Abstract
Aims
Nitrification inhibitors (NI) formulated on granulated ammonium sulphate nitrate (ASN) are an option to minimize nitrate leaching into ground waters and emissions of the greenhouse gas N2O. This paper focuses (a) on the development of an analytic enabling to extract and quantify the NI 3,4-dimethylpyrazolephosphate (DMPP), marketed since 1999. The efficiency of DMPP has been studied in laboratory and field soils. Here the DMPP analytic and the behaviour of a nitrifying bacterial consortium enriched from a field soil and exposed to zero, field applied and a 10 fold higher DMPP concentration than the recommended one for field application are in the focus.
Methods
For extracting DMPP quantitatively from soils a method connected to a HPLC analytic has been developed by us and was standardized in laboratory experiment with a silt clay field soil (allochtone Vega). The method is detailed described here. Its reliability has been tested in a 3 years field trial under varying cropping systems and climatic conditions asides the influence of DMPP on CO2−, CH4− and N2O- emissions, measured by the closed chamber method. Parallel a nitrifying bacterial consortium of the silty clay field soil was enriched and subjected to 0, the recommended DMPP concentration for field applications and a 10 times higher one. In incubation experiments the conversion of ammonium to nitrite and nitrate in presence and absence of DMPP was spectrophotometer determined and pH-shifts with a scaled litmus paper. In sacrificed flasks at the end of incubation morphological changes of the bacteria involved were studied by transmission electron microscope (TEM).
Results
The ammonium, nitrite and nitrate determinations and the TEM pictures show that in presence of the field applied DMPP concentration the nitrifying activity returned around 30 days later than in the control and the cells were slightly enlarged. In presence of a 10 times higher DMPP concentration a recovery was prevented. DMPP prolongs, compared with dicyandiamide (DCD), the period of nitrifiers’ inhibition and reduced N2O− and CO2− the emissions (Weiske et al., Biol Fertil Soils 34:109–117, 2001a, Nutr Cycl Agroecosys 60:57–64, b).
Conclusions
With the method developed by us the stability of DMPP in agricultural soils can be satisfyingly and reproducible studied down to a detection limit of 0.01 μg DMPP g−1 dry soil. The morphological changes in the nitrifying consortium due to DMPP concentrations are in agreement with the recovery rate found by nitrite and nitrate formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic nitrogen fertilizers improve the crop productivity worldwide (Ladha et al. 2011). Since about 40 years N-fertilizers stabilized by nitrification inhibitors (NI) as dicyandiamide (DCD) 3, 4 dimethylpyrazolephosphate (DMPP) introduced and are marketed as ALZON and ENTEC, latter with increasing market share (Pasda et al. 2001; Wissemeier et al. 2002). Both products inhibit the conversion of ammonium into mobile nitrate ions whereby the ratio between nitrifying bacteria and archaea, the soil recycler community, may change and the soil organic matter (SOM) content in agricultural fields decrease (Martens-Habbena et al. 2009; Kleineidam et al. 2011). In soils, not receiving NI-stabilized fertilizer, ammonium converts into mobile nitrate ions and gaseous intermediate products as NO2, NO, N2O. These reactive oxidized N-products may pollute especially during the plant-free period ground, drinking water and the atmosphere whereby N2O is seen as greatest threat to the ozone layer in the 21st Century (Benckiser 1994; Ravishankara et al. 2009). N-containing compounds may influence asides the CH4 emissions (Bollag and Czlonskowski 1973, Weiske et al. 2001a; b). For minimizing N losses, NO3 −- and N2O-impacts N-fertilizer split application is an option, another is to choose crops with a high biological inhibition to inhibit the ammonium oxidase (AMO) and a third the use of nitrification inhibitor (NI)-stabilised N fertilizers (Fig. 1; Amberger 1986; Zerulla et al. 2001; Hatch et al. 2005; Subbarao et al. 2012).
DMPP stabilized N-fertilizers contain 1.6 % DMPP related to NH4 + − N correspondingly to 0.8 mg DMPP L−1 culture solution, about 10 times less than DCD-stabilized N-fertilisers (about 16 % DCD) and field studies revealed that DMPP superior to DCD in lowering NH3-volatilisation, NO3 −-leaching, and NO- and N2O-emissions, inter alia, because its nitrification inhibition efficacy after heavy rainfall simulations is longer lasting and its plant compatibility seems to be better than that of the more mobile DCD, which even increase N losses under hot climates (Pasda et al. 2001; Wissemeier et al. 2002; Chaves et al. 2006; Mahmood et al. 2011).
Ecophysiological tests with DMPP fed to rats showed acute toxic effects only when 5.5 mg L−1 4 h−1 have been inhaled (LC50) and fishes (Brachydanio rerio), water fleas (Daphnia magna), algae (Scenedesmus subspicatus) (LC/EC50), and typical heterotrophic soil and water bacteria (Pseudomonas putida) (EC50) died first at DMPP concentrations of >100 mg L−1 96 h−1, >100 mg L−1 48 h−1, 67.7 mg L−1 72 h−1 and 231 mg L−1 170 h−1, respectively (Andreae 1999). Thus the concentration of DMPP and DCD in NI-stabilized N-fertilisers must be significantly enhanced before under laboratory conditions DMPP side effects are observed (Tindaon et al. 2012). Furthermore, nitrifying archaea seem to be less or not affected by recommended DMPP field application rates and may then numerically outcompete ammonia-oxidizing bacteria (Kleineidam et al. 2011).
NI-effects on soil metabolism can only be studied when a method exist allowing to extract such inhibitors quantitatively from soils after field application. For DCD a method exists (Rajbanshi et al. 1992) and DMPP can be quantified by HPLC in the solution used for stabilizing N-fertilizers (DIN-ISO-5725; Association of German Experimental and Research Stations: Verband Deutscher Untersuchungs- und Forschungsanstalten, VDLUFA; European Committee for Standardization, CEN; VDLUFA-Methodenbuch II.I, 4. Erg., 2008). A reliable method for extracting DMPP quantitatively from the soil matrix we had to develop in order to study the fate of DMPP in agricultural soils.
Here the method, developed by us and tested in a 3 years field experiment, is detailed described. Reported is also how increasing DMPP concentrations influence the bacterial morphology and the NH4 + conversion into nitrate.
Material and methods
Experimental design and soil sampling
Soil samples were taken with an auger to a depth of 15 cm in 10 × 8 m randomly distributed field plots (4 plots per treatment), established within a long-term experiment at the Experimental Station of the Agronomy Faculty of Justus Liebig University, Giessen, Germany. The long-term study was initiated in 1982 to evaluate N2 fixation in different crop rotation systems. The 3 years field experiment was designed as a Latin square and the soil classified as an allochtone brown earth, derived from river sediments, FAO-classification: Fluvisol, German classification: Vega with a clayey loam topsoil, 0–35 cm: clay 31 %, silt 60 %, sand 5 %, pHCaCl2 6.0–6.4, Ct 1.35–1.48 %, Nt 0.15–0.16 %, P 160–450, K 120–330 mg kg−1 dry soil, respectively. On March 24, 1997, the experimental site received 90 kg ammonium sulphate nitrate nitrogen (ASN–N) ha−1 in one dose. On the granulated ASN-fertilizer either DMPP or DCD was formulated (ASN, 26 % N, 18.5 % NH4 +–N and 7.5 % NO3 −–N; DMPP: 1.6 % to NH4 +–N and DCD 16 % to NH4 +–N). The control plots received only ASN without the NI DMPP or DCD. The field plots were cropped with spring barley (Hordeum vulgare L., fertilized on 12 March 1997 with 90 kg ASN-N ha−1), with maize (Zea mays L., fertilized on May 5, 1998, with 160 kg ASN–N ha−1), and with winter wheat (Triticum aestivum L., fertilized on March 18 with 180 kg ASN–N ha−1; for more details see Weiske et al. 2001a; b). In this 3 years field experiment DMPP was regularly quantified by extraction and high pressure liquid chromatography (HPLC) using the method developed by us and detailed described below. Regularly collected random samples of the DMPP treated experimental site were deep-frozen (−20 °C) until DMPP was extracted and quantified from thawed aliquots. Soil samples in the NI-untreated control plots were collected separately for enriching a nitrifying bacteria consortium. The soil dry weight was determined after drying at 105 °C (Schlichting et al. 1995).
CO2−, N2O−, and CH4-emissions after application of DMPP- and DCD-stabilised ASN fertilizer, or ASN fertilizer without NIs, were measured with the closed chamber method in the 3 years field experiment (4 replicate plots; Hutchinson and Mosier 1981; Weiske et al. 2001a), DCD according to Rajbanshi et al. (1992) and DMPP as described below.
DMPP extraction from field soils and its quantification
Equipment and chemicals
Centrifuge (4,000 rpm), refrigerator, balance (μg-g-range), overhead shaker, whirl mix, pH-meter, vacuum pump, high-performance liquid chromatography (HPLC) equipped with a Discovery C18 column, 5 μm (15 cm × 4,6 mm) + pre-column (Supelco, Cataloguenr. 504955), UV-detector: wave length 220 nm, 50 ml tightly closable, tapered polypropylene centrifuge tubes, 10–15 ml tapered glass centrifuge tubes, HPLC-vials + glass or PVC micro-vials to reduce the volume, drilled plastic stoppers with a hole to accommodate a 1 ml pipette tip, 1 ml pipette tips, 10 ml pipette tips, filter equipment to produce HPLC-suitable water, H2O (dest), K3PO4 (pA), DMPP and DMP (purity 99.9 and 96 %, respectively, obtained from BASF Ag, Ludwigshafen, Germany), DCD (purity 96 %, purchased from SKW Trostberg AG, Trostberg, Germany), CaCl2 (pA), NaOH (pA), butyl-methyl-ether (MTBE, pA), Na2SO4 (water free), methanol (HPLC purity), H3PO4 (pA), KH2PO4 (Merck, Germany).
DMPP extraction procedure
The flow chart (Fig. 2) summarizes the single steps during DMPP extraction from soil samples amended with DMPP stabilized N-fertilizer and how DMPP is quantified by HPLC. For testing the reliability of the developed method deep frozen topsoil field samples (0.5 kg) collected in the plots only having received ASN were thawed, with a spatula thoroughly homogenized and 10 g aliquots were weighted into 50 ml tapered, tightly closable polypropylene centrifuge tubes (Nunc, Denmark; 10 replicates). The soil samples were treated with 0, 0.01, 0.02, 0.04 0.2, 0.4, 0.8 or 1.6 μg DMPP g−1 soil (purity 99.9 %), dissolved in 1 ml distilled water, and before it was tried to extract the added DMPP corresponding on dry soil basis to 0, 0.0127, 0.0255, 0.051, 0.2548, 0.5096, 1.0191 or 2.0382 μg DMPP g−1 quantitatively from the soil matrix by the following steps DMPP was allowed to interact in a refrigerator for 24 h with the soil matrix.
DMPP-extraction:
-
a)
10 ml distilled H2O (DMPP-extractant) and 0.2 ml of a pA 1 M K3PO4 solution (dispersing agent) are added in 3 replications to 10 g clayey loam topsoil in 50 ml tapered tubes. To achieve a good soil aggregate dispersion, prerequisite for a satisfying DMPP-extraction, the stoppered tubes are shaken upside down for 2 h at 30 rpm.
-
b)
The humic acids in the dispersed soil solutions are precipitated with 0.2 ml of a pA 1 M CaCl2− solution, followed by another shaking upside down at 30 rpm for 1 h. The largely dispersed soil samples reach a pudding-like consistence allowing a better DMPP liberation from soil exchangers.
-
c)
Subsequently 1 ml of a pA 1 M NaOH solution is added to the soil suspensions (10 g fresh soil, 10 ml H2O, 0,2 ml 1 M K3PO4 solution, 0,2 ml 1 M CaCl2). The base is homogeneously distributed by an upside down shaking for 30 min at 30 rpm during which the pH in the pudding-like suspension increases to about 8 and DMPP disproportionates into DMP ([DMP]+ and H2PO4.
-
d)
For transferring DMP into a t-butyl-methyl-ether phase (MTBE), 15 ml (MTBE) are added to the pudding-like suspensions, the samples for 1 h overhead shaken and afterwards centrifuged for 5 min at 3,000 rpm.
-
e)
The DMP-containing MTBE is separated from the aqueous phase either by freezing or salting out the water. Freezing out the water is achieved by putting the 50 ml centrifuge tubes for 24 h into a deep freezer at −20 °C. Salting out the water is achieved by adding 13 g water-free Na2SO4 and shaking the 50 ml centrifuge tubes heavily by hand until the salt is homogenously distributed. Na2SO4 clumping must be avoided. A semi-solid salt column forms.
-
f)
The MTBE-water phase is separated by centrifugation (5 min at 3,000 rpm) and the DMP-containing MTBE phase (about 10 ml) transferred into fresh glass test tubes, stoppered, and stored as DMP containing MTBE stock in a refrigerator.
-
g)
From the DMP-containing MTBE stock 2 ml aliquots are pipetted into tapered 5 ml centrifugation tubes containing 0.2 ml HPLC eluent (170 g ≅ 212 ml methanol, 788 ml of a 1 mM H3PO4−5 mM KH2PO4−buffer L–1, pH 2.8) and the tubes closed with plastic stoppers, in which a hole is drilled for inserting a 1 ml pipette tip that allows to connect the tube to a vacuum pump.
-
h)
For shifting DMP from the ether into the eluent phase the vacuum pump connected tubes are rotated on a whirl mix under a hood and in presence of a blow-dryer. Is no ether smell detectable anymore the evaporation of the MTBE phase is finished (about 1 min) and a lipophilic component layer becomes visible on the test tube bezel (cell membranes etc. in the ether phase).
-
i)
At expected low DMPP concentrations in the soil samples a second, third or more 2 ml aliquots of the DMP-ether stock can be added to the tubes and evaporated until a HPLC-detectable DMPP concentration is reached.
-
j)
During MTBE-phase evaporation also methanol disappears from the HPLC-eluent. The final volume will be about 160 μl, from which an auto-sampler injects a 100 μl aliquot into the HPLC. For easing the withdrawing of the 100 μl aliquot the addition of a microvial lifting the fluid level may be helpful.
-
k)
The calculation of the DMP concentration on soil dry weight basis affords to determine the weight of the glass test tubes in which proportions of the DMP-MTBE stock are evaporated before and after use. The test tubes can be hanged backwards into a rotating centrifuge at 4,000 rpm for drying them
(about 30 s).
DMPP analytic
DMP is quantified by HPLC (Gynkothek, Germany) using a Supelco C 18 Discovery column (5 μm; 15 cm × 4.6 mm), a pre-column (Supelco C 18 Discovery; 5 μm; 2 cm × 4.0 mm), a pump (Gynkothek P 560 HPG), a detector (Gynkothek UVD 170 S), an auto-sampler (Gynkothek GINA 50), a degassing unit (Gynkothek DG 1310), and a computer software (Chromeleon Version 4.20). The HPLC, DMP, DMPP and the DMPP-stabilised fertilizer were provided by the research partner BASF, which had developed ENTEC and cooperated in finding a way to quantify DMPP in soils. The DMP standards of 0.05, 0.1 and 0.2 μg ml−1 were made by diluting 0.1001 g DMP (purity 96 %) in 100 ml HPLC-eluent (1,000 μg DMP ml−1). From this stock 25 ml were diluted first in 500 ml eluent (50 μg DMP ml−1) and finally 2 ml in 50 ml eluent, from which 25, 50 and 100 μl proportions were injected into the HPLC. The related DMP-peak areas were integrated by the computer software Chromeleon Version 4.20 (Softron GmbH, Germany) and expressed in μg DMPP g−1 dry soil, using the formula
(A = peak area; c0 = intercept with the ordinate; Mk = DMPP concentration μg ml−1; 10 = factor: injected 100 μl to ml; 7.5 = dilution factor by an used ether phase of 2 ml; 2.02 = mol weight DMPP/DMP; c1 = slope; S = soil dry weight in g)
Nitrifying bacteria enrichments from the soil of the field trial
DMPP affects nitrifying bacteria but how? For a better insight into NI-caused changes 20 g soil from the plots of the field trial which never had received DMPP were weighted in 500 ml Pyrex bottles and for soil dispersion 180 ml of a 0.18 % sterile sodium pyrophosphate solution added. In the soil solution the most probable number (MPN) of autotrophic nitrifying bacteria was determined using the following selective medium: 0.5, 0.5, 3.5, 0.7, 0.1, 0.18 0.014 g L−1, (NH4)2SO4, NaHCO3, Na2HPO4, KH2PO4, MgSO4 * 7 H2O, CaCl2 * 2 H2O, FeCl3 *6 H2O, respectively (pH 7.8; 3 replicates; Lorch et al. 1995). In separate samples the soil dry weight was determined after drying at 105 °C (Schlichting et al. 1995). After 4 weeks of incubation at 25 °C turbid tubes of the MPN count were checked for nitrite and nitrate formation and from positively tested tubes 4 ml aliquots, transferred into sterile 50 ml of the above fresh, sterile medium containing 100 ml flasks, served as inoculum (3 replicates). The flasks were incubated in the dark on a horizontal shaker (125 UpM, 25 °C) and after 4 weeks the above procedure repeated and this another time. From the third enrichment culture it was assumed that it may contain the nitrifying bacterial consortium present in the allochtone brown earth.
DMPP inhibitory effects on the nitrifying enrichment culture
Four ml of the nitrifying, allochtone brown earth consortium (third soil enrichment) were transferred into sterile 100 ml Erlenmeyer flasks (3 replicates), which contained 46 ml of the above nutrient broth and zero, 0.005 (recommended field applied DMPP-concentration) and 0.05 g l−1 DMPP. The flasks were incubated for 75 days on a horizontal shaker in the dark (125 UpM, 25 °C). Weekly the pH was checked with a scaled litmus paper (Merck, Germany) and spectrophotometrically NH4 +-N, NO2 −-N and NO3 −-N (Benckiser 2007). For eventual morphological bacterial changes, caused by DMPP, transmission electron microscopy (TEM) at 80 kV (Philips EM300) was employed. At the end of the experiment 1 ml of the respective culture solutions was 50 times diluted with water and a small drop (about 1 μl) of these dilutions was transferred to a 0.3 % formvar resin-coated copper net. After about 2 min the water was removed with filter paper and the bacteria inside of the copper net negative-stained with a 2 % uranylacetate (CH3COOH)2UO2 × 2H2O) solution before the copper nets were launched into the TEM.
Results
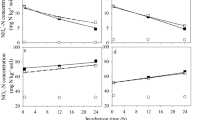
A gram dry soil of the allochtone Vega soil that has never seen DMPP maintains about 105 nitrifying bacteria (MPN counts). The bacteria in a nitrifying enrichment culture from the allochtone Vega soil subdivide under the TEM into 2 morphologically distinct cell structures, into rod-shaped bacteria without flagella and stalk bacterial species with an extremely long prostheca-like appending (Figs. 3a, 4a and b). In presence of increasing DMPP concentrations morphological changes of the cell structures exhibited at the end 75 days, altering ammonium into nitrite and nitrate conversions and pH-shifts (Figs. 3a, b; 4 a-d). At non DMPP-stressed conditions the incorporation of NH4 + into the biomass and the conversion of NH4 + ions into nitrate started between incubation day 6 and 13 (Fig. 3b). Thereby about 40 % of the initial ammonium was oxidized to NO3 − by the rod shaped bacteria and only small amounts of nitrite released. Around day 20 of incubation nitrate formation reached its maximum and the pH dropped from around 7.6 to 7.0 by reaching pH 6.8 at day 75 of incubation.
TEM pictures of bacteria found in a nitrifying enrichment culture from an ammonium-N-fertilizer treated soil of a 3 years field experiment, classified as an allochtone brown earth, derived from river sediments with a clayey loam topsoil (Vega) after 75 days of incubation (Fig. 3a) and conversion of added ammonium to nitrite and nitrate inclusively pH-changes (Fig 3b)
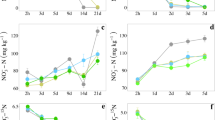
Slightly enlarged the bacterial cell bodies appeared in presence of the field applied DMPP-concentration (Fig. 4a). The formation of nitrite set in delayed at around day 30 of incubation and reached its maximum at day 37 or about 30 days later (Fig. 4c). The nitrite release was 5 times higher than in the control (Figs. 3b, 4c). Such an increased nitrite accumulation, which began to go over into nitrate formation around day 43 of incubation, may affect other metabolic soil processes. The nitrate release peaked significantly delayed compared with the control between day 62 and 70 of incubation and then also the pH began to drop to 6.8 (Fig. 4c). At a 10 times higher DMPP concentration nitrite and nitrate formation was completely blocked, the incurvated bacterial rods considerably enlarged, the prostheca-like attachments widened and broken off and the signalized severe cell damage agrees with the complete inhibition of nitritation and nitratation (Fig. 4b and d).
TEM pictures of bacteria found in a nitrifying enrichment culture from an ammonium-N-fertilizer treated soil of a 3 years field experiment, classified as an allochtone brown earth, derived from river sediments with a clayey loam topsoil (Vega) after 75 days of incubation in presence of 3,4-dimethyl-pyrazolephosphat (DMPP) at field-applied (Fig. 4a) or a 10 times higher DMPP- concentration (Fig. 4b) and conversion of added ammonium to nitrite and nitrate inclusively pH-changes (Fig 4c and d)
The schema of the method, developed by us, is shown in Fig. 2 and the data of the Figs 5, 6 and Weiske et al. (2001a; b) unveil that DMPP can be extracted satisfyingly in laboratory and field experiments from the soil matrix. Fig. 5 exhibits that at an expected low DMPP soil concentration the NI can easily be enriched until a HPLC-detectable concentration is achieved and Fig. 6 that the DMPP recovery rate either by freezing or salting out the DMPP extractant, water, is depending on the chosen procedure and on the DMPP-concentration between 50 % and 80 %, somewhat higher by salting out the water indicating possibilities to methodological improvements. During quantifying DMPP in the field we preferred freezing out the water phase, because deep freezing exhibited no effect on the DMPP recovery rate (data not shown) and freezing out the water is superior to salting out in standardization and sample performance. At Weiske et al. (2001a; b) the disappearing of DMPP and DCD in comparison with CO2, N2O and CH4 emission are presented and reveal that at the end of the vegetation periods in the spring barley field of 1997, the corn field of 1998 and the winter wheat field of 1999 about 16.6 and 15 % of the originally applied DMPP (~ 0.8 mg DMPP kg−1 soil), respectively, were still detectable.
Discussion
Most of the nitrogen in the Earth’s atmosphere is little reactive N2 (78 %). It is introduced into soils as organically bound N and converted to more reactive ammonium and oxidized nitrogen intermediates nitrite (NO2), nitrate (NO3), nitrogen oxides (NO x ) and nitrous oxide (N2O; 310 times greater global warming effect as CO2; IPCC 1995), which may pollute the groundwater or the atmosphere by causing annual costs of around 100 billions US $ (Benckiser 1997; Sutton et al. 2011). DMPP- or DCD-stabilized N-fertilizers are an option to reduce nitrogen impacts (Pasda et al. 2001; Zerulla et al. 2001; Wissemeier et al. 2002; Chaves et al. 2006; Di et al. 2010; Ma et al. 2012). DMPP, binding indiscriminately to the complex of membrane-bound proteins inclusively the ammonium monooxygenase (AMO; Fig. 1), and DCD, blocking the electron transport in the cytochromes of AMO during the conversion of NH3 to hydroxylamine, could become under hot climate regimes, for example in Pakistani summer crops with soil temperatures of around 35 °C, relatively instable and as found for DCD even increase the N-losses rather than reducing those (Ali et al. 2008; Mahmood et al. 2011). The European Commission, having launched a cost/benefit analysis of farming practices (PICCMAT 2011), has evaluated DCD and DMPP stabilized N-fertilizers and came to the following conclusions: though these relatively expensive fertilizers reduce nitrate formation and losses through greenhouse gas emissions by 26 to 49 %, their N-saving effectiveness is insufficiently tested and their cereal and maize crop yield improvements are not conclusively documented. Thus, the present evaluation is a worthwhile investment (PICCMAT 2011). Also in our summer barley (1997), maize (1998) and winter wheat (1999) field experiments the yields after DMPP-stabilized N-fertilization were more variable within one treatment than among the NI-treatments (Weiske et al. 2001a; b).
DMPP interacts in soils primarily with the nitrifying population on which many other organisms are depending. The nitrifying enrichment from the brown earth soil, we have studied in presence and absence of DMPP (Figs 3 and 4), comprises non-flagellated autotrophic ammonia-oxidizing bacteria (AOB), which may belong to the β- and γ-subgroups of Proteobacteria, and bacteria with a narrow, cytoplasm containing extension of the cell wall, a prostheca, which morphologically resemble rather the ubiquitous and frugal α-Proteobacterium Caulobacter crescentius family (Stove and Stanier 1962; Staley 1968; Schmidt 1971; Poindexter 1978). A prostheca increases the bacterial surface area, enhances the nutrient uptake, decreases cell sedimentation and is thus helpful for surviving in aquatic and terrestrial environments. Thus, it is not surprising that prostheca carrying species are also found in the archaeal world (Miroshnichenko et al. 1998). AOA seemingly prefer areas low in NH3 rather than AOB and are in temperate climates evidently less strong inhibited by DMPP than AOB but this needs to be proven (Valentine 2007; Dekas et al. 2009; Prosser and Nicol 2008; Agogué et al. 2008; Di et al. 2010; Fuhrman 2011; Brochier-Armanet et al. 2011; Jarell et al. 2011; Kleineidam et al. 2011; He et al. 2012). Also the accumulation of nitrite in presence of a field applied DMPP-concentration (Fig. 4c) needs to be proven under field conditions, the more because an increased nitrite concentration may interfere with soil respiration (Rinaldo et al. 2008).
On nitrification depends not only the denitrification of nitrifies but also the heterotrophic nitrate respiration, if nitrate is not fertilized. Thus DMPP controls the N2O release of both bacterial groups by a reduced NO3 −-availability and consequently were in our 3 years field experiment the greenhouse gas N2O reduced by around 51 % (Fig. 1; Benckiser 1997; Weiske et al. 2001a; b). Reduced N2O emissions in their dependency of a decreasing NI-concentration were are also observed after field applications of NIs like DCD, nimin, an active agent in the extract of the neem tree Azadiracta indica, furanoflavonoid karanjin (3methoxyfurano 2′,3′,7,8 flavone). nitrapyrin or C2H2 (Figs 3 and 4; IPCC 1995; Weiske et al. 2001a; b; Majumder et al. 2001 and 2004; Chaves et al. 2006; Benckiser 2007; Di et al. 2010; Abasi et al. 2011; Mahmood et al. 2011; PICCMAT 2011). Oxidized nitrogen intermediates as NO2 −, NO3 − or N2O may also inhibit methane formation while organic bound nitrogen may stimulate this process (Bollag and Czlonskowski 1973). In our field experiment this process stayed unaffected by DMPP and DCD (Weiske et al. 2001a; b), but all these NI influences on soil metabolic processes and their inhibition efficiency, diffusion behaviour, their fate in soils can only conclusively be understood when a reproducible and routined method exist allowing to extract quantitatively NI-compounds like DMPP from the soil matrix. Our above described method detects DMPP down to a concentration of 0.01 μg g−1 dry soil and the detection limit is not yet reached as salting out the water implies (Figs 2, 5 and 6). After Azam et al. (2001) had an analytic in hand allowing to study the behaviour of DMPP in soils they found that DMPP in the silty clay soil of the field experiment remains at a water holding capacity of 18 % or 24 % to 80 % within the 0- to 5-mm region around the fertilizer granule and moved only to about 5 to 15 % into the 5− to 20-mm region and to <3 % into the 25− to 40-mm region. In reverse, NH4 + diffused about 4 cm in the same 10 days incubation interval and nitrate showed a fairly uniform distribution. Thus, nitrifying/denitrifying soil AOB and AOA can convert ammonium into mobile NO3 −-ions and further into N2O or N2 DMPP-uninfluenced from a 4 cm zone on but due to the lower scattering of DMPP compared to DCD the N-fertilizer is prolonged protected and that may explain the extended nitrification inhibiting efficiency of DMPP into the plant growth period as found by Weiske et al. (2001a; b).
Different from temperate climate conditions NIs may behave under soil temperature regimes above 30 °C (Ali et al. 2008; Mahmood et al. 2011). At such conditions the NIs seem to be less stabile and only efficient when their application rates are significantly increased. At a 10 times higher concentration than the recommended field apllied concentrations side effects are observed (Fig. 4), which needs to be studied in more details. Further, there are gaps in our knowledge about (a) the other factors determining the nitrifying potential of soils except NIs, (b) the N2O emissions rate ratio of AOA and AOB and (c) DMPP soil side effects (PICCMAT 2011).
References
Abasi MK, Hina M, Tahir MM (2011) Effect of Azadirachta indica (Neem), sodium thiosulphate and calcium chloride on changes in nitrogen transformation and inhibition of nitrification in soil incubated under laboratory conditions. Chempsphere 82:1629–1635
Agogué H, Brink M, Dinasquet J, Herndl GJ (2008) Major gradients in putatively nitrifying and non-nitrifying archaea in the deep North Atlantic. Nature 456:788–791
Ali R, Iqbal J, Tahir GR, Mahmood T (2008) Effect of 3,5-dimetylpyrazole and nitrapyrin on nitrification under high soil temperature Pak. J Bot 40:1053–1062
Amberger A (1986) Potentials of nitrification inhibitors in modern N-fertilizer management. Z. Pflanzenernaehr. Bodenkd 149:469–484
Andreae, M (1999) ENTEC (DMPP – ein neuer Ammoniumstabilisator: Ökotoxikologische Bewertung. In: Düngen mit einer neuen Technologie – Innovation in der Düngung. Wissenschaftliches Kolloquium Agrarzentrum der BASF Limburgerhof, 17. bis 18. Mai, 1999, 3–10
Azam F, Benckiser G, Müller C, Ottow JCG (2001) Release, movement and recovery of 3,4-dimethylpyrazole phosphate (DMPP), ammonium, and nitrate from stabilized nitrogen fertilizer granules in a silty clay soil under laboratory conditions. Biol Fertil Soils 34:118–125
Benckiser G (1994) Relationships between field-measured denitrification losses, CO2 formation and diffusional constraints. Soil Biol Biochem 26:891–899
Benckiser G (1997) Organic inputs and soil metabolism. In: Benckiser G (ed) Fauna in soil ecosystems. Marcel Dekker, New York, USA
Benckiser G (2007) Growth, denitrification and nitrate ammonification of the rhizbial strain TNAU 14 in presence and absence of C2H4 and C2H2. Ann Microbiol 57:509–514
Bollag JM, Czlonskowski ST (1973) Inhibition of methane formation in soil by various nitrogen-containing compounds. Soil Biol Biochem 5:673–678
Brochier-Armanet C, Forterre P, Gribaldo S (2011) Phylogeny and evolution of the archaea: one hundred genomes later. Curr Opin Microbiol 14:274–281
Chaves B, Opoku A, De Neve S, Boeckx P, Van Cleemput O, Hofman G (2006) Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biol Fertil Soils 43:62–68
Dekas AE, Poretsky RS, Orphan VJ (2009) Archaea fix and share nitrogen in methane-consuming consortia. Science 326:422–426
Di HJ, Cameron KC, Shen JP, Winefield CS, O'Callaghan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394
Fuhrman JD (2011) Oceans of Crenarchaeta: a personal history describing this paradigm shift. Microbes 6:531–537
Hatch D, Trindade H, Cardenas L, Carneiro J, Hawkins J, Scholefield D, Chadwick D (2005) Laboratory study of the effects of two nitrification inhibitors on greenhouse gas emissions from a slurry-treated arable soil: impact of diurnal temperature cycle. Biol Fertil Soils 41:225–232
He J-Z, Hu H-W, Zhang L-M (2012) Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils Soil Biol. Biochem 55:146–154
Hutchinson GL, Mosier AR (1981) Improved soil cover methods for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J 45:311–316
IPCC (1995) Climate change 1995: The science of climate change. Contribution of working group 1 to the second assessment of the intergovernmental panel on climate change. Cambridge University Press, UK
Jarell KF, Walters AD, Bochiwal C, Borgia JM, Dickinson T, Chong JPJ (2011) Major players on the microbial stage: why archaea are important. Microbiol 157:919–936
Kleineidam K, Košmrlj K, Kublik S, Palmer I, Pfab H, Ruser R, Fiedler S, Schloter M (2011) Infuence of the nitrifcation inhibitor 3,4-dimethylpyrazolephosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere 84:182–186
Ladha JK, Reddy CK, Padre AT, van Kessel C (2011) Role of nitrogen fertilization in sustaining organic matter in cultivated soils. J Environ Qual 40:1756–1766
Lorch HJ, Benckiser G, Ottow JCG (1995) Basic methods for counting microorganisms in soil and water. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic, London, pp 146–161
Ma Y, Sun L, Xhang X, Yang B, Wang J, Yin B, Yan X, Xeong Z (2012) Mitigation of nitrous oxide emissions from paddy soil under conventional and no-till pratices using nitrification inhibitors during the winter wheat-growing season. Biol Fertil Soil 33:438–442
Mahmood T, Ali R, Latif Z, Ishaque W (2011) Dicyandiamide increases the fertilizer N loss from an alkaline calcareous soil treated with 15 N-labelled urea under warm climate and under different crops. Biol Fertil Soils 47:619–631
Majumder D, Datta A, Kumar S, Pathak H, Jain MC (2001) Mitigation of N2O emission from an alluvial soil by application of karanjin (3 methoxyfurano 2′,3′,7,8 flavone) as nitrification inhibito in different soil types. Biol Fertil Soil 33:438–442
Majumder D, Pandiya B, Arora A & Dhara S (2004) Potential use of karanjin
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying archaea and bacteria. Nature 461:976–979
Miroshnichenko IML, Gongadze GM, Rainey FA, Kostyukova AS, Lysenko M, Chernyhl NA, Bonch-Osmolovskaya EA (1998) Thermococcus gorgonarius sp. nov. And Thermococcus pacificus sp. nov.: heterotrophic extremely thermophilic archaea from New Zealand submarine hot vents. Int J Syst Bacteriol 48:23–29
Pasda G, Hähndel R, Zerulla W (2001) The effect of fertilizer with the new nitrification inhibitor DMPP (3,4-dimethylpyrazolephosphate) on yield and quality of agricultural and horticultural crops. Biol Fertil Soils 34:85–97
Poindexter JS (1978) Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol 135:1141–1145
Politic Incentives for Climate Change Mitigation Agricultural Techniques for an agricultural policy towards the year 2020; PICCMAT, 2011. http://www.climatechangeintelligence.baastel.be/piccmat/index.php
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Rajbanshi SS, Benckiser G, Ottow JCG (1992) Effects on concentration, incubation temperature and repeated applications on degradation kinetics of dicyandiamide (DCD) in soils. Biol Fertil Soils 13:61–97
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Rinaldo S, Brunori M, Cutruzzolà F (2008) Ancient hemes for ancient catalysts. Plant Signal Behav 3:135–136
Schlichting E, Blume HP, Stahr K (1995) Bodenkundliches Praktikum. Blackwell, Berlin
Schmidt JM (1971) Prosthecate bacteria. Annu Rev Microbiol 25:93–110
Staley JT (1968) Prosthecomicrobium and Ancalomicrobium: new prosthecate fresh water bacteria. J Bacteriol 95:1921–1942
Stove JL, Stanier RY (1962) Cellular differentiation in stalked bacteria. Nature 96:1189–1192
Subbarao GV, Sahrawat KL, Nakahara K, Rao IM, Ishitani M, Hash CT, Kishii M, Bonnett DG, Berry WL, Lata JC (2012) A paradigm shift towards low-nitrifying production systems: the role of biological nitrification inhibition (BNI). Ann Bot. doi:10.1093/aob/mcs230
Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W (2011) Too much of a good thing. Nature 472:159–161
Tindaon F, Benckiser G, Ottow JCG (2012) Evaluation of the effect of the nitrification inhibitors 3,4-dimethylpyrazole phosphate (DMPP), 4-chloro methylpyrazole (CIMP) in comparison to dicyandiamide (DCD) on non-target microbial activity in soils as assessed by dehydrogenase- and dimethylsulfoxide reductase activity. Biol Fertil Soils 48:643–650
Valentine DL (2007) Adaption to energy stress dictates the ecology and evolution of the archaea. Nature Rev Microbiol 5:316–323
VDLUFA-Methodenbuch II.I, 4. Erg. (2008) Bestimmung von 3, 4-Dimethyl-1H-pyrazol-Phosphat. 12.2.2, VDLUFA-Verlag, Darmstadt, Germany
Weiske A, Benckiser G, Herbert T, Ottow JCG (2001a) Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol Fertil Soils 34:109–117
Weiske A, Benckiser G, Ottow JCG (2001b) Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr Cycl Agroecosys 60:57–64
Wissemeier A, Linzmeier W, Gutser R, Weigelt W & Schmidhalter U (2002) The new nitrification inhibitor DMPP (ENTEC®) — Comparisons with DCD in model studies and field applications. Plant Nutri. Develop. Plant Soil Sci. 92, Symposium 10: 702–703, doi: 10.1007/0-306-47624-X_340
Zerulla W, Barth T, Dressel J, Erhardt K, Horchler von Locquenghien K, Pasda G, Rädle M, Wissemeier AH (2001) 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture. An Introduction Biol Fertil Soils 34:79–84
Acknowledgment
We highly regret that Prof Johannes CG Ottow, project leader when the method was developed, died unexpectedly on August 20, 2011, and we dedicate posthum this paper to him. We thank Dr. Pasda, Prof Dr. Wissemeier, BASF, PD Dr. Rod Snowdon, Department of Plant Breeding, and Prof Sylvia Schnell, Department of Applied Microbiology for helpful suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Matthias Wissuwa.
Rights and permissions
About this article
Cite this article
Benckiser, G., Christ, E., Herbert, T. et al. The nitrification inhibitor 3,4-dimethylpyrazole-phosphat (DMPP) - quantification and effects on soil metabolism. Plant Soil 371, 257–266 (2013). https://doi.org/10.1007/s11104-013-1664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1664-6