Abstract
Klebsiella species are the most extensively studied among a number of 2,3-butanediol (2,3-BDO)-producing microorganisms. The ability to metabolize a wide variety of substrates together with the ease of cultivation made this microorganisms particularly promising for the application in industrial-scale production of 2,3-BDO. However, the pathogenic characteristics of encapsulated Klebsiella species are considered to be an obstacle hindering their industrial applications. Here, we removed the virulence factors from three 2,3-BDO-producing strains, Klebsiella pneumoniae KCTC 2242, Klebsiella oxytoca KCTC1686, and K. oxytoca ATCC 43863 through site-specific recombination technique. We generated deletion mutation in wabG gene encoding glucosyltransferase which plays a key role in the synthesis of outer core lipopolysaccharides (LPS) by attaching the first outer core residue d-GalAp to the O-3 position of the l,d-HeppII residue. The morphologies and adhesion properties against epithelial cells were investigated, and the results indicated that the wabG mutant strains were devoid of the outer core LPS and lost the ability to retain capsular structure. The time profile of growth and 2,3-BDO production from K. pneumoniae KCTC 2242 and K. pneumoniae KCTC 2242 ΔwabG were analyzed in batch culture with initial glucose concentration of 70 g/l. The growth was not affected by disrupting wabG gene, but the production of 2,3-BDO decreased from 31.27 to 22.44 g/l in mutant compared with that of parental strain. However, the productions of acetoin and lactate from wabG mutant strain were negligible, whereas that from parental strain reached to ~5 g/l.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing energy costs and environmental concerns have stimulated the development of possible alternative renewable material sources for fuel and chemical production. (Steen et al. 2010; Ji et al. 2011). Microbial production of 2,3-butanediol (2,3-BDO) is one example of producing useful chemical using biological process from renewable resources. It provides environmentally attractive ways to produce the compound, which is traditionally derived from petroleum sources. 2,3-BDO can be converted to a number of useful derivatives in chemical industry, including butadiene (synthetic rubber), methyl ethyl ketone (industrial solvent), and tetramethyl compound (a possible gasoline blending agent) (Winfield 1945; Stinson 1979; Emerson et al. 1982). In addition, 2,3-BDO has potential applications in the manufacture of printing inks, moistening and softening agents, plasticizer, cosmetics, foods, and pharmaceuticals (Garg and Jain 1995; Ji et al. 2011). Microbial 2,3-BDO production has recently attracted a great deal of attention worldwide as a renewable biomass is a promising route for the development of low carbon economy and sustainable future.
Among a number of microorganisms that produce 2,3-BDO, Klebsiella, Enterobacter, Bacillus, Pseudomonas, and Serratia have been considered as industrially important genera that produce significant amount (Long and Patrick 1963; Ji et al. 2011). Especially, Klebsiella pneumoniae and Klebsiella oxytoca have been unbeatable in the efficient production of 2,3-BDO. Klebsiella species are able to metabolize a wide variety of sugars and grows quickly in simple medium (Anderson and Wood 1962). However, the pathogenic characteristics of encapsulated Klebsiella species are considered to be an obstacle hindering the industrial applications of this microorganism in the production of 2,3-BDO.
Klebsiella is well known as a cause of community-acquired bacterial pneumonia (Carpenter 1990; Podschun and Ullmann 1998) and accounts for 6 to 17 % of all nosocomial urinary tract infection. A group of patients with neurophatic bladders or diabetes mellitus in particular have high risk upon Klebsiella infection (Lye et al. 1992; Bennette et al. 1995). A number of bacterial factors that contribute to the pathogenesis of the bacteria have been identified. A capsule that forms thick bundles of fibrillous structures covering the bacterial surface in massive layers is essential to the virulence of Klebsiella. It protects the bacteria from phagocytosis by polymorphonuclear granulocytes and bactericidal serum factors (Podschun and Ullmann 1998). As an outer component of the bacterial surface, lipopolysaccharide (LPS) is another important pathogenic determinant in K. pneumoniae-caused pneumonia and bacteremia (Hsieh et al. 2012). LPS comprises three parts, lipid A, core oligosaccharide (OS), and O-antigenic polysaccharide (O-PS). The O-antigen is the outermost component of LPS and consists of a polymer of oligosaccharide repeating units. O-antigen is responsible for the resistance of the bacteria to complement-mediated killing (Alberti et al. 1996). The outer core LPS of K. pneumoniae is also involved in the attachment of capsules to the surface (Izquierdo et al. 2003). Izquierdo et al. (2003) demonstrated that the mutation in genes involved in LPS synthesis converted pathogenic K. pneumoniae into an avirulent one when tested in various animal models.
In an effort to remove the virulence factors from 2,3-BDO-producing Klebsiella species, we constructed outer core LPS-truncated mutant strains from K. pneumoniae KCTC 2242, K. oxytoca KCTC1686 (ATCC8724) and K. oxytoca ATCC 43863 which have been reported to produce 2,3-BDO from various substrates (Jansen et al. 1983; Cho et al. 2012). Using homologous recombination technique, we generated deletion mutation in wabG gene encoding glucosyltransferase in Klebsiella species, which is involved in the attachment of α-l-glycero-d-manno-heptopyranose II (l,d-HeppII) to the O-3 position of an α-d-galactopyranosyluronic acid (α-d-GalAp). In this study, we examined the changes in cellular morphology and interaction characteristics with human epithelial cells in core LPS-truncated mutant strains in comparison to those of their parental strains. The time profiles of fermentation are also examined to determine 2,3-BDO productivity in core LPS-truncated Klebsiella species.
Materials and methods
Bacterial strains and plasmids
K. pneumoniae KCTC 2242 and K. oxytoca KCTC1686 were obtained from the Korean Collection for Type Cultures (Daejeon, Korea). K. oxytoca ATCC 43863 was obtained from the American Type Culture Collection (Manassas, VA, USA). Bacteria were grown routinely at 37 °C in Luria-Bertani (LB) broth or on LB agar plates containing the appropriate antibiotics at the following concentration: chloramphenicol 20 μg/ml and tetracycline 3 μg/ml. The pRedET (Gene Bridge, Germany) and 707-FLPe (Gene Bridge, Germany) plasmids were used for homologous recombination.
Cell lines and culture conditions
The human ileocecal epithelial cell line HCT-8 and the bladder epithelial cell line T-24 were purchased from Korean cell line bank (Seoul, Korea). The cells (1 × 104 cells/well) were grown with RPMI 1640 medium (Welgene, Korea) supplemented with 10 % heat-inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml). For cell adhesion assay, HCT-8 or T-24 cells were seeded at ~104 cells/well in a 96-well tissue culture plate and grown at 37 °C, 5 % CO2 for 24 h.
PCR synthesis of a chloramphenicol cassette with flaking homologous region of the Klebsiella wabG
The wabG gene of K. pneumoniae KCTC 2242, K. oxytoca KCTC1686, and K. oxytoca ATCC 43863 were sequenced to design precise primer sets for homologous recombination. In this study, we employed chloramphenicol-resistant gene as a selection marker to screen wabG mutant. In order to obtain a PCR fragment formed of a chloramphenicol-resistant cassette flanked by 34-bp of flippase recognition site (FRT, 5′-GAA GTT CCT ATT CTC TAG AAA GTA TAG GAA CTT C-3′) and 50-bp of region complementary to the target sequence, two-step PCR procedure was used. In the first step, an 891-bp PCR fragment containing chloramphenicol cassette flanked by 34-bp of flippase recognition site was generated using pKOV (Link et al. 1997) as a template with primer pair FRT-CMP/Forward (5′-GAA GTT CCT ATT CTC TAG AAA GTA TAG GAA CTT CTG AGA CGT TGA TCG GCA CGT-3′) and FRT-CMP/Reverse (5′-GAA GTT CCT ATA CTT TCT AGA GAA TAG GAA CTT CAT TCA GGC GTA GCA CCA GGC-3′). The 20 nucleotides homologous to the extremities of the chloramphenicol gene are indicated in italic. The PCR reaction was carried out with 95 °C for 5 min followed by 25 cycles at 95 °C for 30 s, 66 °C for 55 s, and 72 °C for 60 s. In this study, FRT was introduced to the cassette to remove the chloramphenicol selection marker from the chromosome after disrupting the target gene wabG by transforming the cells with FLP expression plasmid. The PCR products (FRT-flanked chloramphenicol-resistant gene, FCF) were ligated into T-vector (Enzynomics, Korea). In the second step, the FCF was used as a template for PCR reaction with the primer pair containing 50-bp homology arms at their 5′ extremity corresponding to the both ends of target gene wabG. The sequences of primer pairs are as follows: wabG/foward (5′-ATG AGT AAA TTC AGG CTG GCT CTG GTG CGG CAG AAG TAC CGC CCG GAC GGA ATT AAC CCT CAC TAA AGG G-3′) and wabG/reverse (5′-TTA ATT CAC CAG ATC CTG ATA AAG AGA AAG CAG CTG GGT TGA GAG TCG CTT AAT ACG ACT CAC TAT AGG G-3′) for K. pneumoniae 2242, and wabG-2/foward (5′-ATG AGT AAA TTC AGG CTG GCT CTG GTA CGC CAG AAG TAT CGC CCG GAC GGA ATT ACC CTC ACT AAG GG -3′) and wabG-2/reverse (5′-TTA TTT CAC CAA ATC CTG ATA GAG GGA GAG CAG TGC GCC GAG AGA CGT TTA ATA CGA CTC ACT ATA GGG-3′) for K. oxytoca 1686 and K. oxytoca 43863. The homology arms were indicated in italic. The final PCR product contains chloramphenicol-resistant cassette flanked by FRT and 50-bp arms homologous to both ends of wabG gene (WFCFW). The PCR reaction was carried out with 95 °C for 5 min followed by 25 cycles at 95 °C for 30 s, 65 °C for 60 s, and 72 °C for 60 s. Purifications of plasmid DNA and PCR products were performed with the Qiagen Plasmid Kit (Qiagen) and PCR purification Kit (Qiagen). All the PCR reactions were carried out with 0.6 U of Taq polymerase (Takara, Japan).
Site-specific recombination and removal of chloramphenicol selection marker from Klebsiella
At the first step, Klebsiella were transformed with pRedET expressing gam, exo, and bet genes. The Klebsiella were cultured in fresh LB medium supplemented with 0.7 mM of EDTA. EDTA was added to the culture to increase the electroporation efficiency (Fournet-Fayard et al. 1995). The cells were harvested when the cultures reached to an optical density at 600 nm (OD600) of 0.2 ~ 0.3 and washed with pre-chilled dH2O containing 10 % glycerol three times. Forty microliters of cells resuspended in dH2O was mixed with 150 ng of pRedET plasmids and transferred to a pre-chilled electro-cuvette. The mixture was electroporated with a 1.8-kV, 5-ms pulse. After incubating at 30 °C with shaking for an hour, the electroporated cells were spread on LB agar plate supplemented with tetracycline (3 μg/ml) and incubated at 30 °C overnight. The transformed Klebsiella was inoculated into fresh LB media and grown to an OD600 of 0.2 ~ 0.3 at 37 °C. Then l-arabinose was added to the culture to the final concentration of 0.5 % and incubated with shaking at 37 °C for an hour for the induction of Red/ET proteins. The cells were harvested and washed with pre-chilled dH2O containing 10 % glycerol. Forty microliters of cells resuspended in dH2O was mixed with 150 ng of WFCFW and transferred to a pre-chilled electro-cuvette. The mixture was electroporated as described above. After incubating at 37 °C with shaking for 3 h, the electroporated cells were spread on LB agar plate supplemented with chloramphenicol (20 μg/ml) and incubated at 37 °C overnight. Chloramphenicol-resistant colonies were selected and wabG-deleted Klebsiella mutants were confirmed by colony PCR with the primer set: wabG-1 (forward, 5′-ATG AGT AAA TTC AGG CTG G-3′ and reverse, 5′-TTA ATT CAC CAG ATC CTG AT-3′) for K. pneumoniae 2242, and wabG-2 (forward, 5′-ATG AGT AAA TTC AGG CTG GC-3′ and reverse, 5′-TTA TTT CAC CAA ATC CTG AT-3′) for K. oxytoca 1686 and K. oxytoca 43863. The sequences for these primer sets were complementary to the 20-base region of each homologous arm. To remove the FRT-flanked chloramphenicol cassette from the chromosome of Klebsiella, the cells were transformed with FLP expression plasmid (707-FLPe, Gene Bridges, Germany) by electroporation as described above. Electroporated cells were grown in LB media at 30 °C with shaking for an hour and then spread on LB agar plate supplemented with tetracycline (3 μg/ml). After incubating at 30 °C overnight, few colonies were picked, inoculated into fresh 1 mL LB media (antibiotic free), and incubated at 30 °C for 3 h. The cultures were then transferred to 37 °C incubator and grown for 16 h. An aliquot of the culture was streaked on antibiotic-free LB agar plate and incubated at 37 °C overnight. The removal of FRT-flanked chloramphenicol cassette from the chromosome of Klebsiella was confirmed by colony PCR with the same primer set, wabG-1 and wabG-2.
Capsule staining with crystal violet
Klebsiella cells were taken from 1-day-old LB plates and resuspended in 1 mL PBS. Ten microliters of the resuspended cells was mixed with equal volume of India ink (Winsor & Newton, UK) and evenly spread on slide glass to form uniform thin film using another slide glass. The samples were air-dried at room temperature for 15 min. Crystal violet was carefully added onto the film and removed after 1 min by rinsing with distilled water three times. The film was air-dried at room temperature, and images were taken with inverted bright field microscope (Nikon TE2000, Japan).
LPS isolation and electrophoresis
Cultures for LPS analysis were grown in LB broth at 37 °C overnight. LPS from wild-type and wabG mutant Klebsiella were extracted with LPS extraction kit (Intron Biotechnology, Korea) and separated by sodium dodecyl sulfate tricine polyacrylamide gel electrophoresis (SDS-tricine-PAGE). Briefly, extracted LPS was mixed with loading buffer (0.1 M Tris (pH 6.8), 2 % SDS, 20 % sucrose, 2-mercaptoethanol, and 0.001 % Bromophenol blue) and heated up to 100 °C in water bath for 5 min. The samples were loaded into 15 % SDS gel and run at 10 mA, 100 V for 2 h. The LPS was visualized by silver staining as described elsewhere (Tsai and Frasch 1982).
Electron microscopy analysis
The surface morphologies of wild-type and wabG mutant Klebsiella were examined with field emission scanning electron microscopy (FE-SEM, LEO Supra 55, Carl Zeiss, Germany). The bacterial specimens for FE-SEM analysis were prepared according to the work by Monson et al. (2010) with a slight modification. Briefly, an aliquot of culture solution was spread onto polycarbonate (0.2 μm) filter paper (Whatman, UK) and treated with primary fixing solution containing 75 mM lysine (Sigma Aldrich, USA), 2.5 % glutaraldehyde (Sigma Aldrich, USA), and 2 % paraformaldehyde (Deagung, Korea) in 0.1 M sodium cacodylate buffer (pH 7.4) at 4 °C for 2 h. The specimens were rinsed in 0.1 M sodium cacodylate buffer (Wako, Richmond, USA) three times at 4 °C for 10 min each and postfixed with secondary fixative containing 1.5 % potassium ferricyanide (Sigma Aldrich, USA) and 1 % osmium tetroxide (Wafa, USA) at 4 °C for 2 h. Specimens were dehydrated in a graded ethanol series (10, 30, 50, 60, 70, 80, 90, and 100 %) for 20 min each. The specimens were dried overnight at room temperature after rinsing twice with hexamethydisilizane (DaeJung, Korea). The dried samples were mounted on a carbon stub and sputter coated with platinum. Images were obtained using FE-SEM at 5 kV.
Cell adhesion assay
The adhesion ability of Klebsiella toward ileocecal epithelial cells and bladder epithelial cells were tested based on the method reported earlier (Farvre-Bonte et al. 1999a, b; Sahly 2000; Struve and Krogfelt 2003). For cell adhesion assay, ileocecal epithelial cell HCT-8 and bladder epithelial cell T-24 were seeded at ~104 cells/well in a 96-well tissue culture plate and grown with RPMI 1640 media (Welgene, Korea) supplemented with 10 % heat-inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37 °C, 5 % CO2 for 24 h. Wild-type and wabG mutant Klebsiella (3 × 105 bacteria) were resuspended in RPMI 1640 media. The media were supplemented with 2 % d-mannose to prevent fimbriae interaction with epithelial cells. The plates were incubated at 37 °C for 3 h. To remove unbound bacteria, the epithelial cells were washed with DPBS (Welgene, Korea) three times. The cells were treated with 20 % Trypsin (Welgene, Korea) and 0.5 % Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for cell lysis and releasing attached Klebsiella to the media. Lysed samples were serially diluted and spread onto LB agar plate with tetracycline (3 μg/ml) for colony counting. All experiments were carried out triplicate.
Fermentation of Klebsiella for 2,3-BDO production
The batch fermentation was conducted in a 5 L bioreactor (Biotron, Korea) in minimal medium (5 g/l yeast extract, 0.33 M glucose, 0.019 M K2HPO4, 0.083 M KH2PO4, 0.049 M (NH4)2HPO4, 0.049 M (NH4)SO4, 1.01 mM MgSO4·7H2O, 0.9 mM FeSO4·7H2O, 3.5 μM ZnSO4·7H2O, 5.07 μM MnSO4·7H2O, and 6.8 μM CaCl2·2H2O, pH 5.5) supplemented with 70 g/l glucose at 37 °C for 72 h. Samples were withdrawn periodically and centrifuged at 4,500 rpm for 10 min. The amount of carbon source, meso-2,3-BDO, and organic acids in the supernatants were measured by HPLC (Waters Co., USA) with an RI2414 detector (Waters Co., USA) and an Aminex HPX-87H organic acids column (300 mm × 7.8 mm; Bio-Rad). Sulfuric acid (0.01 M) was used as the mobile phase at 60 °C and with a flow rate of 0.6 mL/min. All solutions were filtered through a 0.2 μm membrane before use.
Results
Construction of Klebsiella wabG knockout mutant
Capsules and LPS O-antigens play important roles in the pathogenicity of Klebsiella (Williams and Tomas 1990). The capsular polysaccharides are important in resistance to phagocytosis and complement-mediated lysis of K. pneumoniae (Clements et al. 2008), whereas O-antigens of LPS have been implicated in resistance to complement-mediated lysis (Tomas et al. 1986; Merino et al. 1992). Here, we generated a deletion mutation in wabG gene of K. pneumoniae 2242, K. oxytoca 1686, and K. oxytoca 43863 by homologous recombination. wabG gene encodes for glucosyltransferase involved in the transfer of the first outer core LPS residue in Klebsiella species. The resulting Klebsiell spp. wabG mutants were expected to be devoid of the outer core LPS as well as the cell-attached capsular polysaccharides (Izquierdo et al. 2003). To construct the Klebsiella spp. wabG nonpolar mutants, Red disruption system was used (Datsenko and Wanner 2000). Chloramphenicol-resistant cassette flanked by 34-bp flippase recognition site (FRT) and 50-bp regions complementary to the target sequence were generated by using two pairs of primers that included homology extensions and priming sequences as shown in Fig. 1a. The PCR products were purified and then transformed into bacteria carrying the Red helper plasmid as described in experimental section. CmR colonies were isolated after transformation of three Klebsiella spp. with chloramphenicol-resistant cassettes that were generated independently. PCR test using locus-specific primers revealed that the middle region of wabG gene was successfully replaced with the FRT-flanked CmR cassette, which was reflected by the PCR products of 1 kb (Fig. 1b). Upon elimination of the CmR cassette by transforming the host cell with FLP expression plasmid, the resulting mutants gave the expected size of PCR products (0.1 kb) in a PCR analysis with the same locus specific primers (Fig. 1b). These results together with nucleotide sequencing data confirm that wabG gene of the three Klebsiella spp. was successfully knocked out.
A strategy for the disruption of wabG gene of Klebsiella species. a A diagram showing the generation of wabG/FRT-flanked chloramphenicol-resistant (CmR) cassette by PCR and subsequent homologous recombination using the resistance cassette. The chloramphenicol-resistant cassette was later removed by FLP helper plasmid. wabG-U and wabG-D refer to the homology extensions complementary to both extremities of wabG gene. b Agarose gel electrophoresis of PCR products from K. pneumoniae 2242, K. oxytoca 1686, and K. oxytoca 43863. wabG genes were amplified from the genomic DNA of the three Klebsiella strains before and after recombination using a primer set that is complementary to both ends of wabG gene. The size of wild-type wabG gene is 1.2 kb (1, 4, 7) whereas those after recombination and removal of CmR cassette were 1 kb (2, 5, 8) and 0.1 kb (3, 6, 9), respectively
Characterization of wabG mutant strains
LPS from K. pneumoniae 2242, K. pneumoniae 2242ΔwabG, K. oxytoca 1686, K. oxytoca 1686ΔwabG, K. oxytoca 43863, and K. oxytoca 43863ΔwabG were extracted and analyzed by SDS-tricine-PAGE. The LPS from the mutant strains migrated faster than that from the wild-type counterpart strains, suggesting that the wabG mutants contain a truncated LPS (Fig. 2, lane 2, 4, and 6). The chemical characterization of wabG mutants of other Klebsiella spp. using mass spectroscopy and matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectroscopy revealed that the LPS is devoid of the outer core region (Izquierdo et al. 2003). Nonmucoid colony morphology is another characteristic of wabG mutant strains. Here, we examined the surface morphologies of wild-type and mutant Klebsiella spp. using high magnification SEM. Wild-type strains were shown to have a thick layer of capsular polysaccharides around bacterial surface (Fig. 3). The boundaries of bacteria are not clear due to the presence of capsular polysaccharides covering the bacteria. On the other hand, the three mutant strains showed a drastic reduction in capsular polysaccharides. SEM analysis confirmed that LPS outer core and O-antigens of Klebsiella species are necessary for the encapsulation of bacteria with capsular polysaccharides. The non-capsulated phenotype of wabG mutant strains was also confirmed by light microscopy after negative staining with crystal violet (Fig. 4). The wild-type strains were shown to express a prominent capsule structure surrounding the bacterial cell, whereas the capsule structure is not evident in wabG mutants. The capsular structure is visualized as a white layer around cells by negative staining. The affinity between capsular polysaccharides and LPS outer core components is presumed to be responsible for the encapsulation. As a result, the LPS mutants are unable to retain cell-associated capsule. From the results, it is clear that, without intact LPS in mutant strains, encapsulation would be partially or completely inhibited even though all the genes related with the synthesis of capsule components remain intact. The inability of retaining capsular polysaccharide on the cell surface of wabG mutant strains could be explained by the lack of attachment site for capsule linkage. There is also a possibility of direct linkage of the capsule to LPS core. In this case, the alteration of outer membrane components in wabG mutant strains could preclude the attachment of capsular polysaccharide to the bacterial surfaces.
SDS-tricine-PAGE analysis of LPS from K. pneumoniae 2242 (lane 1), K. pneumoniae 2242ΔwabG (lane 2), K. oxytoca 1686 (lane 3), K. oxytoca 1686 1686ΔwabG (lane 4), K. oxytoca 43863 (lane 5), and K. oxytoca 1686 43863ΔwabG (lane 6). LPS from wabG mutant strains moved down faster than that from wild-type strains due to the absence of outer core part of LPS
FE-SEM analysis of the surface morphology of K. pneumoniae 2242 (a), K. pneumoniae 2242ΔwabG (b), K. oxytoca 1686 (c), K. oxytoca 1686ΔwabG (d), K. oxytoca 43863 (e), and K. oxytoca 43863ΔwabG (f). The surfaces of wild-type Klebsiella species were shown to be covered with a thick layer of capsular polysaccharide. On the other hand, wabG mutant strains were absent of such layer and thus showed distinctive cell to cell boundaries. Scale bar is1 μm
Visualization of the capsules expression in K. pneumoniae 2242 (a), K. pneumoniae 2242ΔwabG (b), K. oxytoca 1686 (c), K. oxytoca 1686ΔwabG (d), K. oxytoca 43863 (e), and K. oxytoca 43863ΔwabG (f). The capsules were shown in white layer around bacterial surface. The capsules were visualized by negative staining with crystal violet. wabG mutant strains were shown to be absent of such capsular layer. Scale bar is 10 μm
The wabG mutants also showed a characteristic colony morphology when examined in high magnification with optical microscopy (Fig. 5). K. pneumoniae is known to have large moist colonies due to the large mucoid polysaccharide capsule that protect it from phagocytosis. The colonies are circular and convex with an entire margin. But the colonies of wabG mutants are irregular with an undulate margin. The frizzy colony morphology of wabG mutant strains on solid media is a result of cell-to-cell aggregation and commonly found in biofilm-forming bacteria. There are various bacterial adhesins, protein structures that recognize a wide range of molecular motifs. While most adhesins provide targeting of the bacteria to a specific nonself tissue surface in the host, some mediate self-recognition (Klemm and Schembri 2000). Those self-recognizing adhesins confer bacterial cell-to-cell aggregation and enhance biofilm formation (Schembri et al. 2004). But the presence of capsule in wild-type strains would sterically shield the possible self-recognizing adhesins and abolish the close cell contact needed for intercellular interaction, resulting in a smooth and circular colony morphology.
Magnified colony edges of K. pneumoniae 2242 (a), K. pneumoniae 2242ΔwabG (b), K. oxytoca 1686 (c), K. oxytoca 1686ΔwabG (d), K. oxytoca 43863 (e), and K. oxytoca 43863ΔwabG (f) grown on LB agar plate. The colony of wild-type Klebsiella species shows smooth edge whereas that of capsule-defective Klebsiella species presents rugged and somewhat irregular edge. Scale bar is 60 μm. Inset shows the shape of whole colonies for each strain. Scale bar is 500 μm
Cell association properties
The ability of the bacteria to associate with host cells is an important process in bacterial infection. Association with epithelial cell allows resistance to mucocilliary clearance and thus facilitates colonization of bacteria (Pizarro-Cerda and Cossart 2006). We investigated the ability of wild-type and wabG mutant Klebsiella spp. to associate with human ileocecal epithelial cell HCT-8 and bladder epithelial cell T-24. As shown in Fig. 6, wabG mutant strains showed increased cell association to both cell lines compared with the parental strains. K. pneumoniae 2242ΔwabG and K. oxytoca 1686ΔwabG showed over 10-fold increased association with HCT-8 compared with their parental strains. K. pneumoniae 2242ΔwabG also showed over 10-fold increased adhesion to T24 compared with its parental wild-type strain, whereas K. oxytoca 1686ΔwabG and K. oxytoca 43863ΔwabG showed around 1.5–3-fold increased adhesion with the same cell line. These results show that the mutant strains lacking both outer core LPS and capsular polysaccharide have an increased ability of association with epithelial cell, which confirms previous findings that the capsule reduce bacterial adherence to epithelial cells in vitro (Farvre-Bonte et al. 1999a, b; Sahly et al. 2000; Struve and Krogfelt 2003). There are mainly two mechanisms proposed for the interference of capsule with bacterial adhesions. One is a masking effect in which adhesin molecules on the surface of bacteria interacting with epithelial cells are blocked by the thick capsule layer. The other may be the interference of capsule with the biosynthesis or assembly of type 1 fimbrial subunits into mature fimbriae on the bacterial surface (Matatov et al. 1999).
Association of wild-type and wabG mutant Klebsiella species to human epithelial cell lines. Ileocecal epithelial cell HCT-8 (a) and bladder epithelial cell T-24 (b) were exposed to the wild-type and wabG mutant Klebsiella species for 3 h. The number of Klebsiella cells that were adhered and internalized to the epithelial cells was recovered and measured by conventional colony counting method
Growth and 2,3-BDO production of wabG mutant strains
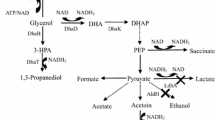
Batch cultures were performed with an initial concentration of 70 g/l glucose. The production of 2,3-BDO together with acetate, acetoin, ethanol, and lactate from the culture of wild-type and wabG mutant K. pneumoniae 2242 was monitored. The growth of K. pneumoniae 2242 and K. pneumoniae 2242ΔwabG was monitored by measuring optical density of the culture at 600 nm (OD600) over the course of 72-h fermentation. As shown in Fig. 7, the growth of K. pneumoniae 2242 in batch culture was not affected by wabG knockout. OD600 of both cultures reached to 6 ~ 7 in 12 h and remained constant over the course of 72-h fermentation. The dry cell weight of the two strains after 72-h batch culture were 5.84 and 5.98 g/l, respectively. These results indicate that the removal of outer core LPS has no distinctive effect on cell growth. wabG mutant strain rapidly consumed glucose at an early stage of fermentation, but the consumption did not occur after 24 h for the rest of fermentation period. On the other hand, the overall glucose consumption by wild-type strain was greater even though the consumption rate at an initial stage was lower compare with that by wabG mutant strain. The product spectrum of mutant strain changed regarding to the concentration of 2,3-BDO, acetate, acetoin, ethanol, and lactate. Overall production of 2,3-BDO was slightly lower in K. pneumoniae 2242ΔwabG compared with that of wild-type strain. The production of 2,3-BDO from wild-type and ΔwabG mutant strain was 31.27 and 22.44 g/l, respectively. The production of 2,3-BDO was decreased about 28.23 % by inactivating wabG gene. The production of acetoin and lactate from K. pneumoniae 2242ΔwabG was negligible, whereas those from wild-type strain reached to ~5 g/l. The gap in 2,3-BDO productivity might be overcome through various metabolic engineering tools.
Discussion
Among a number of 2,3-BDO-producing microorganisms, Klebsiella species have gained a great deal of attention due to their ability to produce large amount of 2,3-BDO from various substrates. In addition, the ease of cultivation made this microorganisms particularly promising for the application in industrial-scale production of 2,3-BDO. However, K. pneumoniae and K. oxytoca are Bio-safety Level 2 microorganisms which are responsible for nosocomial infections including pneumonia and urinary tract infection. Therefore, the pathogenic characteristics of Klebsiella species should be removed for safe applications of this microorganism in industry. In previous report, Izquierdo et al. demonstrated that the outer core LPS is essential for the virulence of K. pneumoniae. Mutation to the genes, such as waaC, waaF, or wabG, which are involved in LPS synthesis abolished the highly virulent characteristics of pathogenic K. pneumonia 52145 according to in vivo and animal model studies (Izquierdo et al. 2003). In this study, we generated deletion mutation in wabG gene of three 2,3-BDO producers, K. pneumoniae KCTC 2242, K. oxytoca KCTC 1686, and K. oxytoca ATCC 43863, using homologous recombination technique. wabG gene encodes a glucosyltransferase which is responsible for the transfer of first outer core LPS residue. The resulting wabG mutants lack the ability to synthesize the outer core LPS including O-antigen. The deletion mutation in wabG gene in three mutants was confirmed by PCR and sequencing analysis of the target gene. SDS-tricin-PAGE analysis of the LPS from the three wabG mutants together with their parental strains revealed that the outer core LPS of mutant strains were clearly truncated (Fig. 2). wabG mutant strains that are devoid of outer core LPS were also found to be deficient of capsular polysaccharides covering the bacterial surface which is obvious in their parental strains. The nonmucoid morphology is evident when the bacteria were examined with high magnification scanning electron microscopy as shown in Fig. 3. The absence of capsules in mutant strains was confirmed again by light microscopy after negative staining with crystal violet (Fig. 4). On the other hand, wild-type Klebsiella species were shown to express a prominent capsule structure surrounding the bacterial cell. These results suggest that there is a strong correlation between the presence of outer core LPS including O-antigen and the encapsulation of the bacteria. The loss of capsules in K. pneumoniae 52145 with truncated outer core LPS was also confirmed by its inability to react with capsular-specific serum as described elsewhere (Izquierdo et al. 2003). All of these data provide strong evidences that the alteration of outer membrane components in wabG mutants could preclude the capsular polysaccharide attachment.
Capsular polysaccharide and LPS have been shown to be essential for the virulence of K. pneumoniae-caused pneumonia and bacteremia. We investigated the ability of wabG mutant strains to associate with a human ileocecal epithelial cell line (HCT-8) and a human bladder epithelial cell line (T-24). As expected, the three unencapsulated wabG mutants showed increased cell association to both cell lines compared with their parental strains. The increased ability of association with epithelial cell lines in mutant strains lacking capsular polysaccharide agrees well with previous reports (Farvre-Bonte et al. 1999a, b; Sahly et al. 2000; Struve and Krogfelt 2003). However, in vivo tests using animal model showed opposite results according to the previous studies (Struve and Krogfelt 2003). The colonization ability of a capsule defective K. pneumoniae in the intestine and bladder was significantly attenuated compared with that of their capsulated parental strains suggesting that capsule plays a critical role in their virulence (Farvre-Bonte et al. 1999a, b; Struve and Krogfelt 2003). The study using mucus producing HT-29-Rev MTX 10−6 differentiated cells also revealed that the capsule defective K. pneumoniae mutant adheres less strongly than that the encapsulated parental strain. These results support the idea that the presence of mucus components on the cell surface would favor the establishment of interaction with encapsulated bacteria.
All the results in this study confirm the generation of three 2,3-BDO-producing Klebsiella species devoid of pathogenic factors such as an outer core LPS and capsular structure by deletion mutation in wabG gene through site-specific recombination. The growth of mutant strains was not affected by knocking out wabG gene. In batch culture, both wild-type and mutant strains reached to an optical density of 6–7 at 600 nm in 12 h and remained constant over the course of 72-h fermentation period (Fig. 7). Overall production of 2,3-BDO was slightly lower in K. pneumoniae 2242ΔwabG compared with that of parental strain. There is no direct evidence that glucosyltransferase associates with 2,3-BDO pathway. The decrease in 2,3-BDO productivity from wabG mutant could be caused by various reasons. The absence of outer core LPS may alter the membrane permeability in wabG mutant strain, which could have influence on its substrate utilization properties. 2,3-BDO productivity of wabG mutant strain could be further improved by optimization of culture condition and possible adaptation process. In addition, the production of acetoin and lactate from K. pneumoniae 2242ΔwabG was negligible whereas that from parental strain reached to ~5 g/l (Fig. 8). The lower production of byproducts such as acetoin and lactate would be advantageous since more substrates or intermediate molecules could be channeled for the production of 2,3-BDO. On the other hand, 2,3-BDO productivities of K. oxytoca 1686ΔwabG and K. oxytoca 43863ΔwabG were comparable with those of their parental strains when batch-cultured with initial glucose concentration of 25 g/l (Fig S-1). The production of capsule and LPS is essential for the pathogenicity but requires high-energy expenditure in Klebsiella species. Aside from the biosafety issues, the Klebsiella strains developed in this study which are devoid of capsule and outer core LPS have a high potential to be developed into efficient 2,3-BDO producers through sophisticated metabolic engineering tools in near future.
References
Alberti S, Alvarez D, Merino S, Casado MT, Vivanco F, Tomas JM, Benedi VJ (1996) Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect Immun 64:4726–4732
Anderson RL, Wood WA (1962) Pathway of l-xylose and l-lyxose degradation in Aerobacter aerogenes. J Biol Chem 287:296–303
Bennette CJ, Young MN, Darrington H (1995) Differences in urinary tract infection in male and femail spinal cord injury patients on intermittent catheterzation. Paraplegia 33:69–72
Carpenter JL (1990) Klebsiella pulmonary infections: occurence at one medical center and review. Rev Infect Dis 12:672–682
Cho J-H, Rathnasingh C, Song H, Chung B-W, Lee HJ, Seung D (2012) Fermentation and evaluation of Klebsiella pneumoniae and K. oxytoca on the production of 2,3-butanediol. Bioproc Biosyst Eng. doi:10.1007/s00449-012-0691-7
Clements A, Gaboriaud F, Duval JFL, Farn JL, Jenney AW, Lithgow T, Wijburg OLC, Hartland EL, Strugnell RA (2008) The major surface-associated sacharides of Klebsiella pneumoniae contribute to host cell association. PLoS One 3(11):e3817
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. PNAS 97(12):6640–6645
Emerson RR, Flickinger MC, Tsao GT (1982) Kinetics of dehydration of aqueous 2,3-butanediol to methyl ethyl ketone. Ind Eng Chem Prod Res Dev 21:473–477
Farvre-Bonte S, Lichr TR, Forestier C, Krogfelt KA (1999) Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice. Infect Immun 67:6152–6156
Favre-Bonte S, Joly B, Forestier C (1999) Consequences of reduction of Klebsiella pneumoniae capsule expression in interaction this bacterium with epithelial cells. Infect Immun 67(2):554–561
Fournet-Fayard S, Joly B, Forestier C (1995) Transformation of wild type Klebsiella pnenumoniae with plasmid DNA by clectroporation. J Microbiol Methods 24:49–54
Garg SK, Jain A (1995) Fermentative production of 2,3-butanediol: a review. Bioresour Technol 51:103–109
Hsieh PF, Lin TL, Yang FL, Wu MC, Pan YJ, Wu SH, Wang JT (2012) Lipopolysaccharide O1 antigen contributes to the virulence in Klebsiella pneumoniae causing pyogenic liver abscess. PLoS One 7:e33155
Izquierdo L, Coderch N, Pique N, Bedini E, Corsaro M, Merino S, Fresno S, Tomas JM, Regue M (2003) The Klebsiella pnenumoniae wabG gene: role in biosynthesis of the core lypopolysaccharide and virulence. J Bacteriol 185(24):7213–7221
Jansen NB, Flickinger MC, Tsao GT (1983) Production of 2,3-butanediol from d-Xylose by Klebsiella oxytoca ATCC 8724. Biotechnol Bioeng 26:362–369
Ji XJ, Huang H, Ouyang PK (2011) Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol Adv 29:351–364
Klemm P, Schembri MA (2000) Bacterial adhesins: function and structure. Int J Med Microbiol 290:27–35
Link AJ, Phillips D, Church GM (1997) Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol 179:6228–6237
Long SK, Patrick R (1963) The present status of the 2,3-butylene glycol fermentation. Adv Appl Microbiol 5:135–155
Lye WC, Chan RKT, Lee EJC, Kumarasinghe G (1992) Urinary tract infections in patients with diabetes mellitus. J Infect 24:169–174
Maatov R, Ofek I, Skutelsky E, Schechter I, Perry R, Posdchun R, Sahly H, Thankavel K, Abraham SN, Goldhar J (1999) Inability of encapsulated Klebeiella pneumoniae to assemble functional type 1 fimbriae on their surface. FEMS Microbiol Lett 179:123–130
Merino S, Camprubi S, Alerti S, Benedi VJ, Tomas JM (1992) Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun 60:2529–2535
Monson BK, Stringham J, Jones BB, Abdel-Aziz S, Cutler Peck CM, Olson RJ (2010) Scanning electron microscopy visualization of methicillin-resistant Staphylococcus aureus after contact with gatifloxacin with and without preservative. J Ocul Pharmacol Ther 26(2):133–136
Pizarro-Cerda J, Cossart P (2006) Bacterial adhesion and entry into host cells. Cell 124:715–727
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenecity factors. Clin Microbiol Rew 11(4):589–603
Sahly H, Podschun R, Oelschlarger TA, Greiwe M, Parolis H, Hasty D, Kekow J, Ullmann U, Ofek I, Sela S (2000) Capsule impedes adhesion to invasion of epithelial cells by Klebsiella pnenumoniae. Infect Immun 68(12):6744–6749
Schembri MA, Dalsgaard D, Klemm P (2004) Capsule shields the function of short bacterial adhesins. J Bacteriol 186(5):1249–1257
Steen EJ, Kang Y, Bokinsky G, Hu Z, Shirmer A, McClure A, del Cardayre SB, Deasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562
Stinson SC (1979) New plants set for octane booster. Chem Eng News 57(26):35–36
Struve C, Krogfelt KA (2003) Role of capsule in Klebsiell pneumoniae vitulence: lack of correlation between in vitro and in vivo studies. FEMS Microbiol Lett 218:149–154
Tomas JM, Benedi VJ, Ciurana B, Jofre J (1986) Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun 54:85–89
Tsai CM, Frasch CE (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119:115–119
Williams P, Tomas JM (1990) The pathogenicity of Klebsiella pneumoniae. Rev Med Microbiol 1:196–204
Winfield ME (1945) The catalytic dehydration of 2,3-butanediol to 1,3-butadien. J Council Sci Ind Res 18:412–423
Acknowledgments
This research was supported by the R&D Program of MKE/KEIT (No. 10035578, Development of 2,3-butanediol and derivative production technology for the C-Zero bio-platform industry).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sung-Geun Jung and Jun-Ho Jang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material
(DOCX 155 kb)
Rights and permissions
About this article
Cite this article
Jung, SG., Jang, JH., Kim, AY. et al. Removal of pathogenic factors from 2,3-butanediol-producing Klebsiella species by inactivating virulence-related wabG gene. Appl Microbiol Biotechnol 97, 1997–2007 (2013). https://doi.org/10.1007/s00253-012-4284-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4284-9