Abstract
The microbiological production of 2,3-butanediol (2,3-BDO) has attracted considerable attention as an alternative way to produce high-value chemicals from renewable sources. Among the number of 2,3-BDO-producing microorganisms, Klebsiella pneumoniae has been studied most extensively and is known to produce large quantity of 2,3-BDO from a range of substrates. On the other hand, the pathogenic characteristics of the bacteria have limited its industrial applications. In this study, two major virulence traits, outer core LPS and fimbriae, were removed through homologous recombination from 2,3-BDO-producing K. pneumoniae 2242 to expand its uses to the industrial scale. The K. pneumoniae 2242 ∆wabG mutant strain was found to have an impaired capsule, which significantly reduced its ability to bind to the mucous layer and evade the phagocytic activity of macrophage. The association with the human ileocecal epithelial cell, HCT-8, and the bladder epithelial cell, T-24, was also reduced dramatically in the K. pneumoniae 2242 ∆fimA mutant strain that was devoid of fimbriae. However, the growth rate and production yield for 2,3-BDO were unaffected. The K. pneumoniae strains developed in this study, which are devoid of the major virulence factors, have a high potential for the efficient and sustainable production of 2,3-BDO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution and the gradual exhaustion of natural resources have led scientists to look for renewable energy sources and alternative processes to produce useful chemicals from non-petroleum-based materials. The bulk chemical, 2,3-butanediol (2,3-BDO), is mostly derived from petroleum sources and can be converted to a number of useful derivatives, including butadiene, methyl ethyl ketone, and tetramethyl compounds, which have a wide range of applications in the chemical, food, cosmetic, and pharmaceutical industries (Perego et al. 2000; Xiu and Zeng 2008). The microbiological production of 2,3-BDO has attracted considerable attention in the chemical industry because it can provide an alternative way of producing these chemicals from renewable resources through an eco-friendly process (Celińska and Grajek 2009). Among the microorganisms that produce 2,3-BDO, Bacillus polymyxa (De Mas et al. 1988), Enterobacter aerogenes (Zeng et al. 1990), Klebsiella oxytoca (Afschar et al. 1993), and Klebsiella pneumoniae (Lee and Maddox 1986) have the potential for 2,3-BDO production on an industrial scale. The non-pathogenic Saccharomyses cerevisiae has also been reported to possess 2,3-BDO biosynthetic pathways which were further improved by in silico metabolic engineering (Nan et al. 2014; Ng et al. 2012). In particular, K. pneumoniae can grow quickly in simple media with a wide variety of sugars. Together with the capability of producing significant amounts of 2,3-BDO, K. pneumoniae is considered the best candidate 2,3-BDO producer in industry (Zeng and Sabra 2011).
K. pneumoniae is an opportunistic pathogen that causes up to 10 % of all nosocomial bacterial infections (Jarvis et al. 1985; Spencer 1996; Strettoti et al. 1984). K. pneumoniae infections can occur at almost any body site; however, the urinary and respiratory tracts are the most frequent infection sites. Klebsiella is second only to Escherichia coli as a cause of nosocomial bacteremia (Yinnon et al. 1996). In particular, a respiratory tract infection by K. pneumoniae has a mortality rate of up to 50 % (Fresno et al. 2006).
The pathogenic factors of Klebsiella include capsular polysaccharide (CPS), lipopolysaccharide (LPS), and pili (fimbriae). Klebsiella develops prominent capsules composed of an acidic polysaccharide complex, which covers the bacterial surface and protects itself from phagocytic action and as well as killing by bactericidal serum factors (Podschun and Ullmann 1998; Strettoti et al. 1984). The size of the capsule, as well as the rate of CPS production, has been shown to influence its virulence considerably (Cryz et al. 1984; Highsmith 1985). In addition, the capsule plays an important role in the adhesion to the mucous membrane of epithelial cells. LPS is the main component of the outer membrane of Klebsiella spp., consisting of lipid A, core oligosaccharide, and O-antigen. Lipid A and core oligosaccharide are essential for the bacteria to spread through the circulatory system and cause sepsis (Williams and Tomas 1990). O-antigen, also called O-polysaccharide or O-side chain, is a glycosyl-repeating unit linked to the core polysaccharide of LPS. Gram-negative bacteria can avoid the defense system of the host by modification of these basal O-repeat unit structures (Raetz and Whitfield 2002). The adhesive feature of Enterobacteriaceae to the host cell is also represented by the types of fimbriae, also known as pili, which are non-flagellar filaments up to 10 μm long, consisting of globular protein subunits. K. pneumoniae has two predominant fimbrial types. Type 1 fimbriae are the best-studied bacterial adhesins, which bind to the d-mannose-containing trisaccharides of the host glycoproteins (Firon et al. 1984). Type 1 fimbriae also play an important role in the pathogenesis of urinary tract infections (Fader et al. 1982; Gerlach et al. 1989; Maayan et al. 1985). Type 3 fimbriae are known to adhere to the epithelia of the respiratory tract, uroepithelial cells, and endothelial cells (Hornick et al. 1988; Tarkkanen et al. 1997).
K. pneumoniae KCTC 2242 has been reported to produce large quantities of 2,3-BDO (Rim et al. 2012). To remove the major virulence factors from this industrially useful 2,3-BDO producer, a mutant strain that is devoid of fimbriae and outer core LPS was constructed by knocking out the fimA and wabG genes, which encode major type 1 fimbrial subunit protein and glucosyltransferase, respectively. The wabG gene is involved in the synthesis of outer core LPS by attaching α-l-glycero-d-manno-heptopyranose II to the O-3 position of an α-d-galactopyranosyluronic acid. This study examined the virulence traits of the mutant strains, such as fimbriae, capsule, binding affinity to human epithelial cells and mucous layer, as well as their susceptibility to phagocytosis by human macrophage-like cells, in comparison to their parental strain. The influence of removing these virulence factors on 2,3-BDO production is also evaluated by a fermentation experiment.
Materials and methods
Bacterial strains, plasmids, and growth conditions
K. pneumoniae KCTC 2242 was obtained from the Korean Collection for Type Cultures (Daejeon, South Korea). K. pneumoniae KCTC 2242 ∆wabG mutant strain was constructed by Jung et al. (2013). The bacteria were grown in LB media (Difco, Lawrence, KS, USA) at 37 or 30 °C containing either chloramphenicol (20 μg/ml) or tetracycline (3 μg/ml). Homologous recombination was carried out using pRedET (Gene Bridge, Germany) and 707-FLPe (Gene Bridge, Germany) plasmids.
Cell lines and culture conditions
The human ileocecal epithelial cell line, HCT-8, and the human bladder epithelial cell line, T-24, were provided by Korean cell line bank (Seoul, Korea). The cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Welgene, Korea) supplemented with 10 % heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 mg/ml). The monocyte cell line, THP-1, was purchased from the American Type Culture collection (Manassas, VA, USA). The cells were grown in RPMI 1640 medium supplemented with 2 mM l-glutamine, 10 % FBS, penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37 °C, 5 % CO2. The differentiation of THP-1 into macrophage-like cells was induced by treating the cells with 100 nM phorbol myristate acetate (PMA) for 3 days (Gasser et al. 2003).
Construction of chloramphenicol-resistant cassette
The fimA gene of K. pneumoniae KCTC 2242 and K. pneumoniae KCTC 2242 ∆wabG was knocked out, as described earlier (Jung et al. 2013; Sawitzke et al. 2007). The target gene, fimA, was sequenced to design primer sets for homologous recombination. In this study, the chloramphenicol-resistant gene was used as a selection marker to screen the ∆fimA mutant. A two-step PCR procedure was carried out to obtain a PCR fragment formed from a chloramphenicol-resistant cassette flanked by 34 bp of the flippase recognition site (FRT, 5′-GAA GTT CCT ATT CTC TAG AAA GTA TAG GAA CTT C-3′) and 50 bp of the region complementary to the target sequence (Link et al. 1997). An 891-bp PCR fragment containing the chloramphenicol-resistant cassette flanked by the 34 bp flippase recognition site was generated using the primer pair FRT-Cm/Forward (5′-GAA GTT CCT ATT CTC TAG AAA GTA TAG GAA CTT CTG AGA CGT TGA TCG GCA CGT-3′) and FRT-Cm/Reverse (5′-GAA GTT CCT ATA CTT TCT AGA GAA TAG GAA CTT CAT TCA GGC GTA GCA CCA GGC-3′). The 20 nucleotides homologous to the extremities of the chloramphenicol gene are indicated in italics. FRT was introduced to the cassette to remove the chloramphenicol selection marker from the chromosome after disrupting the target gene fimA by transforming the cells with the FLP expression plasmid. The PCR products (FRT-flanked chloramphenicol resistant gene, FCF) were used as a template for the PCR reaction with the primer pair containing 50 bp homology arms at their 5′ extremity corresponding to both ends of the target gene, fimA. The sequences of the primer pairs are as follows: fimA/forward (5′-ATG AAA ATC AAA ACA CTG GCA ATG ATT GTT GTG TCA GCC CTG TCA CTG AGG AAG TTC CTA TTC TCT AGA A-3′) and fimA/reverse (5′-TTA CTC GTA CTG CAC TTT GAA CGT GGC ATC CGC GTT CGC TAT ACC AGC CGG AAG TTC CTA TAC TTT CTA G-3′). The homologous overhangs are shown in italics. The final PCR product (AFCFA) contained chloramphenicol-resistant cassette flanked by FRT and 50 bp arms homologous to both ends of the fimA gene.
Site-specific recombination
K. pneumoniae 2242 was transformed with the pRedET expressing gam, exo, and bet genes. K. pneumoniae 2242 was cultured in fresh LB medium supplemented with 0.7 mM of EDTA. The cells were harvested when the optical density of the culture reached 0.2–0.3 at 600 nm (OD600), followed by washing three times with deionized water containing 10 % glycerol. After mixing with 150 ng of the pRedET plasmid, the cells were electroporated with a 1.8-kV, 5-ms pulse. The electroporated cells were incubated at 30 °C with shaking for 1 h and then spread over the LB agar plate supplemented with tetracycline (3 μg/ml) followed by incubation overnight at 30 °C. The colonies were inoculated into fresh LB media and grown to an OD600 of 0.3 at 37 °C. l-Arabinose was added to the culture to a final concentration of 1 % and incubated with shaking at 37 °C for 1.5 h. The cells were harvested and washed with pre-chilled dH2O containing 10 % glycerol. Forty microliters of the cells resuspended in dH2O was mixed with 150 ng of AFCFA and electroporated with a 1.8-kV, 5-ms pulse. After incubating at 37 °C with shaking for 3 h, the electroporated cells were spread over a LB agar plate supplemented with chloramphenicol (20 μg/ml) and incubated overnight at 37 °C. The chloramphenicol-resistant colonies were selected and the fimA-deleted Klebsiella mutants were verified by colony PCR with the primer set: fimA-F (5′-ATG AAA ATC AAA ACA CTG GC-3′) and fimA-R (5′-TTA CTC GTA CTG CAC TTT GA-3′), which are complementary to the 20 bp region of each homologous arm. To remove the FRT-flanked chloramphenicol cassette from the chromosome of the mutant, the cells were transformed with the FLP expression plasmid (707-FLPe, Gene Bridges, Germany) by electroporation, as described above. The electroporated cells were grown in LB media at 30 °C with shaking for 1 h and then spread over a LB agar plate supplemented with tetracycline (3 μg/ml). After incubating overnight at 30 °C, a few colonies were chosen, inoculated into fresh 1 ml LB media, and incubated at 30 °C for 3 h, followed by additional incubation overnight at 37 °C. An aliquot of the culture was streaked on an antibiotic-free LB agar plate and incubated overnight at 37 °C. The removal of the FRT-flanked chloramphenicol cassette from the chromosome of the mutant was confirmed by colony PCR using the same primer set, fimA-F and fimA-R.
Atomic force microscopy
Overnight cultures of K. pneumoniae 2242 cells were harvested by centrifugation at 25 °C for 5 min followed by washing with distilled water. The cells were resuspended in distilled water to a final concentration of 108 CFU/ml. Five microliters of the resuspended cells was applied to a clean slide glass and dried at room temperature for imaging. The morphology of the cells and fimbriae was examined by atomic force microscopy (AFM, Park System XE-70) in non-contact mode.
Extraction and quantitation of the capsule polysaccharides
The capsular polysaccharides of K. pneumoniae 2242 were obtained using a slight modification of the method described elsewhere (Domenico et al. 1989). Briefly, 5000 μl of overnight bacterial cultures, corresponding to 109 CFU, was mixed with 100 μl of 1 % Zwittergent 3-14 detergent (Sigma-Aldrich, USA) in 100 mM citric acid pH 2.0 (Biosesang, Korea). After incubating the mixture at 50 °C for 30 min, the pellet was removed by centrifugation at 15,000 rpm for 5 min. Three hundred microliters of supernatant was transferred to a new tube, and absolute ethanol was added to a final concentration of 80 %. The mixture was precipitated at 4 °C for 1 h, followed by centrifugation at 4 °C, 15,000 rpm for 15 min. The pellet was dissolved in 200 μl of distilled water.
The capsule polysaccharides were quantified by determining the concentration of uronic acid in the samples, using the method described previously (Blumenkrantz and Asboe-Hansen 1973). Two hundred microliters of the CPS sample was mixed with 1200 μl of 0.0125 M tetraborate (Sigma-Aldrich, USA) in absolute H2SO4 (Daejung, Korea). The samples were heated in boiling water for 5 min. After cooling at room temperature, 20 μl of 0.15 % 3-hydroxydiphenol (Sigma-Aldrich, USA) and 0.5 % NaOH were added and mixed thoroughly. The absorbance measurements were taken at 520 nm. Serially diluted solutions of d-mannuronic acid (A Johnson Matthey, Korea) were used for the standard curve.
Epithelial cell adherence assay
The cell adhesion assay was carried out using the method reported previously ( Favre-Bonté et al. 1999; Sahly et al. 2000). The ileocecal epithelial cell line, HCT-8, and bladder epithelial cell line, T-24, were seeded at 104 cells/well in a 96-well tissue culture plate and grown with RPMI 1640 media (Welgene, Korea) supplemented with 10 % heat-inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37 °C and 5 % CO2 for 24 h. Resuspended 3 × 105 bacterial cells in RPMI 1640 media were introduced to each well and incubated at 37 °C for 3 h. The unbound bacterial cells were removed by washing the well three times with DPBS (Welgene, Korea). The cells were treated with 250 μl of 0.05 % Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) in PBS containing 20 % trypsin for cell lysis and releasing the bound K. pneumoniae to the media. The lysed samples were diluted serially and spread over a LB agar plate for colony counting. All experiments were carried out in triplicate.
Mucin binding assay
Mucin (Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved in an acetate buffer (pH 5.0) to a final concentration of 100 μg/ml. The surface of the 96-well plate was coated with mucin by filling each well with 100 μl of a mucin solution followed by incubation at 37 °C for 24 h (Ryan et al. 2001). The unbound mucin was removed by washing the wells three times with PBS (pH 7.0) (Vishwanath and Ramphal 1984). Overnight cultures of K. pneumoniae were harvested by centrifugation, washed three times with PBS, and resuspended in 0.1 M phosphate buffer at pH 8.5. Carboxyfluorescein diacetate (CFDA, Dojindo Molecular Technologies, USA) was added to a final concentration of 2 μg/ml, and the suspension was stirred at 37 °C in the dark for 30 min. The labeled bacteria were washed three times with PBS to remove the unconjugated fluorophore and resuspended in PBS. Cells 5 × 106 in PBS were introduced to each well and incubated at 37 °C for 1 h for adhesion. The wells were washed five times with PBS, and the fluorescence from the cells bound to the mucin surface was measured using a fluorescence spectrophotometer (Tecan, Infinite 200, Grodig, Austria) with an excitation and emission wavelength of 485 and 535 nm, respectively. The adhesion test was performed three times.
Phagocytosis assay
The phagocytosis assay was carried out by flow cytometry, as described earlier (Kabha et al. 1995). The THP-1 cells were differentiated into macrophage-like cells by treating the cells with 100 nM phorbol myristate acetate (PMA) for 3 days (Gasser et al. 2003). The differentiated macrophage-like cells were harvested from the cell culture flask, washed with DPBS, and suspended in RPMI 1640 medium to a final concentration of 2 × 105 cells/ml. The cells were incubated at 37 °C for 30 min in the presence of 5 % CO2 with the CFDA-tagged K. pneumoniae 2242 wild type and its mutant counterpart to a final concentration of 2 × 106 CFU/ml. The ratio of macrophage-like cells to K. pneumoniae 2242 was set to 1:10. After incubation, the cells were washed three times with DPBS, resuspended in 200 μl PBS, and analyzed by flow cytometry (Guava easyCyte 8, EMD Millipore Co). Phagocytosis of K. pneumoniae to macrophage-like cells was determined by calculating the percentage of fluorescent macrophage-like cells from the entire macrophage-like cell population.
Fermentation of K. pneumoniae for 2,3-BDO production
The batch fermentation was carried out in a 2.5-l bioreactor (Kobiotech, Korea) in minimal medium (5 g/l yeast extract, 0.33 M glucose, 0.019 M K2HPO4, 0.083 M KH2PO4, 0.049 M (NH4)2HPO4, 0.049 M (NH4)2SO4, 1.01 mM MgSO4 · 7H2O, 0.9 mM FeSO4 · 7H2O, 3.5 μM ZnSO4 · 7H2O, 5.07 μM MnSO4 · 7H2O, and 6.8 μM CaCl2 · 2H2O) supplemented with 30 g/l glucose at 37 °C for 72 h. The pH of the minimal medium was set to 6.5 over the course of fermentation. The samples were withdrawn periodically and centrifuged at 5000 g for 10 min. The amount of meso-2,3-BDO, carbon source, and organic acids in the supernatants were analyzed by HPLC (Younglin, Korea) with an RI2414 detector (Waters Co, USA) and an Aminex HPX-87H organic acids column (300 mm × 7.8 mm; Bio-Rad). Sulfuric acid (0.01 M) was used as the mobile phase at 60 °C at a flow rate of 0.6 ml/min. All solutions were filtered through a 0.2-μm membrane prior to use.
Results
Construction of K. pneumoniae ∆fimA and ∆wabG/∆fimA mutants
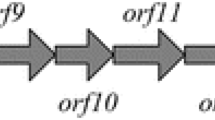
K. pneumoniae 2242 expresses type 1 fimbriae which are encoded on a gene cluster containing all the genes necessary for the fimbriae structure and assembly (Fig. 1). The expression of the major fimbrial subunit FimA protein in type 1 was regulated by the on-off switch of the promoter (Di Martino et al. 2003). Here, fimA gene, which encodes the major fimbrial subunit FimA protein in K. pneumoniae 2242, was knocked out. The resulting K. pneumoniae 2242 ∆fimA mutants were devoid of fimbriae, which are otherwise abundant in the wild-type strains. The red disruption system with chloramphenicol-resistant cassette flanked by 34-bp recognition site (FRT), together with 50-bp homologous regions complementary to the both ends of target gene was used, as shown in Fig. 2. After the transformation of K. pneumoniae 2242 with the chloramphenicol-resistant (Cm) cassettes, the Cm colonies were isolated. The PCR test using locus-specific primers showed that the middle region of the fimA gene of the isolate had been replaced with the FRT-flanked Cm cassette, which was reflected by the PCR product of 991 bp (Fig. 2). Upon elimination of the Cm cassette by transforming the host cell with the FLP expression plasmid, the resulting mutants gave the expected size of the PCR products (134 bp) in PCR analysis with the same locus-specific primers. The fimA gene of the K. pneumoniae 2242 ∆wabG mutant was also knocked out the same way as described above. These results together with the DNA sequencing data confirmed that the fimA gene of K. pneumoniae 2242 and K. pneumoniae 2242 ∆wabG had been successfully knocked out.
Construction of K. pneumoniae KCTC 2242 ∆fimA mutant strain. a Diagram showing the generation of fimA/FRT-flanked chloramphenicol-resistant (Cm) cassette by PCR and subsequent homologous recombination using the cassette. The Cm cassette was removed by FLP helper plasmid. fimA-U and fimA-D refer to the homology extensions complementary to both extremities of fimA gene. b Agarose gel electrophoresis of PCR products amplified from the genomic DNA of K. pneumoniae 2242 and K. pneumoniae 2242 ∆wabG mutant before and after recombination, using a primer set that is complementary to both ends of fimA gene. The size of wild-type fimA gene is 549 bp (lane 1) whereas those after recombination and removal of Cm cassette were 991 bp (lane 2 and lane 4) and 134 bp (lanes 3 and 5), respectively
Characterization of fimbriae
The expression and morphological features of the fimbriae at K. pneumoniae 2242 and its mutant counterpart strains were examined by AFM (Fig. 3). The tapping mode AFM images showed that the rigid fimbriae radiated peritrichously from the surface of the K. pneumoniae 2242 wild type and ∆wabG mutant strain. In contrast, the K. pneumoniae 2242 ∆fimA mutant strain was devoid of fimbriae, which shows that the fimA gene encoding the main structural protein of fimbriae had been knocked out successfully. The absence of fimbriae in the K. pneumoniae 2242 ∆wabG/∆fimA double mutant strain was also observed. Interestingly, the expression level of fimbriae in the K. pneumoniae 2242 ∆wabG mutant strain was higher than that of its wild-type counterpart strain. The capsule and fimbriae are characteristic features of K. pneumoniae and are also involved in the pathogenicity and survival of the bacteria in the host tissues. Several studies have reported the inverse relationship between the capsule and fimbriae expression in K. pneumoniae (Matatov et al. 1999; Sahly et al. 2000; Schembri et al. 2005). Considering these reports, the relatively lower level of fimbriae expression in the wild-type strain compared to its ∆wabG mutant counterpart may be caused by the presence of a thick capsule layer on the surface of the bacteria.
Interactions with epithelial cells
The ability of wild-type K. pneumoniae 2242 and its mutant counterpart strains to interact with the human ileocecal epithelial cell line, HCT-8, and the bladder epithelial cell line, T-24, was examined because the bacterial association with the host cells is an important process in bacterial infections. As shown in Fig. 4, ∆wabG mutant strains revealed significantly increased association to both epithelial cell lines compared to the parent strain. This is in agreement with several studies showing that the presence of a capsule in K. pneumoniae reduces its adhesion ability to epithelial cells (Sahly et al. 2000). The masking of adhesion molecules on the bacterial surface by a capsule has been reported to be responsible for the reduced adhesion to epithelial cells. The expression of the capsule was also found to downregulate CF29K adhesion at the transcriptional level in K. pneumoniae ( Favre-Bonte et al. 1999). This study examined the role of fimbriae on the adhesion ability of K. pneumoniae 2242 to epithelial cells in vitro. Both ∆fimA and ∆wabG/∆fimA mutants showed a more than fourfold and fivefold decrease in the association with the human ileocecal epithelial cell line, HCT-8. Their adhesion ability with the human bladder epithelial cell line, T-24, also decreased approximately twofold and fourfold, respectively, compared to their parent strain. These results suggest that the ∆wabG mutant lacking an outer core LPS and capsule has increased the adhesion to epithelial cells, which is in good agreement with previous studies (Struve and Krogfelt 2003). On the other hand, type 1 fimbriae are clearly related to the adhesion ability of K. pneumoniae 2242 to cultured epithelial cells because the adhesion of the ∆fimA mutant to epithelial cells was diminished greatly.
Interaction with mucin surface
A previous study reported that the ability of K. pneumoniae to adhere to cultured epithelial cells is not always associated with the infectivity or virulence of the bacteria because an in vivo study using a mouse model showed no correlation with an in vitro study (Shi et al. 2000). Therefore, this study investigated the adhesion or colonization ability of bacteria onto the surface of the mucous layer. As a major component of mucous, mucin is a family of glycoprotein that covers the luminal surfaces of the epithelial organs and serves as physical barrier between the extracellular milieu and plasma membrane (Shi et al. 2000). The association of bacteria with the mucous layer on epithelial tissues increases the resistance to mucocilliary clearance and facilitates the infection process. To test the adhesion ability of bacteria onto the artificial mucous layer, a 96-well plate coated with mucin was prepared. Each well was filled with the same number (5 × 106) of CFDA-labeled K. pneumoniae 2242. After 1 h incubation, the wells were washed thoroughly with PBS and the fluorescence intensity from each well was measured to determine the number of bacteria adhered to the mucin surface. The results showed that the binding ability the K. pneumoniae 2242 ∆wabG mutant strain to the mucin surface was significantly lower than its parental strain (Fig. 5). The ∆fimA mutants showed slightly lower binding ability compared to the wild type but the degree of the reduction was not significant. According to the results, the capsule of K. pneumoniae 2242 appears to exert more pronounced effect on binding to the mucin surface and resembles the mucous layer of epithelial tissues. Favre-Bonté et al. examined the role of K. pneumoniae capsular polysaccharides during the colonization of the mouse intestine ( Favre-Bonté et al. 1999). They reported that the colonization ability of the bacteria decreased significantly in the isogenic capsule-defective mutant, which is in good agreement with the present results. Most epithelial tissues, such as the large intestine or bladder, are covered with a mucous layer. Therefore, the binding characteristics of K. pneumoniae to the mucous layer are more relevant to measuring the virulence or pathogenicity of the bacteria.
Roles of capsule and fimbriae in phagocytosis by macrophage-like cell
To examine the role of the capsule and fimbriae in bacterial phagocytosis by macrophage-like cell, the degree of phagocytosis of ∆wabG and ∆fimA mutants by differentiated THP-1 cells (dTHP-1) was compared with that of their parental strain. THP-1 is a human monocytic leukemia cell line that can develop macrophage functions after the addition of stimulators. The dTHP-1 behaves more like native monocyte-derived macrophages (Auwerx 1991). Thus, the susceptibility of K. pneumoniae 2242 and its isogenic mutants, ∆wabG and ∆fimA, to one of the human innate defense systems can be assessed using dTHP-1. The dTHP-1 cells were incubated with CFDA-labeled K. pneumoniae wild type and its isogenic mutant strains at a ratio of 1:10 for 30 min. As expected, the ∆wabG strain, which lacks outer core LPS with impaired capsule formation, showed higher phagocytosis by dTHP-1 (Fig. 6). After 30 min incubation, the ratio of the dTHP-1 containing ∆wabG mutant to the total dTHP-1 was 93.5 %, which is much higher than that containing its parental strain (47.2 %). The presence of a capsule is likely to influence the susceptibility of bacteria to phagocytosis by macrophage-like cells. The bacteria internalized within dTHP-1 were imaged by fluorescence microscopy (Fig. 6c). An aliquot of the dTHP-1 cells incubated with the CFDA-labeled K. pneumoniae 2242 wild type and its isogenic mutant strains was taken before flow cytometry analysis and investigated by fluorescence microscopy. The number of wild-type bacteria in each dTHP-1 ranged from 0 to 5, whereas that of the ∆wabG mutant exceeded 10. Fluorescence microscopy also showed that the rate of internalization or phagocytosis of the ∆wabG mutant, whose outer core LPS is missing with impaired CPS formation, by dTHP-1 was much higher than that of its parental strain. The fimbriae, however, did not appear to influence the susceptibility or resistance of the bacteria to macrophage-like cells. The rate of internalization of the ∆fimA mutant by the dTHP-1 cells was similar to that of its parental strain. The K. pneumoniae 2242 ∆wabG/∆fimA double mutant presented similar characteristics to the ∆wabG mutant in terms of its susceptibility against macrophage-like cells. The results suggested that the outer core LPS or the amount of CPS is important for the bacteria to exert a resistance to macrophage-like cells, whereas the fimbriae have little or no role.
The susceptibility of K. pneumoniae 2242 and its isogeneic mutant strains to the phagocytic activity of dTHP-1 cells. Following coculture of dTHP-1 cells and bacteria, phagocytosis was assessed by flow cytometric methods. dTHP-1 cells were gated by FSC vs SSC scatter plot (a), and phagocytized bacteria were quantified in FL1 channel (b). The dTHP-1 cells after coculture with the bacteria were imaged by fluorescence microscopy (c)
Growth and 2,3-BDO production
Batch cultures were performed with an initial glucose concentration of 30 g/l. The production of 2,3-BDO together with lactate, acetate, and ethanol from a culture of the wild-type, ∆wabG, ∆fimA, and ∆wabG/∆fimA double mutant strains was monitored. The growth of these four strains was monitored by measuring the optical density of the culture at 600 nm (OD600) over the course of 48 h fermentation. As shown in Fig. 7a, the growth rate of the K. pneumoniae 2242 wild type and its isogenic mutants in the batch culture was similar. The OD600 of the cultures reached 6 ∼ 8 in 12 h and remained constant over the course fermentation. The overall production of 2,3-BDO for 48 h fermentation from the wild type, the ∆wabG mutant, ∆fimA mutant, and the ∆wabG/∆fimA double mutant strains was 6.87, 6.51, 7.24, and 7.35 g/l, respectively. The production of 2,3-BDO was increased by approximately 5.38 and 6.98 % in the ∆fimA mutant and ∆wabG/∆fimA double mutant strains, respectively. The amount of lactate, acetate, and ethanol from the four tested strains was similar. The ∆wabG mutant strain showed a slightly increased level of acetate production along with decreasing 2,3-BDO at later stage. Other than that, the characteristics of K. pneumoniae 2242 in growth and 2,3-BDO production were maintained after knocking out the representative virulence factors, such as the outer core LPS and fimbriae.
Discussion
The two most prominent virulence factors associated with pathogenic K. pneumoniae are the fimbriae and capsule, which are responsible for the adherence to epithelia as well as evading the host defense system (Tarkkanen et al. 1992; Williams and Tomas 1990). In this study, strains devoid of fimbriae and outer core lipopolysaccharide were constructed by homologous recombination, and their roles in the virulence were investigated. AFM showed that the ∆fimA mutant completely lost its ability to express fimbriae, whereas its parental strain possessed a number of fimbriae expressed radially from the surface of the cell membrane (Fig. 3). Interestingly, the level fimbriae expression in the ∆wabG mutant was higher than that of its parental strain. The outer core LPS was reported to be essential for the formation of a capsule around the cell (Jung et al. 2013). As shown in Fig. 5, the amount of capsular polysaccharides in ∆wabG mutant is approximately a half that of its parental strain. The thick capsule layer in the wild-type strain may impede the full expression of fimbriae, whereas the expression of fimbriae is less inhibited in the ∆wabG mutant having a partially impaired form of capsule. This is in a good agreement with a previous study, which reported an inverse relationship between the capsule synthesis and type 1 fimbriae expression (Schembri et al. 2005). On the other hand, the amount of capsular polysaccharides in the ∆fimA mutant was similar to that of the wild-type strain.
The ability to associate with the host cells is another important characteristic of pathogenic bacteria. The binding characteristics of K. pneumoniae KCTC 2242 and its isogenic mutants to the human ileocecal epithelial cell line, HCT-8, and the bladder cell line, T-24, were tested (Fig. 4). The ability of the ∆wabG mutant strain to associate with the human epithelial cell lines was significantly higher than that of its parental counterpart (Fig. 4). Several researchers have also reported an increased association of the ∆wabG mutant strain having a partially impaired capsule toward human epithelial cells in vitro ( Favre-Bonté et al. 1999; Sahly et al. 2000; Struve and Krogfelt 2003). On the other hand, the association of the ∆fimA and ∆wabG/∆fimA mutants to the same cell lines decreased dramatically, suggesting that the fimbriae are responsible for the interaction between the bacteria and host cell in vitro. In addition, an infection takes place mostly in the epithelia covered with a thick layer of mucous. This is why an in vitro test sometimes produces inconsistent results when investigating the pathogen’s colonization ability in the host tissues.
Epithelial cells have a mucous layer to protect the gut, bladder, and respiratory tract as a barrier to prevent pneumococcal colonization (Nelson et al. 2007; Snyder and Walker 1987). The study using a mucous-producing cell line, HT-29-Rev MTX 10−6, showed that the capsule is required for the initial steps of colonization by binding to the mucous layer (Schild et al. 2005). As predicted, the binding ability of the ∆wabG mutant strains to the mucous layer was significantly lower than that of the wild type, whereas the binding ability of K. pneumoniae to the mucous layer was not affected significantly by the presence or absence of fimbriae (Fig. 5b). The binding ability of K. pneumoniae 2242 wild type and its isogenic mutants are closely related to the amount of capsular polysaccharides that each strain possesses (Fig. 5). In other words, the more capsular polysaccharides that the bacteria possess, the more the bacteria can associate with the mucous layer. These results show that the fimbriae are responsible for the direct association of the bacteria with epithelial cells, whereas the capsule is required for the bacteria to interact with the mucous layer as the first step of colonization and subsequent infection process.
K. pneumoniae has developed survival strategies to resist the host defense system, such as macrophage-mediated phagocytosis. Therefore, this study investigated the susceptibility of K. pneumoniae 2242 wild type and its isogenic mutant strains to macrophage-like cell dTHP-1. As shown in Fig. 6, the susceptibility of the ∆wabG mutants to the macrophage-like cell was approximately two times higher than that of its parental strain. On the other hand, the phagocytic rate of the ∆fimA mutant strain was similar to that of wild type. This suggests that the outer core LPS and capsular polysaccharide on the surface of the bacteria is the key factor that confers the bacteria the ability to resist the host immune system. The role of fimbriae on the resistance of the bacteria to phagocytic activity by macrophage-like cells is insignificant.
Cellular components, such as LPS, capsule, and fimbriae are sometimes associated with the cellular integrity and growth ability of the bacteria, particularly when it comes to industrial applications. Therefore, this study examined the growth and production ability of 2,3-BDO. The growth rate of the ∆wabG mutant strain is comparable to that of the wild type. Interestingly, the growth rate the ∆wabG/∆fimA double mutant was highest among the four tested strains. On the other hand, all the strains reached an optimal density of 6 ∼ 8 at 600 nm within 12 h and remained constant over the course of fermentation. The correlation between OD and cell mass was not notably changed after knocking out the genes that are responsible for the synthesis of outer core LPS and fimbriae, suggesting that the OD represent actual cell mass for the four strains (Fig. S1). The overall production of 2,3-BDO from the four strains was similar to one another. The yield of 2,3-BDO from the ∆wabG/∆fimA double mutant strain was slightly higher than that of its parental strain. The production patterns of the byproducts, such as lactate, acetate, and ethanol, are also relatively unaffected by knocking out the virulence-associated genes. The production of the outer core LPS, capsule, and fimbriae is essential for the bacteria to achieve its virulence but they also requires high-energy expenditure for K. pneumoniae. Therefore, the strains developed in this study will have high potential as an efficient 2,3-BDO producer on an industrial scale. In conclusion, two major virulence factors, outer core LPS and fimbriae, were removed from K. pneumoniae KCTC 2242, which has a high potential in the industrial production of 2,3-BDO. The ability to produce 2,3-BDO was unaffected by removing these pathogenic factors. This non-virulent K. pneumoniae 2242 strain is suitable for industrial applications and may contribute to the sustainable production of high-value chemicals from renewable resources.
References
Afschar A, Rossell CV, Jonas R, Chanto AQ, Schaller K (1993) Microbial production and downstream processing of 2, 3-butanediol. J Biotechnol 27(3):317–329
Auwerx J (1991) The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 47(1):22–31
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–9
Celińska E, Grajek W (2009) Biotechnological production of 2, 3-butanediol—current state and prospects. Biotechnol Adv 27(6):715–725
Cryz S, Pitt T, Fürer E, Germanier R (1984) Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun 44:508–513
De Mas C, Jansen NB, Tsao GT (1988) Production of optically active 2, 3‐butanediol by Bacillus polymyxa. Biotechnol Bioeng 31(4):366–377
Di Martino P, Cafferini N, Joly B, Darfeuille-Michaud A (2003) Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res Microbiol 154(1):9–16
Domenico P, Schwartz S, Cunha BA (1989) Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun 57(12):3778–3782
Fader RC, Duffy L, Davis CP, Kurosky A (1982) Purification and chemical characterization of type 1 pili isolated from Klebsiella pneumoniae. J Biol Chem 257(6):3301–3305
Favre-Bonté S, Licht TR, Forestier C, Krogfelt KA (1999) Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice. Infect Immun 67(11):6152–6156
Favre-Bonte S, Joly B, Forestier C (1999) Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect Immun 67(2):554–561
Firon N, Ofek I, Sharon N (1984) Carbohydrate-binding sites of the mannose-specific fimbrial lectins of Enterobacteria. Infect Immun 43(3):1088–1090
Fresno S, Jiménez N, Izquierdo L, Merino S, Corsaro MM, De Castro C, Parrilli M, Naldi T, Regué M, Tomás JM (2006) The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide. Microbiology 152(6):1807–1818
Gasser O, Hess C, Miot S, Deon C, Sanchez J-C (2003) Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res 285(2):243–257
Gerlach G-F, Clegg S, Allen BL (1989) Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol 171(3):1262–1270
Highsmith RC (1985) Floating and algal rafting as potential dispersal mechanisms in brooding invertebrates. Mar Ecol Prog Ser Oldendorf 25(2):169–179
Hornick D, Dayton C, Bedell G, Fick R (1988) Nontuberculous mycobacterial lung disease. Substantiation of a less aggressive approach. CHEST J 93(3):550–555
Jarvis WR, Munn VP, Highsmith AK, Culver DH, Hughes JM (1985) The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect Cont 6(02):68–74
Jung S-G, Jang J-H, Kim A-Y, Lim M-C, Kim B, Lee J, Kim Y-R (2013) Removal of pathogenic factors from 2, 3-butanediol-producing Klebsiella species by inactivating virulence-related wabg gene. Appl Microbiol Biotechnol 97(5):1997–2007
Kabha K, Nissimov L, Athamna A, Keisari Y, Parolis H, Parolis L, Grue RM, Schlepper-Schafer J, Ezekowitz A, Ohman DE (1995) Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immun 63(3):847–852
Lee H, Maddox I (1986) Continuous production of 2, 3-butanediol from whey permeate using Klebsiella pneumoniae immobilized in calcium alginate. Enz Microbial Technol 8(7):409–411
Link AJ, Phillips D, Church GM (1997) Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol 179(20):6228–6237
Maayan MC, Ofek I, Medalia O, Aronson M (1985) Population shift in mannose-specific fimbriated phase of Klebsiella pneumoniae during experimental urinary tract infection in mice. Infect Immun 49(3):785–789
Matatov R, Goldhar J, Skutelsky E, Sechter I, Perry R, Podschun R, Sahly H, Thankavel K, Abraham SN, Ofek I (1999) Inability of encapsulated Klebsiella pneumoniae to assemble functional type 1 fimbriae on their surface. FEMS Microbiol Lett 179(1):123–130
Nan H, Seo S-O, Oh EJ, Seo J-H, Cate JH, Jin Y-S (2014) 2, 3-Butanediol production from cellobiose by engineered Saccharomyces cerevisiae. Appl Microbiol Biotechnol 98(12):5757–5764
Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN (2007) Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun 75(1):83–90
Ng CY, M-y J, Lee J, Oh M-K (2012) Production of 2, 3-butanediol in Saccharomyces cerevisiae by in silico aided metabolic engineering. Microbial Cell Fact 11:68
Perego P, Converti A, Del Borghi A, Canepa P (2000) 2,3-Butanediol production by Enterobacter aerogenes: selection of the optimal conditions and application to food industry residues. Bioproc Eng 23(6):613–620
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11(4):589–603
Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Ann Rev Biochem 71:635
Rim KB, Park JH, Oh MK, Lee JW (2012) Enhanced 2, 3-butanediol production in recombinant Klebsiella pneumoniae via overexpression of synthesis-related genes. J Microbiol Biotechnol 22(9):1258–1263
Ryan PA, Pancholi V, Fischetti VA (2001) Group A Streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors. Infect Immun 69(12):7402–7412
Sahly H, Podschun R, Oelschlaeger TA, Greiwe M, Parolis H, Hasty D, Kekow J, Ullmann U, Ofek I, Sela S (2000) Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect Immun 68(12):6744–6749
Sawitzke JA, Thomason LC, Costantino N, Bubunenko M, Datta S, Court DL (2007) Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol 421:171–199
Schembri MA, Blom J, Krogfelt KA, Klemm P (2005) Capsule and fimbria interaction in Klebsiella pneumoniae. Infect Immun 73(8):4626–4633
Schild S, Lamprecht A-K, Fourestier C, Lauriano CM, Klose KE, Reidl J (2005) Characterizing lipopolysaccharide and core lipid A mutant O1 and O139 Vibrio cholerae strains for adherence properties on mucus-producing cell line HT29-Rev MTX and virulence in mice. Int J Med Microbiol 295(4):243–251
Shi L, Ardehali R, Caldwell KD, Valint P (2000) Mucin coating on polymeric material surfaces to suppress bacterial adhesion. Coll Surf B: Biointerfaces 17(4):229–239
Snyder J, Walker W (1987) Structure and function of intestinal mucin: developmental aspects. Int Arch Allergy Immunol 82(3–4):351–356
Spencer R (1996) Predominant pathogens found in the European prevalence of infection in intensive care study. Europ J Clin Microbiol Infect Dis 15(4):281–285
Strettoti CW, Ristuccia PA, Cunha BA (1984) Topics in clinical microbiology Klebsiella. Infect Cont 5(07):343–348
Struve C, Krogfelt KA (2003) Role of capsule in Klebsiella pneumoniae virulence: lack of correlation between in vitro and in vivo studies. FEMS Microbiol Lett 218(1):149–154
Tarkkanen A-M, Virkola R, Clegg S, Korhonen TK (1997) Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. Infect Immun 65(4):1546–1549
Tarkkanen A, Allen BL, Williams P, Kauppi M, Haahtela K, Siitonen A, Orskov I, Orskov F, Clegg S, Korhonen T (1992) Fimbriation, capsulation, and iron-scavenging systems of Klebsiella strains associated with human urinary tract infection. Infect Immun 60(3):1187–1192
Vishwanath S, Ramphal R (1984) Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun 45(1):197–202
Williams P, Tomas J (1990) The pathogenicity of Klebsiella pneumoniae. Rev Med Microbiol 1:196–204
Xiu Z-L, Zeng A-P (2008) Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl Microbiol Biotechnol 78(6):917–926
Yinnon A, Butnaru A, Raveh D, Jerassy Z, Rudensky B (1996) Klebsiella bacteraemia: community versus nosocomial infection. QJM 89(12):933–942
Zeng A-P, Biebl H, Deckwer W-D (1990) Effect of pH and acetic acid on growth and 2,3-butanediol production of Enterobacter aerogenes in continuous culture. Appl Microbiol Biotechnol 33(5):485–489
Zeng A-P, Sabra W (2011) Microbial production of diols as platform chemicals: recent progresses. Curr Opin Biotechnol 22(6):749–757
Funding
This study was funded by the R&D Program of MKE/KEIT (No. 10035578, development of 2,3-butanediol and derivative production technology for the C-Zero bio-platform industry).
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The correlation between OD and cell mass of K. pneumoniae 2242 and its isogenic mutant strains in LB culture at 37°C for 16 h (PDF 58 kb)
Rights and permissions
About this article
Cite this article
Huynh, D.T.N., Kim, AY., Seol, IH. et al. Inactivation of the virulence factors from 2,3-butanediol-producing Klebsiella pneumoniae . Appl Microbiol Biotechnol 99, 9427–9438 (2015). https://doi.org/10.1007/s00253-015-6861-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6861-1