (1.09–7.06 %), Bacteroidetes (3.79–4.86 %), candidate division WS3 (2.80–3.98 %), Actinobacteria (0.6–1.85 %), and Gemmatimonadetes (1.37–1.52 %) in decreasing order. Members of the phyla Spirochaetae, Firmicutes, Verrucomicrobia, and Elusimicrobia were also presented in most samples, but at low abundance.
Relative abundance of the dominant bacterial phyla in arsenic-contaminated paddy soil samples. Si-L: silica fertilization at the level of 1.8 kg/m2; Si-H: silica fertilization at the level of 18 kg/m2; Mn-L: nano-MnO2 amendment at the level of 1.8 kg/m2; Mn-H: nano-MnO2 amendment at the level of 18 kg/m2
The effects of silica fertilization and nano-MnO2 amendment on the bacterial richness and diversity in the paddy soil samples depend on the amendment materials and also the level applied. Proteobacteria was the highest presented phylum in all these paddy soil samples, and silica fertilization or nano-MnO2 amendment showed no significantly influence on its relative abundance. Silica fertilization decreased the relative abundance of Chloroflexi but increased the relative ratio of Acidobacteria in these paddy soil samples, and such effects were also observed on the treatment of high level nano-MnO2 amendment (Mn-H). This positive effect on Acidobacteria and negative effect against Chloroflexi led to the substitution of Chloroflexi with Acidobacteria as the second highly presented phylum in the two silica fertilization treatments and the Mn-H treatment. Cyanobacteria was another phylum significantly influenced by silica fertilizer and nano-MnO2 amendments. The relative abundance of Cyanobacteria decreased drastically in response to silica fertilization or nano-MnO2 amendment, and the richness of Nitrospirae increased in response to two silicon fertilization treatments and the treatment of Mn-H.
The alpha diversity of bacteria in paddy soil samples was also modified by silica fertilization and nano-MnO2 amendments. The Chao1 estimator, ACE estimator, Shannon index, and Simpson index were all presented in the same pattern to silica fertilization or nano-MnO2 amendments (Fig. 3). The α-diversity of bacteria in the paddy soil samples increased to silica fertilization at an amendment level of 1.8 kg/m2 but decreased at the amendment level of 18 kg/m2; nano-MnO2 amendment decreased α-diversity at the amendment level of 18 kg/m2.
Effect of silica fertilization and nano-MnO2 amendment on the α-diversity of bacteria in arsenic-contaminated paddy soil samples. a Chao1 estimator. b ACE estimator. c Shannon index. d Simpson index. Different lowercase letters within columns indicate statistically significant difference by one-way ANOVA (p < 0.05)
The number of OTUs shared by all these soil samples, according to Venn analysis, was 1705, accounting for 32.42 % of the total identified OTUs (Fig. 4). In order to analyze the total number of shared OTUs between two samples, the percentage of shared OTUs (accounted for the total observed OTUs of two target samples) were calculated based on Venn analysis. The results indicated that the shared OTUs between the control and the treatment of silica fertilization or nano-MnO2 amendment were 38.6 % for low level of silica fertilization (Si-L), 38.6 % for high level of silica fertilization (Si-H), 38.0 % for low level of nano-MnO2 amendment (Mn-L), and 35.0 % for Mn-H, respectively.
A heatmap, based on the level of class, was generated using R packages pheatmap, which divided these five samples into two groups at first levels (Fig. 5). One is composed of the samples Si-L and Si-H; the other of control, Mn-L and Mn-H. Bacterial community in the control treatment shared high similarity with the treatment of Mn-L, and they grouped into a branch apart from the treatment of Mn-H in the group.
The results of PCoA analysis are shown in Fig. 6. The first principal coordinate (PC1) and second principal coordinate (PC2) account for 60.7 and 23.3 % of the intersample variances, respectively. The samples are located in three loci with the control and the treatment of Mn-L located in locus 1, the treatments of Si-L and Si-H in locus 2, and the treatment Mn-H in locus 3 alone.
Discussion
Silicon and arsenic are metalloids. They may share the same transport route during uptake by cell, and this has been proven in rice (Ma et al. 2008). The increase of silicon concentration could depress the uptake of arsenic and alleviate the toxicity of it. Nano-MnO2 and the complex of oxides and silica all could effectively adsorb arsenic and decrease its concentration in water (Con et al. 2013; Bian et al. 2012; Han et al. 2011; Mahmood et al. 2012). Thus, silica fertilizer and nano-MnO2 could lower the toxicity of arsenic through inhibiting the competitive uptake of arsenic by cells or decrease the concentration of bioavailable arsenic in soils.
According to the results of pyrosequencing and analysis, Proteobacteria, Chloroflexi, and Acidobacteria were the dominant phyla in all these paddy soil samples of this study (Fig. 2). Such results are in agreement with previous studies (Xuan et al. 2012; Feng et al. 2015). The relative abundance and constituent of Proteobacteria was hardly influenced by silica fertilization or nano-MnO2 amendment (Figs. 2 and 5), and the main OTUs shared by all these soil samples in Fig. 4 are belonging to the phylum Proteobacteria. Some previous studies indicated that Proteobacteria showed resistance to arsenic contamination (Lorenz et al. 2006; Das et al. 2013), and this may be a reason for its relatively high and constant abundance in these soil samples.
Acidobacteria and Chloroflexi are the other two highly presented phyla in these paddy soils. Silica fertilization and nano-MnO2 amendment increased the relative ratio of Acidobacteria but decreased the relative abundance of Chloroflexi (Fig. 2). Previous studies suggested that the abundance of Acidobacteria was strongly regulated by soil pH, and low pH was in favor of Acidobacteria (Jones et al. 2009; Tripathi et al. 2012). Results of RAD analysis in this study indicated that Acidobacteria showed a negative correlation with soil pH value, whereas Chloroflexi showed a positive correlation with soil pH value in this study (Fig. 7). As shown in Table 1, the pH values of the soils that received silica or nano-MnO2 amendment were lower than the control. The decrease of pH value in paddy soils may be a reason for the positive effect of silica and nano-MnO2 amendment on Acidobacteria. As indicated in Fig. 7, the content of bioavailable arsenic in soil is also negatively correlated with Acidobacteria but positively with Chloroflexi. The reduction of biotoxicity of arsenic by silica fertilization and nano-MnO2 amendment may be another reason for this phenomenon observed.
Actinobacteria are widely distributed in soils and play an important role in organic matter turnover, carbon cycling, and suppression of some fungal pathogens (Jenkins et al. 2010). Results of RAD analysis also suggested that the relative abundance of Actinobacteria was closely related with the contents of TOC in paddy soils (Fig. 7). Some early studies indicated that Actinobacteria had high abundance (always more than 10 %) in the soils (Nacke et al. 2011; Davinic et al. 2012). However, the relative abundance of Actinobacteria in this study ranged only from 0.6 to 2.25 % (Fig. 2). Other studies reported that Actinobacteria had low relative abundance in arsenic-contaminated soils, whereas it had high relative abundance in the control soils (Sheik et al. 2012; Lorenz et al. 2006). Thus, arsenic contamination may be a plausible reason for the low relative abundance of Actinobacteria in soils of this study. The paddy soils in this study were under flooding during the whole experiment period, and the anaerobic condition in paddy soils may be another reason for the lower relative abundance of Actinobacteria in this study since most Actinobacteria are aerobic. Similar results were also presented in some paddy soils and activated sludge (Xuan et al. 2012; Yang et al. 2014).
Cyanobacteria was highly presented in the control soil in this study and accounted for 7.06 % of total reads in the control (Fig. 2). However, the relative abundance of Cyanobacteria decreased drastically to silica fertilization or nano-MnO2 amendment. Cyanobacteria, such as Microcystis, Nostoc, and Synechocystis, have various strategies to detoxify arsenic, including arsenite methylation, volatilization, arsenite reduction, and efflux (López-Maury et al. 2003; Yin et al. 2011; Pandey et al. 2012). These strategies make Cyanobacteria resistant/tolerant to arsenic contamination. This may be the reason for the high abundant ratio of Cyanobacteria in the control paddy soils.
As indicated in Table 1, the content of bioavailable arsenic decreased under silica fertilization or nano-MnO2 amendment. The decrease of arsenic biotoxicity, in response to silica fertilization or nano-MnO2 amendment, may decrease the competition of Cyanobacteria, which in turn decreased the relative abundance of this microorganism in soils. Cyanobacteria prefer the weak alkaline niches (Nayak and Prasanna 2007). The results of RAD analysis indicated that the richness of cyanobacteria was closely related to the pH of soil (Fig. 7). The decrease of soil pH in response to silica or nano-MnO2 amendment may be another reason for the decrease of relative abundance of Cyanobacteria in paddy soils.
Results of heatmap (Fig. 5) and PCoA (Fig. 6) analysis all indicated that low level of nano-MnO2 amendment had little effect on the structure of bacterial community in arsenic-contaminated paddy soil, whereas high level nano-MnO2 amendment and two silica fertilization treatments all significantly altered the structure of bacterial community. Nano-MnO2 was proposed to remediate heavy metal contamination, while the toxicity of nano-MnO2 had also been emphasized (Li et al. 2014). Though the bioavailable arsenic in the treatment of Mn-L was lower than the control, the toxicity of nano-MnO2 may partially offset the alleviation of toxicity from arsenic. We deduce that this is the reason for the little effect of low level nano-MnO2 on the structure of bacterial community in arsenic-contaminated paddy soil. The α-diversity analysis (Chao1 estimator, ACE estimator, Shannon, and Simpson) indicated that silica fertilization increased soil microbial diversity at low amendment level but decreased it at high amendment level (Fig. 3). The effects of silica fertilization on the structure of bacterial community are still obscure. Ahn et al. (2012) reported that silica fertilizer decreased the α-diversity of bacteria in April in paddy soil but increased it in August. The bioavailable arsenic in two silica fertilization treatments was significantly lower than the control in this study, while two silica fertilization treatments have opposite effects on the bacterial diversity. We presently could not confirm the increase of bacterial diversity in the treatment of Si-L either it was induced by silica fertilizer itself or by the decrease of bioavailable arsenic in paddy soils, but, we at least could deduced that the decrease of bacterial diversity in the treatment of Si-H was induced by high-level silica amendment. As mentioned above, nano-MnO2 may be toxic to bacteria; thus, we deduced that the decrease of bacterial diversity in the treatment of Mn-H was caused by the toxicity of nano-MnO2 itself.
References
Ahn JH, Song J, Kim BY, Kim MS, Joa JH, Weon HY (2012) Characterization of the bacterial and archaeal communities in rice field soils subjected to long-term fertilization practices. J Microbiol 50:754–765
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Mol Biol Rev 59:143–169
Azizur Rahman M, Hasegawa H, Mahfuzur Rahman M, Mazid Miah MA, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicol Environ Saf 69:317–324
Bian ZQ, Xie L, Tian XH, Fan W, Zhang J (2012) Adsorption of As(Ш) and As(V) in water by nano-MnO2 with three kinds of crystal structures. J Environ Health 29:645–648
Chen J (2007) Rapid urbanization in China: a real challenge to soil protection and food security. Catena 69:1–15
Con TH, Thao P, Dai TX, Loan DK (2013) Application of nano dimensional MnO2 for high effective sorption of arsenic and fluoride in drinking water. Environ Sci 2:69–77
Das S, Jean JS, Kar S, Liu CC (2013) Changes in bacterial community structure and abundance in agricultural soils under varying levels of arsenic contamination. Geomicrobiol J 30:635–644
Davinic M, Fultz LM, Acosta-Martinez V, Calderón FJ, Cox SB, Dowd SE, Allen VG, Zak JC, Moore-Kucera J (2012) Pyrosequencing and mid-infrared spectroscopy reveal distinct aggregate stratification of soil bacterial communities and organic matter composition. Soil Biol Biochem 46:63–72
Feng Y, Yu Y, Tang H, Zu Q, Zhu J, Lin X (2015) The contrasting responses of soil microorganisms in two rice cultivars to elevated ground-level ozone. Environ Pollut 197:195–202
Han X, Li YL, Gu J-D (2011) Oxidation of As(III) by MnO2 in the absence and presence of Fe(II) under acidic conditions. Geochim Cosmochim Acta 75:368–379
Jenkins S, Waite I, Blackburn A, Husband R, Rushton S, O’Donnell A (2010) Actinobacterial community dynamics in long term managed grasslands. 19th World Congress of Soil Science, Brisbane
Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer NA (2009) Comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–445
Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ (2009) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43:3778–3783
Li T, Shi T, Li X, Zeng S, Yin L, Pu Y (2014) Effects of nano-MnO2 on dopaminergic neurons and the spatial learning capability of rats. Int J Environ Res Public Health 11:7918–7930
Lohse M, Bolger AM, Nagel A, Fernie AR, Lunn JE, Stitt M, Usadel B (2012) RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res 40:W622
López-Maury L, Florencio J, Reye JC (2003) Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 18:5363–5371
Lorenz N, Hintemann T, Kramarewa T, Katayama A, Yasuta T, Marschner P, Kandeler E (2006) Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol Biochem 38:1430–1437
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A 105:9931–9935
Magoc T, Salzberg S (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Mahmood T, Din SU, Naeem A, Mustafa S, Waseem M, Hamayun M (2012) Adsorption of arsenate from aqueous solution on binary mixed oxide of iron and silicon. Chem Eng J 192:90–98
McClintock TR, Chen Y, Bundschuh J, Oliver JT, Navoni J, Olmos V, Lepori EV, Ahsan H, Parvez F (2012) Arsenic exposure in Latin America: biomarkers, risk assessments and related health effects. Sci Total Environ 429:76–91
Mukherjee A, Sengupta MK, Hossain MA, Ahamed S, Das B, Nayak B, Lodh D, Rahman MM, Chakraborti D (2006) Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr 24:142–163
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36
Nacke H, Thürmer A, Wollherr A, Will C, Hodac L, Herold N, Schöning I, Schrumpf M, Daniel R (2011) Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS One 6:e17000
Nayak S, Prasanna R (2007) Soil pH and its role in cyanobacterial abundance and diversity in rice field soils. Appl Ecol Environ Res 5:103–113
Nocker A, Burr M, Camper AK (2007) Genotypic microbial community profiling: a critical technical review. Microb Ecol 54:276–289
Pandey S, Rai R, Rai LC (2012) Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J Proteome 75:921–937
Pandey S, Ghosh PK, Ghosh S, De TK, Maiti TK (2013) Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J Microbiol 51:11–17
Richaume A, Steinberg D, Jocteur-Monrozier L, Faurie G (1993) Differences between direct and indirect enumeration of soil bacteria: the influence of soil structure and cell location. Soil Biol Biochem 25:641–643
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 6:e27310
Sheik CS, Mitchell TW, Rizvi FZ, Rehman Y, Faisal M, Hasnain S, McInerney MJ, Krumholz LR (2012) Exposure of soil microbial communities to chromium and arsenic alters their diversity and structure. PLoS One 7:e40059
Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C (2012) Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect 120:1527–1531
Somenahally AC, Hollister EB, Loeppert RH, Yan W, Gentry TJ (2011) Microbial communities in rice rhizosphere altered by intermittent and continuous flooding in fields with long-term arsenic application. Soil Biol Biochem 43:1220–1228
Tan M, Li X, Xie H, Lu C (2005) Urban land expansion and arable land loss in China—a case study of Beijing–Tianjin–Hebei region. Land Use Policy 22:187–196
Thomas EC (2006) Bioavailability assessment of trace metal contaminants in urban soils and partitioning of zinc, cadmium, lead, nickel, and copper in the roots, shoots, foliage, and seeds of Chenopodium quinoa. Master Thesis, The University of British Columbia
Tripathi BM, Kim M, Singh D, Lee-Cruz L, Lai-Hoe A, Ainuddin AN, Go R, Rahim RA, Chun J, Husni MHA, Adams JM (2012) Tropical soil bacterial communities in Malaysia: pH dominates in the equatorial tropics too. Microb Ecol 64:474–484
Wang X, Chen X, Yang J, Wang Z, Sun G (2009) Effect of microbial mediated iron plaque reduction on arsenic mobility in paddy soil. J Environ Sci (China) 21:1562–1568
Wang LY, Sun XB, Liu JF, Gu JD, Mu BZ (2014) Comparison of bacterial community in aqueous and oil phases of the water-flooded petroleum reservoir using pyrosequencing and clone library approaches. Appl Microbiol Biotechnol 98:4209–4221
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Xuan DT, Guong VT, Rosling A, Alström S, Chai B, Högberg N (2012) Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol Fertil Soils 48:217–225
Yan Q, Bi Y, Deng Y, He Z, Wu L, Nostrand JDV, Shi Z, Li J, Wang X, Hu Z, Yu Y, Zhou J (2015) Impacts of the Three Gorges Dam on microbial structure and potential function. Sci Rep. doi:10.1038/srep08605
Yang Y, Quensen J, Mathieu J, Wang Q, Wang J, Li M, Tiedje JM, Alvarez PJ (2014) Pyrosequencing reveals higher impact of silver nanoparticles than Ag+ on the microbial community structure of activated sludge. Water Res 48:317–325
Yin XX, Chen J, Qin J, Sun GX, Rosen BP, Zhu YG (2011) Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol 156:1631–1638
Zhou S, Peng L, Lei M, Pan Y, Lan D (2015) Control of As soil to rice transfer (Oryza sativa L.) with nano-manganese dioxide. Acta Sci Circumst 35:855–861
Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, Meharg AA, Williams PN (2008) High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol 42:5008–5013
Acknowledgments
This work was supported by the Foundation of National Water Science and Technology Projects of China (2014ZX07206001-03), the Foundation of Furong Scholar Project of Hunan Province, and an honorary professorship.
Funding
This study was funded by National Water Science and Technology Projects of China (NO. 2014ZX07206001-03), Furong Scholar Project of Hunan Province, and an honorary professorship (J-D Gu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shao, J., He, Y., Zhang, H. et al. Silica fertilization and nano-MnO2 amendment on bacterial community composition in high arsenic paddy soils. Appl Microbiol Biotechnol 100, 2429–2437 (2016). https://doi.org/10.1007/s00253-015-7131-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7131-y