Abstract
This review provides an analysis of recent published work on interactions between microorganisms, especially the ones involving mainly nutrient exchanges and at least with one microalga species. Examples of microbial partners are given, with a remark to the potential application of cultures of an autotroph and a heterotroph, which grow simultaneously, taking advantage of the complementary metabolisms. These are particularly interesting, either due to economic or sustainable aspects, and some applications have already reached the commercial stage of development. The added advantages of these symbiotic cultures are biomass, lipid, and other products productivity enhancement a better utilization of resources and the reduction or even elimination of process residues (including carbon dioxide and other greenhouse gases) to conduct an increasingly greener biotechnology. Among the several symbiotic partners referred, the microalgae and yeast cultures are the most used. The interaction between these two microorganisms shows how to enhance the lipid production for biodiesel purposes compared with separated (stand-alone) cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are symbioses in nature since the beginning of life on Earth and mankind grasped the opportunity to apply these phenomena in biotechnology processes. Symbiosis, which simply means living together, is a relationship wide spread in nature that defines an association between dissimilar organisms, divided in types according to the advantages that the partners obtain from it. Mutual symbiosis occurs when both partners get advantages from the association. Commensalism occurs when one partner gains an advantage but the other is not damaged in the association. When one of the partners is damaged, the association is called parasitism (Schlegel 1986; Black 1996; Norton 1985). This review focuses only on the positive interaction types, such as mutualism and commensalism.
There are examples of symbioses in all kingdoms of living beings and the most widespread reason for that is nutrient supply (Honegger 2008; Ahmadjian and Paracer 2000). The same interactions can be found among fungi, bacteria, microalgae, and cyanobacteria, which are the main target of biotechnology processes.
A well-known example from nature is that of lichens, commonly found on rocks and trees as crusts, which are associations of fungi with photosynthetic algae or cyanobacteria (Prescott et al. 1999; Oksanen 2006). The fungus and alga are so closely associated that they make up a unitary vegetative body growing attached to rocks, tree trunks, and other unlikely habitats. The fungus obtains its organic nutrients from an alga that fixes CO2 by photosynthesis. In turn, the fungus protects the alga and supplies it with water and minerals obtained from the atmosphere. These partners in lichen symbiosis can be separated and will grow as individual fungi or algae. It is also possible to construct lichens (Schlegel 1986).
Some of the most interesting characteristics of lichens are the production of secondary metabolites, tolerance to extreme environments, and sensitivity to pollution. Industrially important secondary metabolites isolated from lichens are unique to this symbiosis and have application in medicine, perfume industry, brewing, dying industry, and food industry (Oksanen 2006; Müller 2001). Some authors report that more than 8,000 tonnes annually of two specific species of lichens are harvested for the purpose to enhance the persistence of a fragrance on the skin (Lutzoni and Miadlikowska 2009). An interaction as such is designated as mutualism, as both alga and fungus are benefiting from it (Schlegel 1986; Ahmadjian and Paracer 2000). This is one of the oldest symbioses that have been applied to biotechnology, where the outcome is a valuable product to market.
The aim of this review is the symbiotic interactions between microorganisms that are currently under research because of their role and contribution to biofuels production from microorganisms in particular the biodiesel production from single-cell oil (SCO).
Syntrophism
A special kind of symbiosis is syntrophism, a biological association in which microorganisms are mutually dependent on one another for nutritional requirements (Prescott et al. 1999).
A very important syntrophism occurs in the anaerobic methanogenic consortium, where fatty acids are degraded to produce molecular hydrogen (H2) and methane by two different bacterial groups (Prescott et al. 1999). This interaction falls in the syntrophic symbioses which are cross-feeding symbioses: metabolic products of one organism are required by another. The H2 is produced by a variety of anaerobic fermentative bacteria; then H2 is required by anaerobic methanogenic bacteria in order to carry out anaerobic respiration, according to the following sequence of reactions:
The metabolism of methanogens maintains a low concentration of H2 in the immediate environment of both bacteria (Eq. 2); only when H2 generated by H2-producing bacteria is consumed can the latter bacteria gain sufficient energy for growth, as continuous removal of H2 promotes further fatty acid fermentation and H2 production (Eq. 1) (Kim and Gadd 2008).
An example of syntrophic bacteria is Clostridium ultunense and Thermoacetogenium phaeum that oxidize acetate to CO2 and H2 in association with H2-consuming sulfidogens or methanogens (Kim and Gadd 2008). The oxidation of butyrate, propionate, and ethanol to acetate are endergonic reactions that do not occur spontaneously and therefore cannot support growth of the syntrophic bacteria under standard conditions. However, oxidation of those substrates becomes an exergonic reaction when the reaction product concentration (H2) is kept very low. The co-culture of methanogens and sulfidogenic bacteria removes hydrogen efficiently, keeping its partial pressure low as these reactions become exergonic. Co-culture of syntrophic bacteria and methanogens on butyrate and propionate is well documented. These mixed cultures are referred to as syntrophic associations or described as interspecies hydrogen transfer (Kim and Gadd 2008).
The H2-producing bacteria associated with CH4-producing bacteria are part of a consortium on anaerobic digestion: in the absence of the former, methanogens cannot grow, and only when methanogens uptake the H2 can the former grow. This is a very important association between microorganisms for biotechnology as it is the basis of biogas production, composed mainly by methane, a renewable energy source (Marques 2001).
Nutritional interactions applied in biotechnology
It is known from the experience of the food industry that the more complex microbial communities cope better with environmental perturbations. This can be explained by the exchange of information and metabolites between the interacting microbes in mixed cultures. Most of the fermentations for the production of fermented food products use mixed bacterial cultures because they provide better quality products. Important interactions bacteria–bacteria in dairy products (yogurt) fermentation have a crucial role in final product quality (Smid and Lacroix 2013). Improved microbial stability on the obtained final product is one of the most relevant among these qualities, which is achieved after symbiotic interaction has been established. As a result of these findings, the authors concluded that there is a need to use mixed cultures in fermentation processes, and they highlighted the difficulties in propagation of such a mixed culture starter (Smid and Lacroix 2013). The use of high throughput genomic tools can elucidate about the mechanisms of interaction between microorganisms in mixed cultures. This knowledge can be used to optimize or control processes in all biotechnology subjects.
A number of applications based on the use of complementary metabolisms have been investigated by several authors, and some are already giving their contribution to an ecologically sustainable solution to biotechnology industry.
This has been applied to aquaculture, for example, establishing a symbiosis between fish and algae in a culture pond exposed to light resulted in a win–win situation for the industry and ecology. In this case, fish provide dissolved carbon dioxide, organic nitrogen (ammonia), physical agitation, and removal of protozoa, which are beneficial to microalgae. In turn, microalgae provide dissolved oxygen, nutrients, and consumption of excreted compounds to clean the water, which are beneficial to fish. The food chain in this pond can be described shortly as small fish (zooplanktivorous fish) that eat protozoa that in turn eat microalgae, which can be sustainable if the fish is removed periodically (Kazamia et al. 2012a). Both organisms benefit from this interaction and so a mutual symbiosis is achieved with commercial aim. The improvement in aquaculture of fish and mass cultivation of microalgae raises the income of the process as claimed by the author (Pack 1991).
There is also a practical application of laboratory model studies of closed ecosystems based on microorganisms in space science, which began in the 1960s, to show the feasibility of synthesizing micro-ecosystems and to know their longevity (Pisman and Somova 2003). Those works evidenced that a community must include a microalgae as the autotroph and two or three species of heterotrophs. Furthermore, it was demonstrated that closed gas cycle systems had a longer life than the separate cultivation of the same microorganisms (Pisman and Somova 2003).
Recently, in order to provide the necessary nitrogen required for microalgae growth for carbon dioxide fixation purposes, the bacteria Azotobacter vinelandii that is able to fix nitrogen from the atmosphere, was grown in co-culture with microalgae (Villa et al. 2013). Two strains of green algae, Neochloris oleoabundans and Scenedesmus sp. BA032, were able to utilize the A. vinelandii siderophore azotobactin as a source of nitrogen, demonstrating to support microalgae growth. This interaction between bacteria and microalgae which could be classified as a commensalism may be applied to mass culture of microalgae reducing the nitrogen source cost.
Nutritional interactions based on metabolism complementarity
The idea of symbiosis between microorganisms based on complementary metabolisms was first approached in 1958, as a method to improve the oxygen supply to the oxidation ponds for wastewater treatment, using algae symbiosis with bacteria (Oswald et al. 1953). The secondary treatment processes of wastewater involve the oxidation of organic matter by microorganisms under conditions wherein oxygen does not become depleted so that, to supply the necessary large amounts of oxygen from atmosphere, the air is forced to enter the liquid phase at an accelerated rate, consuming high quantities of energy to achieve it. The author disclosed that photosynthetic oxygenation by microalgae growing in ponds is a completely different method for supplying the required oxygen to grow bacteria in the same pond, avoiding intensive consumption of energy (Oswald et al. 1953). The symbiosis established between microalgae (autotrophic microorganisms) and bacteria (heterotrophic microorganisms) is mutual because both organisms benefit from each other (CO2 and O2, respectively) to improve their growth. The use of symbiosis for wastewater treatment in high-rate oxidation ponds (HROP) is a known technology since the 1960s (Oswald et al. 1953), and so it will not be covered in this paper. Further details can be found elsewhere because this subject has been already reviewed by other authors (Abeliovich 1986; Larsdotter 2006; Su et al. 2011).
Mixed cultures of microalgae and bacteria
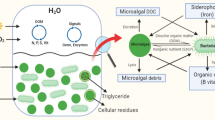
Interactions between microalgae and bacteria are thought to exist in natural habitats and that those are responsible for the failure of microalgae isolation in the laboratory, as the partner has been discarded in the process. It is quite common that unicellular microalgae grow in the presence of bacteria. A survey of 326 algal species revealed that 171 species require exogenous vitamin B12 for growth, implying that more than half of the algal kingdom is cobalaminauxotrophic (Croft et al. 2005). The authors showed that the role of vitamin B12 in algal metabolism is primarily as a cofactor for vitamin B12-dependent methionine synthesis, and that cobalaminauxotrophism has arisen numerous times throughout evolution, probably owing to the loss of the vitamin B12-independent form of the enzyme. The source of cobalamin seems to be bacteria, indicating a symbiotic interaction between microalgae and bacteria (Croft et al. 2005).
A recent study of the bacteria associated with mass culture of Botryococcus braunii (both the planktonic bacteria in the water column and those forming biofilms adhered to the surface of the microalgal cells) found ∼107–108 cultivable cells/g of microalgae (Rivas et al. 2010). The authors found that at least eight different cultivable species of bacteria were detected in the biofilm and two species, Pseudomonas sp. and Rhizobium sp., were present in the bacterial biofilm associated with B. braunii. These bacteria were not detected in the planktonic bacteria isolated from the water and the bacterium, Rhizobium sp., acted as a probiotic bacterium to significantly encourage the growth of B. braunii. A direct application of these beneficial bacteria associated with B. braunii could be to use them as inoculants for large-scale microalgal cultures. They could optimize biomass production by enhancing growth, especially when combined with this microalga that has a low growth rate. Few studies have considered the implications of this phenomenon as regards the interaction between bacteria and microalgae (Rivas et al. 2010).
More recently, the study of algal-bacterial interactions for vitamin B12 in a model laboratory system has enlightened this theme (Kazamia et al. 2012b). The model co-culture was composed by the following microorganisms: the vitamin B12-dependent green algae Lobomonas rostrata and the rhizobial bacterium Mesorhizobium loti. These two were grown together in a medium, neither containing vitamin B12 nor an organic carbon source, as reported by the author (Kazamia et al. 2012b). The upshot was that the algae grew well and, moreover, the algae and the bacteria reached an equilibrium in their population densities which was stable in both batch and chemostat conditions. In this case bacteria provide vitamin B12 to algae and algae give photosynthetic products like organic carbon source to bacteria, so this is a mutual symbiosis. The authors suggest that bacterial consortia that will enhance the growth of algae, through provision of expensive nutrients (vitamin B12 and iron), may be applied to the industrial algae cultivation with the referred advantages over pure cultures.
Another important aspect of using the symbiotic bacteria in algae cultures, with carefully chosen co-habitants, is the exclusion of bacterial contaminants. The bacteria in the culture discourage other bacteria from invading it, as that niche is already occupied. This can be explained by the competitive exclusion principle of community ecology, as referred by the authors (Kazamia et al. 2012a). The single-species approach to algal cultivation on a large scale in open photobioreactors is difficult because biological communities will inevitably increase in complexity over time as a nature principle. On the other hand if species with complementary niches are chosen to form a stable synthetic community, then it is predictable that these species will grow without the presence of contaminant species (Kazamia et al. 2012a). This is called synthetic ecology as the microbial community has been chosen to solve a specific problem. The overall lipid yield can be maximized by choosing the most lipid producing algae in the bigger proportion in this mixed community. This topic is of great interest for algae production in large scale aimed at biodiesel production and it highlights the importance of microorganism interactions when sterile environments are not allowed. Once more the symbioses between microorganisms can be allies or tools to improve growth and productivity.
Mixed cultures of microalgae and yeast
The co-culture of microalgae and yeast is being studied for various purposes such as aquaculture feed (Cai et al. 2007) and fine chemical production (Dong and Zhao 2004). The authors showed that there are significant advantages over monoculture of the same species, like an improved yield of high value products and a higher growth rate and biomass concentration. The mixed cultures performed better due to the higher carbon dioxide available for microalgae use in photosynthesis, and due to the higher oxygen availability for heterotrophy of yeast, leading to reduced microalgae production costs while maintaining alga production reliability.
Many applications based on the use of complementary metabolisms have been applied also to SCO production for biodiesel purposes, as this objective does not necessarily demand pure cultures, but the requirements of a low price product and a carbon dioxide neutral process are of major importance. Also, in natural habitats mixed cultures of microorganisms are common.
The symbiosis between a culture of the oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris was shown to enhance lipid production from industrial wastes (Cheirsilp et al. 2011). The authors reported that, in the mixed culture, the yeast grew faster and the lipid production was higher than that obtained in the pure cultures (Table 1). This could be because the microalga acted as an oxygen generator for the yeast, whereas the latter provided CO2 to the microalga and both carried out the production of lipids. In the mixed culture, the metabolic reactions of both CO2 release and uptake were combined and complementary. Moreover, with this strategy of mixed culture cultivation, higher concentrations of CO2 and O2 that were reported to have deleterious effects on the yeast and microalga, respectively, are avoided. This is a mutual symbiosis as both microorganisms are benefiting from each other and it is an improved biotechnological process with industrial application.
The mixed cultivation of the microalga Spirulina platensis and yeast R. glutinis significantly increased the accumulation of total biomass and total lipid yield (Table 1), improving the strategy for lipid production based on a mutual symbiosis between these two microorganisms, as reported by the authors (Xue et al. 2010).
The mixed cultures of microalgae Chlorella sp. KKU-S2 and oleaginous yeast Torulaspora maleeae Y30 or Torulaspora globosa YU5/2, using sugar cane juice as organic carbon source, resulted in improved growth toward microbial oil production (Papone et al. 2012). The biomass concentration in the mixed culture of the oleaginous yeast with microalgae increased faster and was higher, when compared with those obtained in monoculture (Table 1). There was a maximum increase of 96 % in lipid yield in the mixed culture as compared with the yeast culture alone. The author explains that the microalga may function as an O2 producer in the mixed culture, enhancing the growth of the yeast, whereas the latter produces CO2 that is used by the microalgae under photoautotrophic cultivation. In the mixed culture, the metabolic reactions of both CO2 release and uptake were combined and complementary (Papone et al. 2012).
The lipid productivity in the mixed cultures is generally higher than the lipid productivity of the yeast culture. This demonstrates the advantage of symbiosis in mixed culture when producing lipids (Table 1).
The autotroph to heterotroph inoculum volume ratio seems to play a key role in the CO2 production and uptake control in mixed cultures. The authors used different strategies: some (Cheirsilp et al. 2011; Papone et al. 2012) used an equal amount of inoculum (10 % inoculation volume ratio) for the autotroph and for the heterotroph; others (Xue et al. 2010) used double amount of autotroph (20 %, v/v) to heterotroph (10 %, v/v). The initial proportion of heterotroph to autotroph is one of the factors that certainly determine the performance of the mixed culture growth. Another key factor seems to be the specific growth rate of the autotroph and the heterotroph as has been pointed out by the author (Papone et al. 2012).
Symbiosis between two microorganisms in separated bioreactors
A completely artificial and controlled interaction between microorganisms can be established in biotechnology processes to obtain important products for humanity.
This is the case of a heterotrophic microorganism growing in a fermenter with adequate conditions and a photoautotrophic microorganism growing in a separate photobioreactor. Both organisms are cultured separately and the gases produced while growing are exchanged between the bioreactors, limiting the interaction to this, benefiting both organisms or only one, but not damaging the other (Santos et al. 2011; Santos et al. 2013). Autotrophically grown Chlorella protothecoides improved biomass productivity (0.015 g L−1 h−1) and lipid productivity (2.2 mg L−1 h−1) when aerated with the off-gas from the yeast R. toruloides fermentation (Fig. 1), representing an increase of 87 and 83 %, respectively, compared with the same culture aerated only with air and not exchanging gases (Table 2) (Santos et al. 2013).
Schematic representation of the gas stream from a fermenter to a photobioreactor indicating an artificial symbiosis between the two microorganisms in culture (Santos et al. 2013)
Symbiosis between microorganisms achieved by biosystems engineering leads to high concentrations of a product of the desired quality in the shortest possible time. That is the case of choosing the heterotroph that has the highest lipid and biomass productivity, which can be a heterotrophic microalga (Santos et al. 2011) or a yeast (Santos et al. 2013), and combining it with a microalga that also has the highest lipid and biomass productivity.
The overall productivity improvement of autotrophically grown C. protothecoides aerated with the CO2-enriched air from its heterotrophic culture was 55 % in biomass and 55 % in lipids when compared with the bioreactor aerated only with air (Table 2) (Santos et al. 2011). The inverse case of heterotrophic grown C. protothecoides showed biomass (0.052 g L−1 h−1) and lipid (28.6 mg L−1 h−1) productivities increased by 6 and 96 %, respectively, when aerated with the O2-enriched air from an autotrophic C. protothecoides culture in bubble columns (Fig. 2), as compared with aeration with ambient air (Santos et al. 2011). Although the classic concept of symbiosis mentions an interaction between two different species, the example discussed here uses the same species C. protothecoides, growing as a heterotroph in one bioreactor and as an autotroph in the other, operated independently, connected only by their gas phases. This particular case raises the question if it is still a symbiosis or if we have only a synergetic effect.
Symbiotic association of bubble columns growing Chlorella protothecoides autotrophically (a, A) and heterotrophically (h, H) (Santos et al. 2011)
Other authors have been growing the yeast T. maleeae Y30, that is a SCO producer, and supply the CO2 produced to a photosynthetic microalga, Chlorella sp. KKU-S2, which is also a lipid producer (Puangbut and Leesing 2012). According to them, relatively high values of lipid productivity (0.223 g L−1 h−1) and biomass productivity (0.48 g L−1 h−1) were obtained by using air coupled with CO2 emissions from the yeast fermentation to supply the alga. Chlorella sp. biomass productivity improved by 6 % and lipid productivity by 15 % when the culture was aerated with air and CO2 from the yeast fermentation, when compared with the control growth aerated only with air (Puangbut and Leesing 2012).
The advantage of this technology is that two biomasses will be obtained with different characteristics and can then be processed separately to obtain two or more products. The usage of mixed cultures generates a single biomass type that has a composition equal to the weighted average of those of each one of the species that originated it, and could be directed for biodiesel production.
The autotroph to heterotroph culture volume ratio seems to play a key role in the control of CO2 evolution and uptake in these cases where heterotroph and autotroph cultures in separate reactors are connected by their gas phases. The specific growth rate of the autotroph and the heterotroph are also affecting the CO2 production and uptake, respectively, as these processes are directly related to microbial growth rates.
Conclusions and prospects of symbiosis in biotechnology
Symbiosis is wide spread in nature because of its advantages over the individual living of organisms.
In biotechnology, the symbiotic interactions also bring advantages over the single culture option.
As summarized in Table 3, the number of articles found in the literature has increased considerably in recent years, reflecting the growing interest in the many applications that make use of the symbiotic interactions involving microalgae. From this table, the majority of the research studies concern the interaction between microalgae and yeasts, which is an indicator that they are probably the ideal partners to choose in a synthetic consortium. The use of bacteria in co-culture with algae can reinforce the photoautotrophic microalgae culture in open bioreactors, leading to a more reliable production.
Exploring interactions between complementary metabolism microorganisms into its limits, by separating the microorganisms in different bioreactors, brings a new perspective to biotechnology, offering the possibility to add the advantages of mixed cultures without losing the physical separation between the two biomasses.
The advantage that through a symbiotic interaction between microorganisms we can improve targeted operational variables, for example, productivity, is of major impact because these variables are directly related to capital and operational costs in a biotechnological process.
The published uses of microbial symbiosis at least with one microalga species in the biotechnological exploitation of several compounds such as pigments, biofuels and polyunsaturated fatty acids among others, have commercial and environmental positive impacts. Beyond the obvious biomass and lipid productivity enhancement, there is a more efficient utilization of resources and the reduction or even elimination of process residues (including carbon dioxide and other greenhouse gases) to conduct an increasingly greener biotechnology.
This biotechnological approach is relatively new (started one decade ago) and has not attained yet a stage of development near to commercialization or even to demonstration. However, the interest in these symbiotic interactions remains as an attractive line for further research, development and demonstration in the near future to come.
References
Abeliovich A (1986) Algae in wastewater oxidation ponds. In: Richmond A (ed) Handbook of microalgal mass culture. CRC Press, Boca Raton, pp 331–338
Ahmadjian V, Paracer S (2000) Symbiosis: an introduction to biological associations. Oxford University Press, Oxford
Black JG (1996) Microbiology, principles and applications, Prentice Hall, New Jersey
Cai S, Hu C, Du S (2007) Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J Biosci Bioeng 104(5):391–7. doi:10.1263/jbb.104.391
Cheirsilp B, Suwannarat W, Niyomdecha R (2011) Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. N Biotechnol 28(4):362–368. doi:10.1016/j.nbt.2011.01.004
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438(7064):90–3. doi:10.1038/nature04056
Dong Q-L, Zhao X-M (2004) In situ carbon dioxide fixation in the process of natural astaxanthin production by a mixed culture of Haematococcus pluvialis and Phaffia rhodozyma. Catal Today 98(4):537–544. doi:10.1016/j.cattod.2004.09.052
Honegger R (2008) Mycobionts. In: Nash TH (ed) Lichen biology. Cambridge University Press, Cambridge, pp 27–39. doi:10.1017/CBO9780511790478.004
Kazamia E, Aldridge DC, Smith AG (2012a) Synthetic ecology—a way forward for sustainable algal biofuel production? J Biotechnol 162(1):163–169. doi:10.1016/j.jbiotec.2012.03.022
Kazamia E, Czesnick H, Nguyen TTV, Croft MT, Sherwood E, Sasso S, Hodson SJ, Warren MJ, Smith AG (2012b) Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol 14(6):1466–76. doi:10.1111/j.1462-2920.2012.02733.x
Kim BH, Gadd GM (2008) Anaerobic respiration. In: Bacterial physiology and metabolism. Cambridge University Press, Cambridge, pp. 298–353. doi:10.1017/CBO9780511790461.010
Larsdotter K (2006) Wastewater treatment with microalgae—a literature review. Vatten 62:31–38
Lutzoni F, Miadlikowska J (2009) Lichens. Curr Biol 19(13):502–503. doi:10.1016/j.cub.2009.04.034
Marques IP (2001) Anaerobic digestion treatment of olive mill wastewater for effluent re-use in irrigation. Desalination 137(1–3):233–239. doi:10.1016/S0011-9164(01)00224-7
Müller K (2001) Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 56(1–2):9–16. doi:10.1007/s002530100684
Norton CF (1985) Microbiology. Addison-Wesley Publishing Company, Reading
Oksanen I (2006) Ecological and biotechnological aspects of lichens. Appl Microbiol Biotechnol 73(4):723–34. doi:10.1007/s00253-006-0611-3
Oswald WJ, Gotaas HB, Ludwing HF, Lynch V (1953) Algae Symbiosis in oxidation ponds III Photosynthetic Oxygenation. Sewage Ind Wastes 25(6):692–705
Pack MY (1991) Symbiotic production method for microalgae and Fishes. US Patent 5040486
Papone T, Kookkhunthod S, Leesing R (2012) Microbial oil production by monoculture and mixed cultures of microalgae and oleaginous yeasts using sugarcane juice as substrate. World Acad Sci Eng Technol 64:1127–1131
Pisman TI, Somova LA (2003) Interaction of a mixed yeast culture in an “autotroph-heterotroph” system with a closed atmosphere cycle and spatially separated components. Adv Space Res 31(7):1751–1756
Prescott L, Harley JP, Klein DA (1999) Microbiology. WCB/McGraw-Hill, New York
Puangbut M, Leesing R (2012) Integrated cultivation technique for microbial lipid production by photosynthetic microalgae and locally oleaginous yeast. World Acad Sci Eng Technol 64:975–979
Rivas MO, Vargas P, Riquelme CE (2010) Interactions of Botryococcus braunii cultures with bacterial biofilms. Microb Ecol 60(3):628–35. doi:10.1007/s00248-010-9686-6
Santos CA, Ferreira ME, Lopes da Silva T, Gouveia L, Novais JM, Reis A (2011) A symbiotic gas exchange between bioreactors enhances microalgal biomass and lipid productivities: taking advantage of complementary nutritional modes. J Ind Microbiol Biotechnol 38:909–917. doi:10.1007/s10295-010-0860-0
Santos CA, Caldeira ML, Lopes da Silva T, Novais JM, Reis A (2013) Enhanced lipidic algae biomass production using gas transfer from a fermentative Rhodosporidium toruloides culture to an autotrophic Chlorella protothecoides culture. Bioresour Technol 138:48–54. doi:10.1016/j.biortech.2013.03.135
Schlegel HG (1986) General Microbiology. Cambridge University Press, Cambridge.
Smid EJ, Lacroix C (2013) Microbe-microbe interactions in mixed culture food fermentations. Curr Opin Biotechnol 24(2):148–54. doi:10.1016/j.copbio.2012.11.007
Su Y, Mennerich A, Urban B (2011) Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res 45(11):3351–8. doi:10.1016/j.watres.2011.03.046
Villa JA, Ray EE, Barney BM (2013) Azotobacter vinelandii siderophore can provide nitrogen to support the culture of the green algae Neochloris oleoabundans and Scenedesmus sp. BA032. FEMS Microbiol Lett 1–8. doi:10.1111/1574-6968.12347
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160:498–503. doi:10.1007/s12010-008-8376-z
Acknowledgments
The authors would like to thank Professor Helena Pinheiro for the English language and scientific revision. Carla A. Santos’s PhD scholarship was financed by FCT (SFRH/BD/38516/2007). Portuguese Foundation for Science and Technology supported studies presented here through the SIMBIOALGA project (PTDC/AAC-AMB/112954/2009) FCOMP-01-0124-FEDER-013935 (also supported by FEDER funding through COMPETE-Programa Operacional Factores de Competitividade).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, C.A., Reis, A. Microalgal symbiosis in biotechnology. Appl Microbiol Biotechnol 98, 5839–5846 (2014). https://doi.org/10.1007/s00253-014-5764-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5764-x