Abstract
Nitrification inhibitors (NIs) 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) have been used extensively to improve nitrogen fertilizer utilization in farmland. However, their comparative effects on ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) in agricultural soils are still unclear. Here, we compared the impacts of these two inhibitors on soil nitrification, AOA and AOB abundance as well as their community structure in a vegetable soil by using real-time PCR and terminal restriction fragment length polymorphism (T-RFLP). Our results showed that urea application significantly increased the net nitrification rates, but were significantly inhibited by both NIs, and the inhibitory effect of DMPP was significantly greater than that of DCD. AOB growth was more greatly inhibited by DMPP than by DCD, and the net nitrification rate was significantly related to AOB abundance, but not to AOA abundance. Application of urea and NIs to soil did not change the diversity of the AOA community, with the T-RFs remaining in proportions that were similar to control soils, while the community structure of AOB exhibited obvious shifts within all different treatments compared to the control. Phylogenetic analysis showed that all AOA sequences fell within group 1.1a and group 1.1b, and the AOB community consisted of Nitrosospira cluster 3, cluster 0, and unidentified species. These results suggest that DMPP exhibited a stronger inhibitory effect on nitrification than DCD by inhibiting AOB rather than AOA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrification is a crucial process in the nitrogen (N) cycle, involving the oxidization of ammonia to nitrate through nitrite, and results in significant agricultural and environmental consequences with respect to soil nitrogen available for plant uptake and nitrogen loss via nitrate leaching and greenhouse gas emissions, which has become more and more serious in recent years. Zhao et al. (2010) indicated that nitrate leaching loss from intensively irrigate vegetable production systems was 353.7 kg N ha−1, accounting for 21.7 % of the N applied (1,480 kg N ha−1). One of the technologies showing great prospect of reducing N loss in grassland and agricultural systems is the application of nitrification inhibitors (NIs) (Cui et al. 2011; Di and Cameron 2012).
Dicyandiamide (DCD) and 3,4-dimethylpyrazole phosphate (DMPP) are used extensively as NIs because of their highly effective inhibition of nitrification. DCD has been recommended for large scale adoption in Europe over the past decades, and has gained practical and commercial benefits in agricultural production. DCD is soluble in water, nonvolatile, and highly mobile. DMPP, marketed since 1999, is a heterocyclic N compound with the advantages of high effectiveness at low concentrations (0.5–1.5 kg DMPP ha−1, compared to 15–30 kg DCD ha−1) (Zerulla et al. 2001), low mobility, slow biodegradation and persistence, and thus it is more effective in inhibiting nitrification than DCD (Fangueiro et al. 2009; Wissemeier et al. 2001). The different effects of both inhibitors on nitrification are due to not only their distinct physicochemical properties but also the various modes of action (McCarty 1999). Previous studies suggest that DCD impairs the uptake or utilization of ammonia (Zacherl and Amberger 1990), or acts as a copper chelator of the enzymes involved in ammonia oxidation (Subbarao et al. 2006). DMPP is believed to act by binding indiscriminately to the complex of membrane proteins inclusively ammonia monooxygenase (AMO) (Benckiser et al. 2013) or acts as a readily available C and N substrate (Florio et al. 2014).
Generally speaking, both DMPP and DCD inhibit nitrification by interfering with AMO enzyme which catalyzes ammonia oxidation, the first and rate-limiting step of nitrification. Recent researches about amoA gene, the gene that encodes the first subunit of AMO enzyme, revealed that not only ammonia-oxidizing bacteria (AOB) but also ammonia-oxidizing archaea (AOA) play a major role in soil nitrification (He et al. 2007; Leininger et al. 2006). As AOA and AOB differ in their cellular biochemistry and physiology, these two ammonia-oxidizing microbes tend to occupy different niches in different agroecosystems in terms of sensitivity to soil pH, nitrogen status, and other soil conditions (Morimoto et al. 2011; Shen et al. 2008). Moreover, they might also be differently susceptible to different NIs. By comparing the impacts of varying NIs on nitrifying microbes, Shen et al. (2013) indicated that less effect of DCD was observed on AOA than on AOB, while nitrapyrin significantly inhibited the growth of AOA. Although DMPP is more effective in inhibiting nitrification than DCD, their comparative effects on the abundance of ammonia-oxidizing microbes have never been reported.
Furthermore, community shifts of ammonia oxidizers are closely related to soil nitrification (He et al. 2007; Zhang et al. 2012). However, the previous evidence for ammonia oxidation by AOA and AOB in soils has mostly based on amoA gene copies, and little information is available concerning the differential susceptibility of community diversities of AMO to NIs. O’Callaghan et al. (2010) reported that changes in AOB community in response to DCD application were evident, but community shifts in AOA were infrequent. Yang et al. (2013) indicated that DMPP decreased the diversity of the AOB community. Some studies revealed different responses of AOB community structure, not AOA, to NIs in different soils (Cui et al. 2013; Gong et al. 2013), while small shifts of both AOA and AOB active communities were detected following an application of cattle effluent plus DMPP (Florio et al. 2014).
The annual N fertilizer inputs and water irrigation are significantly higher in the greenhouse vegetable systems than in other cropping systems, and these intensive agricultural practices accompany with higher inner temperature increase nitrogen nitrification, and induce significant shifts of microbial community composition and activity (Lin et al. 2004; Shen et al. 2011; Sheng et al. 2013). Sheng et al. (2013) indicated that the relative abundances of Nitrosospira and Nitrospira genera of ammonia-oxidizing bacteria increased in long-term vegetable cultivation field with its nitrification potential being greater than in other land use patterns. Using NIs to reduce the nitrate leaching and N2O emissions in vegetable soils has been well documented (Cui et al. 2011; Liu et al. 2013; Xu et al. 2005). So far, there has been only one report showing that the application of DCD not only significantly inhibited nitrification but also decreased AOB abundance and altered AOB community (Liu et al. 2014a). Therefore, very limited information is available on the distinct effects of DCD and DMPP on ammonia oxidizers in intensive vegetable soils.
The objective of this study was to determine the effect of DMPP and DCD on nitrification and abundance of AOA and AOB, as well as AOA and AOB community structure in vegetable soil. For this, molecular approaches, including real-time PCR and terminal restriction fragment length polymorphism (T-RFLP) combined with clone library, were used.
Material and methods
Soils and incubation experiment
Soil sample was obtained from a greenhouse vegetable field to a depth of 15 cm, of the urban-rural transitional area (30°17′ N, 120°13′ E) of Hangzhou City, China. The soil, developed from shallow sea facies sediment parent material, is a typical coastal alluvial aggradations soil (Chen et al. 2008a). It is sandy loam (clay 6 %, silt 34 %, sand 60 %) with a pH of 6.9, organic matter (OM) content of 27.2 g kg−1, and total N content of 2.8 g kg−1. The field-moist soil was passed through a 3.5-mm sieve to remove the stones, crop residues, and roots. A portion of the soil was taken to determine the soil water-holding capacity, and the remainder was homogenized and preincubated for 1 week at 25 °C.
To test the effects of DCD and DMPP on nitrification, and on AOA and AOB growth, we set up four treatments with triplicates for each as follows: control, 200 mg kg−1 urea-N, 200 mg kg−1 urea-N + 2 mg kg−1 DMPP, and 200 mg kg−1 urea-N + 20 mg kg−1 DCD. For each treatment, 2,000 g of soil sample was placed in a vessel and thoroughly mixed with urea, DMPP, and DCD, which were dissolved in deionized water before being applied to the soil, then the soil moisture was adjusted to 60 % water-holding capacity (WHC). Soil samples (50 g each) were distributed into 100-mL plastic jars. A plastic lid with aeration holes was placed on each jar to maintain aerobic conditions. All these jars were then incubated inside the incubator at 25 °C for 45 days. Throughout the incubation period, the soil moisture content was adjusted to and maintained at 60 % WHC by monitoring the weight loss of each soil sample and adding deionized water as required.
The destructive sampling was performed in triplication at days 0, 1, 3, 7, 16, 31, and 45. About 5.0 g of fresh soil samples were collected from each replicated and immediately stored in a freezer at −80 °C for molecular analysis. The rest of the soil was homogenized for chemical analysis.
Chemical analysis
Soils samples were extracted with 2 mol l−1 KCl solution to determine soil nitrate N (NO3 −-N) and ammonium N (NH4 +-N) concentrations on a flow injection analyzer. Soil nitrate N and ammonium N on day 0 was determined using preincubated soils. The equation developed according to the description of Persson and Wirén (1995) was used to calculated net nitrification rate (n) as
where (NO3 −-N) t2 is the NO3 −-N concentration in the soil at time 2, (NO3 −-N) t1 is the NO3 −-N concentration in the soil at time 1, and t is the number of days between two sampling dates (t2 and t1, day).
DNA extraction and quantitative PCR of AOA and AOB amoA genes
DNA was extracted using the FastDNA SPIN kit for soil (Bio101, Vista, CA) according to the manufacturer’s instructions.
Real-time quantitative PCR of amoA genes was performed to estimate the abundances of AOA and AOB communities. The primers Arch-amoAF and Arch-amoAR were used for ammonia-oxidizing archaea (Francis et al. 2005); amoA-1F and amoA-2R were used for ammonia-oxidizing bacteria (Rotthauwe et al. 1997). The DNA extracts were tenfold diluted, and the reactions for each sample were performed on a Bio-Rad CFX1000 real-time PCR machine. The quantification of amoA genes was performed in a total volume of 20 μl consisting of 10 μl of SYBR Premix Ex TaqTM, plus 1 μl of each primer and 1.5 μl of template DNA. The fragments for the AOA and AOB were both amplified using an initial denaturation step at 95 °C for 3 min, followed by 40 cycles of 10 s at 95 °C, 30 s at 55 °C, and 40 s at 72 °C with plate reading. The PCR reaction runs had an efficiency of 94 and 99 % for the AOB and AOA, respectively. Standard curves were generated using serial dilutions of linearized plasmids containing cloned amoA genes (R 2 > 0.99 for both standard curves).
T-RFLP of amoA genes for AOA and AOB
T-RFLP analysis was performed on DNA extracted from soil samples of all four treatments at day 3, day 16, and day 45, according to the methods described by Singh et al. (2006) and Yao et al. (2011). Briefly, the archaeal and bacterial amoA gene fragments were PCR amplified using FAM-labeled primers CrenamoA23f and CrenamoA616r (Nicol et al. 2008), amoA-1F and amoA-2R (Rotthauwe et al. 1997), respectively. The AOA samples were digested with restriction enzyme HpyCH4V, and AOB PCR products were restricted with MspΙ. Fragment size was analyzed using an ABI PRISM 3030xl genetic analyzer (Applied Biosystems, Warrington, UK).
Construction of archaeal and bacterial amoA gene fragment libraries
Clone libraries of archaeal and bacterial amoA amplicons obtained from the control soil sample were constructed to analyze the AOA and AOB community structures. Clones were generated using a TOPO® TA Cloning kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. AOA and AOB clones were screened by PCR amplification, and a total of 27 AOA clones and 36 AOB clones were sequenced by ABI 3730xl DNA analyzer (Applied Biosystems®).
Data analysis
Least significant differences (LSD) test was performed to compare differences of AOA and AOB amoA gene copies among the treatments using SPSS 16.0 (SPSS Inc., USA). The community structures of AOA and AOB derived from T-RF relative abundance were analyzed by principle component analysis using SPSS 16.0 (SPSS Inc., USA). Phylogenetic analyses were conducted using MEGA version 4.0, and the neighbor-joining tree was constructed using Kimura 2-parameter distance with 1,000 replicates to produce bootstrap values. The sequences were aligned with BLAST similarity search program in order to find the most similar sequences from the GenBank database.
Accession numbers of nucleotide sequences
Nucleotide sequences of amoA genes for the clone libraries were deposited in the GenBank database with the accession numbers KC662513–KC662536.
Results
Dynamics of NH4 +-N and NO3 −-N concentrations
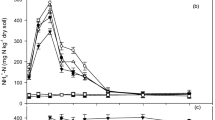
In the control soil, the NH4 +-N concentrations remained low and changed little over time. The urea treatment showed an immediate NH4 + release with the highest NH4 +-N concentration (193 ± 2 mg kg−1 soil) at day 1, and dramatically declined to 55 ± 12 mg kg−1 soil at day 3, and remained higher than in the control treatment until the end of the incubation. NH4 +-N concentrations of the urea + NIs (DCD and DMPP) treatments gradually decreased to about 27 ± 2 mg kg−1 with urea treatment at day 31, and NH4 +-N concentration of the urea + DMPP treatment was significantly greater than that of the urea + DCD treatment from day 3 to day 16 (P < 0.05) (Fig. 1).
The NO3 −-N concentration remained relatively stable at 107 ± 12 mg kg−1 in control soil, but changed in an inverse pattern to that of NH4 +-N concentration in other treatments (Fig. 1). For the urea treatment, the highest concentration was detected at day 7 and then remained at approximately 350 mg kg−1 soil. For the urea + NIs treatments, the NO3 −-N concentration increased with a delay of 24 days. Net nitrification rates for all soils attained the highest values during the time interval d 0–d 1, decreased subsequently and reached the lowest values towards the end of the incubation (Table 1). Urea application increased the net nitrification rate by 4.6 and 12.6 times compared with the control at the interval of d 0–d 1 and d 1–d 3, respectively. The net nitrification rates in urea + DMPP and urea + DCD treatments were 3.6 and 4.7 times higher than the control at the interval of d 1–d 3, respectively.
AOA and AOB abundance
AOA amoA gene copies were in the range of 2.9 to 3.6 × 108 copies g−1 dry soil, while the AOB type amoA gene were in the range of 3.3 to 15.0 × 107 copies g−1 dry soil, and ratios of AOA to AOB ranged from 2.5 to 9.7 (Fig. 2). The results showed that AOA population size was much greater than AOB in the vegetable soil.
Application of urea resulted in significant increase in AOA amoA gene copies compared to the control until day 3, and then had no significant difference with control. The AOA amoA gene copies did not change in urea + NIs treatments compared to the control, but it reached maximum at day 31 and then kept stable. The abundance of the AOA amoA gene from urea + DMPP and urea + DCD remained similar through the whole treatment.
The AOB population in control treatment did not change much over time and remained between 3.4 and 4.8 × 107 copies g−1 dry soil. Different from the AOA amoA gene, the AOB amoA gene copies reached up to threefold higher than control at day 3 in urea treatment, and kept decreasing to the end. In the urea + DMPP and urea + DCD treatments, the AOB amoA gene copies increased 66 and 126 % compared to the control at day 3, respectively, and then decreased according to their decrease of net nitrification rates. Moreover, there was a significant positive correlation between net nitrification rate and AOB abundance (p < 0.05), whereas no relationship was observed between net nitrification rate and AOA abundance (p > 0.05).
Community structure of AOA and AOB
A total of seven AOB T-RFs were obtained from soils, and AOB T-RFs 60 and 264 bp were dominant groups, accounting for about 27.5 and 44.9 % on average, respectively (Fig. 3a). At day 3, the relative abundance of T-RF 264 bp significantly increased after urea application, about 51.5 %, and had no significant difference with control at day 16 and day 45. The relative abundance of T-RF 264 bp did not show any differences between urea + DMPP and control treatments, whereas significant decrease of 264 bp was observed in urea + DCD treatment during incubation. PCA analysis of the AOB T-RFLP profiles showed that the two axes explained 68.1 % of the observed structure, and urea and NIs (DMPP and DCD) had significant effects on the soil AOB community composition (Fig. 3b). A significant separation was found between soils with and without nitrification inhibitors (DMPP and DCD) along PC1 at day 3, and the separation between samples with urea and without urea was significant along PC2.
T-RFLP analyses were used to determine the AOA community structure (Fig. 4). Digestion of AOA amoA sequences by HpyCH4V enzyme produced seven fragments in the T-RFLP profiles (Fig. 4a). In these treatments, a unique T-RF 82 bp was the most abundant. PCA analysis of the T-RFLP data showed that the first and second axes accounted for 78.5 % of the total variance (Fig. 4b). No significant differences were found in the composition of AOA T-RFs profiles at day 3. However, at day 16 and day 45, urea + NIs treatments were distinct from other treatments because of the increase of T-RF 52 bp and the decrease or disappearance of T-RFs 169, 208, and 268 bp.
Phylogeny of AOA and AOB
The AOA phylogenetic analysis indicated that a total of 27 AOA clones were classified into two different clusters, and there were 23 clones belonging to group 1.1b, and only 4 clones belonging to group 1.1a (Fig. 5). A unique T-RF 82 bp belonged to group 1.1a and 1.1b. T-RFs 169, 208, and 268 bp were closely aligned with group 1.1b, and T-RF 250 bp was affiliated with group 1.1a.
Neighbor-joining phylogenetic tree of archaeal amoA sequences retrieved from the vegetable soil. Sequences from this study are shown in bold and are described as clone name (accession number) T-RF size. Reference sequences are described as clone name (environment, accession number). Bootstrap values (>50 %) are indicated at branch points. The scale bar represents 5 % estimated sequence divergence
The AOB phylogenetic tree showed that the 19 clones with T-RFs 60, 156, and 235 bp belong to Nitrosospira cluster 3 and cluster 0, whereas the 17 clones with T-RFs 264 and 75 bp were closely related to unidentified clones (Fig. 6).
Neighbor-joining phylogenetic tree of bacterial amoA sequences retrieved from the vegetable soil. Sequences from this study are shown in bold and are described as clone name (accession number) T-RF size. Reference sequences are described as clone name (environment, accession number). Bootstrap values (>50 %) are indicated at branch points. The scale bar represents 5 % estimated sequence divergence
Discussion
Ammonium, produced by urea hydrolysis, is converted to nitrate via nitrite by nitrifying bacteria, leading to decline of the ammonium concentration in soil (Fig. 1). The presences of DMPP and DCD led to an inhibition of NO3 −-N concentration in vegetable soil, and gave a significant inhibition of 44 ± 0.7 and 37 ± 1.4 % at day 3, and the average of 38 ± 7.4 and 26 ± 10 % within 16 days, respectively, compared to urea treatment (Fig. 1). Moreover, the net nitrification rate in urea + DMPP and urea + DCD treatments was respectively about two and 1.5-fold lower than that in urea treatment at day 3, respectively (Table 1). These results demonstrated that DMPP, due to its persistence and stability, was more effective in inhibiting nitrification in our study although it was applied at a concentration ten times lower than that of DCD. There were no statistically significant differences of nitrate concentrations between urea and urea + NIs treatments after 31 days, which might be due to the decrease or disappearance of inhibitory effect. The persistence of DCD and DMPP in soil varies with environmental conditions, in particular temperature (Irigoyen et al. 2003; Kelliher et al. 2008). At the temperature above 30 °C, NIs seem to be less stabile. Irigoyen et al. (2003) concluded that the inhibitory effect of both DCD and DMPP prevailed for a month at 20 °C, and only for a week at 30 °C. DCD, as a source of N, can be metabolized by soil microbes, and the microbiological decomposition occurred over a temperature range of 10–30 °C (Rajbanshi et al. 1992) with the fast degradation rate at 25 °C (Hauser and Haselwandter 1990). DMPP is nonvolatile and low mobile in soil (Zerulla et al. 2001), and biological decomposition of DMPP might be the reason of its declining inhibitory effect (Florio et al. 2014). However, DMPP is more persistent to microbial degradation than DCD, and the degradation kinetics of DMPP and DCD showed that DCD decreased in soil more rapidly than DMPP (Weiske et al. 2001).
Ammonia oxidizers play important roles in the nitrification processes of most terrestrial ecosystems (Chen et al. 2008b; Di et al. 2009; Paranychianakis et al. 2013; Zheng et al. 2013). Populations of ammonia oxidizers significantly increased after the applications of urea, with approximately 1.2- and 3.1-fold increase in AOA and AOB amoA gene copies, respectively, relative to control soils (Fig. 2), showing greater response of AOB than that of AOA, which confirmed that archaea was at a disadvantage when competing with bacteria in ammonia-rich environment (Höfferle et al. 2010). Greater decrease of AOB amoA gene abundance was observed in the presence of DMPP than DCD (Fig. 2), indicating a coincidence of dynamics between AOB population and net nitrification rate. Previous studies have shown that DMPP and DCD were equally effective in inhibiting the AOB population growth and in inhibiting ammonia oxidation (Di and Cameron 2011; Gong et al. 2013, 2012). AOA amoA gene copies were significantly lower in urea + NIs soils than those in urea soil for about 3 days (Fig. 2), and the inhibitory effect of both DMPP and DCD was similar. Ammonia oxidation in archaea is believed to depend heavily on copper, because of an alternative electron transfer mechanism of AOA consisting of copper-containing proteins, but lacking cytochrome c proteins (Walker et al. 2010). However, DCD as a copper chelator did not have a stronger inhibitory effect on AOA than DMPP, and the inhibitory effects of Cu-chelators nitrapyrin and allyithiourea on archaeon Nitrosotalea devanaterra were contrasting (Lehtovirta-Morley et al. 2013). Moreover, archaea could be able to counteract the inhibiting effect of DMPP, but DMPP application slightly enlarged the bacterial cell bodies, altering ammonium into nitrite and nitrate conversions (Benckiser et al. 2013). Our observations further confirmed the results of DMPP revealing a stronger inhibition of AOB than AOA (Benckiser et al. 2013; Kleineidam et al. 2011), and Florio et al. (2014) indicated that DMPP exerted its greater inhibitory effect against soil bacterial than archaeal ammonia-oxidizing transcriptional activity. It was reported that bacterial might be more susceptible than its archaeal counterpart to different inhibitory substances due to their fundamental metabolic and cellular differences (Shen et al. 2013). Although the comparative effect of DMPP and DCD indicated that DMPP was more effective than DCD in inhibiting soil nitrification (Fangueiro et al. 2009; Wissemeier et al. 2001), the inhibitory effect on ammonia oxidizers was not estimated simultaneously. It is the first finding that the heterocyclic N compound DMPP caused a stronger inhibitory effect on AOB abundance as well as nitrification than DCD.
The AOB phylogenetic tree showed that all the bacterial amoA gene sequences in the vegetable soil fell within Nitrosospira lineage and unidentified species (Fig. 6). Shen et al. (2011) found that all sequences belonged to the genus Nitrosospira in the greenhouse vegetable soil, and Nitrosospira cluster 3 has been detected as the dominant AOB in the neutral pH agricultural soils after long-term N fertilization (He et al. 2007). Schauss et al. (2009) hypothesized that AOB may be more susceptible than AOA to inhibitory substances. Visible changes of AOB community composition after the application of nitrification inhibitors were found in our study (Fig. 3), and the relative abundance of T-RF 60 bp, belonging to Nitrosospira clusters 3a, 3b, and cluster 0, increased after NIs application, but the increase of the relative abundance of T-RF 264 bp, closely related to unidentified clones, after urea application was inhibited by NIs at day 3, reflecting the high versatility of AOB community, and the effects of DCD on T-RFs 60 and 264 bp were greater than those of DMPP. Shen et al. (2013) found a greater inhibitory effect of DCD on Nitrosospira multiformis compared with nitrapyrin, suggesting various effects of diverse NIs on AOB community. Moreover, PCA analysis showed that urea + NIs treatments separated from the urea along PC1, AOB community in urea + DMPP treated soil was significantly different from urea + DCD treatment, mainly due to various shifts in AOB community structure following application of different nitrification inhibitors. By day 16, the proportions of various T-RFs in urea + DMPP treatment appeared to be returning to levels similar to control treatment, the great AOB community diversities between urea + DMPP and urea + DCD were maintained. However, the different responses of AOB community to DCD and DMPP were not observed in Hap-Ustic Luvisol (Gong et al. 2013). O’Callaghan et al. (2010) indicated the changes in AOB community in response to only DCD application, but not to urine + DCD until day 56. It was reported that the shift of AOB community composition after the application of nitrification inhibitors was related to the spatial pattern of NH4 +-N (Yang et al. 2013), or soil types (Chen et al. 2010; Gong et al. 2013). Liu et al. (2014a) demonstrated that DCD caused obvious shifts of AOB community composition in short-term intensive vegetable soil, but did not in long-term intensive vegetable soil. Continuous intensive vegetable production stimulated the formation of a single predominant group (Shen et al. 2011), which might be slightly affected by DCD.
Our results are consistent with the previous findings that the majority of soil derived archaeal sequences were dominated by group 1.1b (23 out of 27 clones) in neutral and alkaline soils (Hu et al. 2014), phylogenetically close to the soil clade containing neutrophilic Nitrososphaera viennensis EN76 and moderately thermophilic Nitrososphaera gargensis (Fig. 5), which isolated from garden soil and hot spring, respectively, and they could heterotrophically or mixotrophically even autotrophically participate in ammonia oxidation in soil and sediment environments (Liu et al. 2014b; Tourna et al. 2011; Xia et al. 2011; Zhang et al. 2012). N. viennensis EN76 was enriched in the presence of ammonia or urea as an energy source (Tourna et al. 2011), and N. gargensis has been frequently observed in tropical soils (Kim et al. 2012). No distinct difference of AOA community structure was observed among treatments (Fig. 4). Likewise, no discernable changes in the AOA community was found between samples with and without N fertilizer and nitrification inhibitors (Gong et al. 2013; O’Callaghan et al. 2010; Wang et al. 2009). Whereas community shifts occurred in urea + DMPP and urea + DCD treatments after 16 days of incubation, as shown in T-RFLP profiles, T-RFs of 82, 268, 208, and 169 bp representing AOA group 1.1b reduced or disappeared, which was confirmed by PCA analysis.
In conclusion, our results indicated clearly that urea application stimulates nitrification in the vegetable soil, and the nitrification inhibitor DMPP exerts greater effect on nitrification than DCD, in accordance with the variation in AOB populations. Significant relationship between net nitrification rate and AOB abundance is observed. AOB community structure in vegetable soil appears to be highly variable with urea and NIs application. However, the abundance and community composition of AOA are less susceptible to urea and NIs than those of AOB. The results showed that the application of DMPP combined with urea is a more effective intervention than DCD in inhibiting nitrification and bacterial ammonia oxidizer rather than archaeal ammonia oxidizer. Therefore, DMPP is considered as an ideal nitrification inhibitor combined with N fertilizer in vegetable production system.
References
Benckiser G, Christ E, Herbert T, Weiske A, Blome J, Hardt M (2013) The nitrification inhibitor 3,4-dimethylpyrazole-phosphat (DMPP)—quantification and effects on soil metabolism. Plant Soil 371:257–266
Chen T, Liu XM, Zhu MZ, Zhao KL, Wu JJ, Xu JM, Huang PM (2008a) Identification of trace element sources and associated risk assessment in vegetable soils of the urban-rural transitional area of Hangzhou, China. Environ Pollut 151:67–78
Chen XP, Zhu YG, Xia Y, Shen JP, He JZ (2008b) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ Microbiol 10:1978–1987
Chen X, Zhang LM, Shen JP, Xu Z, He JZ (2010) Soil type determines the abundance and community structure of ammonia-oxidizing bacteria and archaea in flooded paddy soils. J Soils Sediments 10:1510–1516
Cui M, Sun XC, Hu CX, Di HJ, Tan QL, Zhao CS (2011) Effective mitigation of nitrate leaching and nitrous oxide emissions in intensive vegetable production systems using a nitrification inhibitor, dicyandiamide. J Soils Sediments 11:722–730
Cui PY, Fan FL, Yin C, Li ZJ, Song AL, Wan YF, Liang YC (2013) Urea- and nitrapyrin-affected N2O emission is coupled mainly with ammonia oxidizing bacteria growth in microcosms of three typical Chinese arable soils. Soil Biol Biochem 66:214–221
Di HJ, Cameron K (2011) Inhibition of ammonium oxidation by a liquid formulation of 3,4-dimethylpyrazole phosphate (DMPP) compared with a dicyandiamide (DCD) solution in six new Zealand grazed grassland soils. J Soils Sediments 11:1032–1039
Di HJ, Cameron K (2012) How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures? Soil Use Manag 28:54–61
Di HJ, Cameron K, Shen JP, Winefield C, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Fangueiro D, Fernandes A, Coutinho J, Moreira N, Trindade H (2009) Influence of two nitrification inhibitors (DCD and DMPP) on annual ryegrass yield and soil mineral N dynamics after incorporation with cattle slurry. Commun Soil Sci Plant Anal 40:3387–3398
Florio A, Clark IM, Hirsch PR, Jhurreea D, Benedetti A (2014) Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on abundance and activity of ammonia oxidizers in soil. Biol Fertil Soils 50:1–13
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Gong P, Zhang LL, Wu ZJ, Li D (2012) Laboratory study of the effects of nitrification inhibitors on the abundance of ammonia-oxidizing bacteria and archaea in a Hap-Ustic Luvisol. Afr J Microbiol Res 6:7428–7434
Gong P, Zhang LL, Wu ZJ, Chen ZH, Chen LJ (2013) Responses of ammonia-oxidizing bacteria and archaea in two agricultural soils to nitrification inhibitors DCD and DMPP: a pot experiment. Pedosphere 23:729–739
Hauser M, Haselwandter K (1990) Degradation of dicyandiamide by soil bacteria. Soil Biol Biochem 22:113–114
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Höfferle Š, Nicol GW, Pal L, Hacin J, Prosser JI, Mandić-Mulec I (2010) Ammonium supply rate influences archaeal and bacterial ammonia oxidizers in a wetland soil vertical profile. FEMS Microbiol Ecol 74:302–315
Hu HW, Xu ZH, He JZ (2014) Ammonia-oxidizing archaea play a predominant role in acid soil nitrification. Adv Agron 125:261–302
Irigoyen I, Muro J, Azpilikueta M, Aparicio-Tejo P, Lamsfus C (2003) Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Soil Res 41:1177–1183
Kelliher FM, Clough TJ, Clark H, Rys G, Sedcole JR (2008) The temperature dependence of dicyandiamide (DCD) degradation in soils: a data synthesis. Soil Biol Biochem 40:1878–1882
Kim JG, Jung MY, Park SJ, Rijpstra WIC, Sinninghe Damsté JS, Madsen EL, Min D, Kim JS, Kim GJ, Rhee SK (2012) Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol 14:1528–1543
Kleineidam K, Košmrlj K, Kublik S, Palmer I, Pfab H, Ruser R, Fiedler S, Schloter M (2011) Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere 84:182–186
Lehtovirta-Morley LE, Verhamme D, Nicol GW, Prosser JI (2013) Effect of nitrification inhibitors on the growth and activity of Nitrosotalea devanaterra in culture and soil. Soil Biol Biochem 62:129–133
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Lin XG, Yin R, Zhang HY, Huang JF, Chen RR, Cao ZH (2004) Changes of soil microbiological properties caused by land use changing from rice-wheat rotation to vegetable cultivation. Environ Geochem Health 26:119–128
Liu C, Wang K, Zheng X (2013) Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system. Biogeosci Discuss 10:711–737
Liu Y, Yang Y, Qin HL, Zhu YJ, Wei WX (2014a) Differential responses of nitrifier and denitrifier to dicyandiamide in short- and long-term intensive vegetable cultivation soils. J Integr Agric 13:1090–1098
Liu Y, Zhang JX, Zhang XL, Xie SG (2014b) Depth-related changes of sediment ammonia-oxidizing microorganisms in a high-altitude freshwater wetland. Appl Microbiol Biotechnol 98:5697–5707
McCarty G (1999) Modes of action of nitrification inhibitors. Biol Fertil Soils 29:1–9
Morimoto S, Hayatsu M, Takada HY, Nagaoka K, Yamazaki M, Karasawa T, Takenaka M, Akiyama H (2011) Quantitative analyses of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in fields with different soil types. Microbes Environ 26:248–253
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
O’Callaghan M, Gerard EM, Carter PE, Lardner R, Sarathchandra U, Burch G, Ghani A, Bell N (2010) Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biol Biochem 42:1425–1436
Paranychianakis NV, Tsiknia M, Giannakis G, Nikolaidis NP, Kalogerakis N (2013) Nitrogen cycling and relationships between ammonia oxidizers and denitrifiers in a clay-loam soil. Appl Microbiol Biotechnol 97:5507–5515
Persson T, Wirén A (1995) Nitrogen mineralization and potential nitrification at different depths in acid forest soils. Plant Soil 168:55–65
Rajbanshi S, Benckiser G, Ottow J (1992) Mineralization kinetics and utilization as an N source of dicyandiamide (DCD) in soil. Naturwissenschaften 79:26–27
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Schauss K, Focks A, Leininger S, Kotzerke A, Heuer H, Thiele-Bruhn S, Sharma S, Wilke BM, Matthies M, Smalla K (2009) Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ Microbiol 11:446–456
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Shen WS, Lin XG, Gao N, Shi WM, Min J, He XH (2011) Nitrogen fertilization changes abundance and community composition of ammonia-oxidizing bacteria. Soil Sci Soc Am J 75:2198–2205
Shen TL, Stieglmeier M, Dai JL, Urich T, Schleper C (2013) Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344:121–129
Sheng R, Meng DL, Wu MM, Di HJ, Qin HL, Wei WX (2013) Effect of agricultural land use change on community composition of bacteria and ammonia oxidizers. J Soils Sediments 13:1246–1256
Singh BK, Nazaries L, Munro S, Anderson IC, Campbell CD (2006) Use of multiplex terminal restriction fragment length polymorphism for rapid and simultaneous analysis of different components of the soil microbial community. Appl Environ Microbiol 72:7278–7285
Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM (2006) Scope and strategies for regulation of nitrification in agricultural systems—challenges and opportunities. Crit Rev Plant Sci 25:303–335
Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci 108:8420–8425
Walker CB, De La Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ, Brochier AC, Chain PSG, Chan PP, Gollabgir A (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci 107:8818–8823
Wang YN, Ke XB, Wu LQ, Lu YH (2009) Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization. Syst Appl Microbiol 32:27–36
Weiske A, Benckiser G, Herbert T, Ottow J (2001) Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol Fertil Soils 34:109–117
Wissemeier AH, Linzmeier W, Gutser R, Weigelt W, Schmidhalter U (2001) The new nitrification inhibitor DMPP (ENTEC®)—comparisons with DCD in model studies and field applications. In: Horst WJ, Schenk MK, Bürkert A, Claassen N, Flessa H, Fommer WB, Goldbach H, Olfs HW, Römheld V, Sattelmacher B, Schmidhalter U, Schubert S, von Wirén N, Wittenmayer L (eds) Plant Nutrition, 1st edn. Springer, Netherlands, pp 702–703
Xia WW, Zhang CX, Zeng XW, Feng YZ, Weng JH, Lin XG, Zhu JG, Xiong ZQ, Xu J, Cai ZC (2011) Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5:1226–1236
Xu C, Wu LH, Ju XT, Zhang FS (2005) Role of nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) in NO3 −-N accumulation in greengrocery (Brassica campestris L. ssp. Chinensis) and vegetable soil. J Environ Sci 17:81–83
Yang JB, Li XC, Xu L, Hu F, Li HX, Liu MQ (2013) Influence of the nitrification inhibitor DMPP on the community composition of ammonia-oxidizing bacteria at microsites with increasing distance from the fertilizer zone. Biol Fertil Soils 49:23–30
Yao HY, Gao YM, Nicol GW, Campbell CD, Prosser JI, Zhang LM, Han WY, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microbiol 77:4618–4625
Zacherl B, Amberger A (1990) Effect of the nitrification inhibitors dicyandiamide, nitrapyrin and thiourea on Nitrosomonas europaea. Fertil Res 22:37–44
Zerulla W, Barth T, Dressel J, Erhardt K, von Locquenghien KH, Pasda G, Rädle M, Wissemeier A (2001) 3,4-dimethylpyrazole phosphate (DMPP)—a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soils 34:79–84
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Zhao CS, Hu CX, Huang W, Sun XC, Tan QL, Di HJ (2010) A lysimeter study of nitrate leaching and optimum nitrogen application rates for intensively irrigated vegetable production systems in Central China. J Soils Sediments 10:9–17
Zheng YL, Hou LJ, Liu M, Lu M, Zhao H, Yin GY, Zhou JL (2013) Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl Microbiol Biotechnol 97:8351–8363
Acknowledgments
This study was supported in part by the National Basic Research Program (973 Program) of China (2013CB127403), the National Project of Scientific and Technical Supporting Programs (2012BAC17B02), the Natural Science Foundation of China (31272237, 30971859), the Foundation for University PhD Granting Discipline of the Ministry of Education (20120101110130), and International Plant Nutrition Institute (IPNI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q., Qi, L., Bi, Q. et al. Comparative effects of 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) on ammonia-oxidizing bacteria and archaea in a vegetable soil. Appl Microbiol Biotechnol 99, 477–487 (2015). https://doi.org/10.1007/s00253-014-6026-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6026-7