Abstract
Lactic acid bacteria (LAB) are widely used for the production of a variety of fermented foods, and are considered as probiotic due to their health-promoting effect. However, LAB encounter various environmental stresses both in industrial fermentation and application, among which acid stress is one of the most important survival challenges. Improving the acid stress resistance may contribute to the application and function of probiotic action to the host. Recently, the advent of genomics, functional genomics and high-throughput technologies have allowed for the understanding of acid tolerance mechanisms at a systems level, and many method to improve acid tolerance have been developed. This review describes the current progress in engineering acid stress resistance of LAB. Special emphasis is placed on engineering cellular microenvironment (engineering amino acid metabolism, introduction of exogenous biosynthetic capacity, and overproduction of stress response proteins) and maintaining cell membrane functionality. Moreover, strategies to improve acid tolerance and the related physiological mechanisms are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) are a heterogeneous group of low-GC, nonsporulating Gram-positive bacteria, which ferment a range of carbon sources primarily to lactic acid (Gaspar et al. 2013). In food industry, LAB are mainly used for food and beverage fermentation, production of add-in ingredients, bacteriocins, exopolysaccharides (Zhu et al. 2009; Table 1). In addition, LAB are also used to produce bulk and fine chemicals including organic acids, polyols, and vitamins (Gaspar et al. 2013; Table 1). However, as cell factories, LAB encounter various stress conditions during the industrial production and in the gastrointestinal tract. Among various environmental stresses, acid stress is one of the most important survival challenges, and acid tolerance is one of the criterias to select potential probiotics (Parvez et al. 2006). During fermentation, the growth of LAB is accompanied by lactic acid production leading to acidification of the media, arrest of cell growth, and possibly cell death due to the entrance of undissociated form of lactic acid into the cytoplasm by simple diffusion (Serrazanetti et al. 2009). Intracellular lactic acid dissociates, changes the intracellular pH, and disrupts the cytoplasm anion pool, which affects the integrity of purine bases and results in denaturing of essential enzymes inside the cells (Warnecke and Gill 2005). Thus, improving the acid stress resistance is important for the industrial application of LAB.
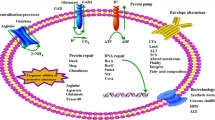
In response to acid stress, LAB have evolved stress-sensing systems and employed numerous mechanisms to withstand harsh conditions and sudden environmental changes, including the maintenance of intracellular pH homeostasis, cell membrane functionality, and upregulation of stress response proteins (Lebeer et al. 2008; Wu et al. 2012b; O'Sullivan and Condon 1997; Fig. 1). In addition, acid tolerance response (ATR) appears to confer protection against environmental stresses by prior exposure of cells to moderately acidic conditions (De Angelis et al. 2001). Meanwhile, omics methods combined with molecular techniques have contributed to the understanding and validation of the molecular mechanisms involved in acid tolerance, and many feasible strategies (engineering general stress response proteins, maintaining cell membrane functionality, and regulating amino acid metabolism) have been proposed to improve the acid stress resistance of LAB (Wu et al. 2012a, 2013b; Trip et al. 2012). Thus, it is necessary to timely summarize the progress to further stimulate the research interest in this field. In this review, we provide an overview of the recent progress in engineering acid stress resistance of LAB, with emphasis on engineering intracellular microenvironment (engineering amino acid metabolism, introduction of exogenous biosynthetic capacity, and overproduction of stress response proteins) and maintaining cell membrane functionality based on physiological and omics analysis.
Engineering intracellular microenvironment of LAB
Regulation of intracellular amino acid metabolism
Regulation of intracellular amino acid metabolism is a common mechanism utilized by LAB upon environmental stresses. Arginine deiminase (ADI) system is a widely reported regulative system in LAB during acid stress. This system converts arginine and subsequently leads to the production of NH3, CO2, and ATP. The generation of ATP enables extrusion of cytoplasmic protons by H+-ATPase (Burne and Marquis 2000). Previous research demonstrated acid stress induced the accumulation of arginine in Lactobacillus casei, and Streptococcus faecium could degrade arginine at extremely low initial pH of 2.5 and raise the pH to nearly 8.0 with 80 mM NH3 accumulation (Wu et al. 2012a). Moreover, the addition of arginine improved the survival of L. casei during acid stress by increasing the activity of H+-ATPase and intracellular ATP levels (Zhang et al. 2012b). An example with Streptococcus suis showed that knockout of arcABC encoding genes involved in the ADI system resulted in decreased ammonia production and decreased cell growth during acidic conditions (Fulde et al. 2011).
Intracellular accumulation of aspartate is another response induced by LAB during acid stress, and the biomass and survival at low pH were significantly improved in the presence of aspartate (Wu et al. 2013a). In addition, an aspartate-dependent acid survival system was also characterized in Yersinia pseudotuberculosis. The expression of aspartase (AspA), which catalyzed the deamination of aspartate to form fumarate and ammonia, increased acid survival of Y. pseudotuberculosis (Hu et al. 2010).
Regulation of branched-chain amino acids (BCAA) leucine, isoleucine, and valine was also an ATR in LAB. During acid stress, the enzymes (IlvA, IlvC2, IlvD, and IlvE) involved in BCAA metabolism were overproduced, and deamination of BCAA was postulated as a mechanism to maintain the internal pH of the cells (Sánchez et al. 2007; Ganesan and Weimer 2004). Knockout of the ilvE gene led to decreased F0F1-ATPase activity and acid tolerance in Streptococcus mutans (Santiago et al. 2012). In addition, decarboxylation was also reported to protect cells against acid damage by generation of ATP and consumption of a single proton (Higuchi et al. 1997). Trip et al. (2012) heterologously expressed the histidine decarboxylation pathway in L. lactis, and this pathway enabled cells to survive at low pH in the presence of histidine.
Introduction of exogenous biosynthetic pathway
Nowadays, a vast genetic toolbox for the regulation of LAB gene expression levels is available, allowing the manipulation of acid tolerance through metabolic engineering (Table 2). Previous researches showed that glutathione can protect LAB against a variety of environmental stresses (acid, oxygen, cold and salt stresses; Kim et al. 2012; Zhang et al. 2010a, b, 2012a; Li et al. 2003). To further investigate the protective roles of glutathione during stressed conditions, two genes gshA and gshB, encoding γ-glutamylcysteine synthetase and glutathione synthetase, respectively, from Escherichia coli, were expressed in L. lactis NZ9000. As expected, the recombinant strain exhibited higher resistance to acid stress compared to the control strain (Zhang et al. 2007; Fu et al. 2006).
Previous study showed that acid stress led to substantial accumulation of trehalose in Propionibacterium freudenreichii during acid stress (Cardoso et al. 2004). Inspired by this observation, Carvalho et al. (2011) introduced the P. freudenreichii trehalose de novo biosynthetic pathway into L. lactis to investigate the effect of trehalose production on the tolerance of host strain to acid stress. As expected, the mutant exhibited higher tolerance to acid (pH 3.0) and cold (4 °C) shock, as well as to heat stress (45 °C; Carvalho et al. 2011).
Overproduction of stress response proteins by genetic modification
With the development of genome sequencing and other high-throughput technologies, it has enabled us to engineer the robustness of industrial microbes at a global or systems biology levels (Zhu et al. 2012). At present, several systems biology approaches (e.g., genomics, transcriptomics, proteomics, metabolomics), combined with the molecular techniques have been employed to further understand the physiological mechanisms of LAB, and based on these, strategies to improve the physiological functions and engineer stress tolerance of LAB were proposed (Fig. 2). For example, Broadbent et al. (2010) investigated the acid stress response of L. casei ATCC334 during acid stress by transcriptional analysis, and the results showed that the two genes involved in malolactic fermentation (mleS, malolactic enzyme; mleP, malate/lactate antiporter) and eight genes cluster for histidine biosynthesis (LSEI_1426–1434) were significantly upregulated. To further validate the microarray data, 30 mM malate or 30 mM histidine were supplemented into the acid challenge medium, and the presence of either malate or histidine in the medium at pH 2.5 resulted in a more than 100-fold increase in cell survival after 60-min incubation, and greater than 107-fold improvement after 2 h (Broadbent et al. 2010).
Generally, bacteria maintain protein homeostasis under normal or stressed conditions using various mechanisms including the action of a group of regulatory proteins. Likely, LAB upregulated the expression of general stress response proteins in response to environmental stress (Wu et al. 2011, 2012a), among which molecular chaperones and DNA repair proteins were widely investigated (Table 3). Heterologous expression of dnaK from E. coli in L. lactis NZ9000 resulted in improved tolerance to lactic acid, NaCl, and ethanol stresses (Abdullah-Al-Mahin et al. 2010). Tian et al. (2012) expressed a small shock protein (shsp) gene from Streptococcus thermophilus in L. lactis, and the recombinant strain displayed significantly higher survival rate under acid, heat, ethanol, bile salt, and H2O2 stresses. In addition, comparative proteomic analysis with L. casei parental strain and its acid-resistant mutant demonstrated that higher expression of DNA repair proteins (e.g., MutL, MutS2, UvrC, RecO) were observed in the mutant. Engineering the overproduction of DNA repair protein RecO in L. lactis NZ9000 was carried out, and the recombinant strain exhibited higher tolerance to lactic acid, salt, and H2O2 stresses (Wu et al. 2013b).
The whole-genome sequencing has yielded increasing numbers of completed genomes of LAB, many of which are publicly available on the Internet. This allows us to characterize their gene expression profiles and to identify the genes during environmental stresses. In addition, it provides an effective platform for us to engineer LAB with improved robustness. However, it should be noted that the strains obtained by genetic engineering may be hampered by legal issues and the general public opinion during industrial application. Therefore, further efforts should be made to ensure the acceptability of recombinant LAB in industrial manufacture, especially in food industry.
Engineering cell membrane functionality
As the first barrier of the cell, cell membrane separates cells from their environments and is a primary target for damage during environmental stresses. Changes in the cell membrane can protect the cell from environmental damage by modifying the physicochemical properties of membrane (Mykytczuk et al. 2007). Upon acid stress, L. casei increased the fluidity of cell membrane and increased the proportions of monounsaturated fatty acids, as well as mean chain length (Wu et al. 2012b). Therefore, engineering the production of unsaturated fatty acids could be a potential method to increase the acid tolerance of LAB. FabM, a novel enzyme, responsible for the production of monounsaturated fatty acids, was identified in S. mutans, and the FabM-defective mutant was extremely sensitive to acid stress compared with the wild type (Fozo and Quivey 2004). However, the acid-sensitive phenotype was relieved by growth in the presence of monounsaturated fatty acids or through genetic complementation (Fozo and Quivey 2004). In addition, production of cyclopropane fatty acids (CFA) was also a general stress response to acid stress (Broadbent et al. 2010; Wu et al. 2012b). Previous work with E. coli demonstrated that the CFA-defective mutant exhibited decreased resistance to acid stress, and the acid tolerance was restored by incorporation of a functional cfa gene (Chang and Cronan 1999). Conversely, recent work with L. lactis subsp. cremoris wild-type strain, the cfa mutant, and the complemented strain showed that the cyclopropanation of unsaturated fatty acids was not essential for survival under acidic conditions (To et al. 2011). Thus, further investigation concerning the detailed protective mechanisms of CFA during acid stress is necessary.
Adaptive evolution
Adaptive evolution, as a convenient approach to study many microbial phenomena, such as the emergence of new pathogens and the acquisition of environmental resistance factors, can address fundamental question on adaptation to selection pressures and evolution, and it has also become a widely used tool for biotechnological applications, improving yields and reducing costs in industrial settings (Fig. 3; Portnoy et al. 2011; Fong et al. 2005; Wang et al. 2011). Recently, adaptive evolution has been used with great success to gain insight into the genetic basis and dynamics of adaptation (Teusink et al. 2009). An example with L. casei, Zhang et al. (2012b) isolated acid-resistant mutant lb-2 by adaptive evolution for 70 days, and the evolved mutant exhibited higher biomass and survival during acid stress. Analysis of the intracellular microenvironments showed that the acid tolerance mutant displayed higher intracellular pH and NH4 + concentration in acidic conditions. In addition, at least 40.0 % and 23.9 % higher contents of intracellular arginine and aspartate were observed in the mutant compared with that in the parental strain (Zhang et al. 2012b). Moreover, proteomic analysis showed that higher expressions of many proteins including chaperonin (groEL), DNA repair protein (RecO) were observed in the evolved strain, and the overproduction of RecO in L. lactis led to increased tolerance to acid and NaCl stresses (Wu et al. 2012a, 2013b). These results suggest that adaptive evolution might be a useful method to engineer robust LAB.
Application of adaptive evolution. a Investigation of the mechanisms to environmental adaptation. b Engineering cellular metabolism for enhanced biosynthetic capacity of desired product (a) and decreased amount of by-product (b). c Improved robustness to environmental stresses. d Schematic representation of the procedure for adaptive evolution
Pre-adaptation and cross-protection
In response to environmental stress, LAB employ sophisticated mechanisms to combat stress. In many cases, similar responses were induced during different stressful conditions (heat, acid, oxygen, and cold), and as such, these mechanisms of resistance were interconnected. In this respect, preadaptation (pretreatment of a strain to a sublethal level can improve its resistance toward a potential severe stress) and cross-protection (one kind of stress tolerance confers protection against other stresses). Notably, Broadbent et al. (Broadbent et al. 2010) enhanced the survival of L. casei ATCC 334 to severe acidic conditions (pH 2.0) by prior exposure of the cells at pH 4.5 for 20 min. Moreover, a dramatic increase in survival to a severe acid stress (pH 3.9) was obtained by pre-exposing the L. lactis subsp. lactis cells for 30 min to a mildly acid shock at pH 5.5 (Hartke et al. 1996). Cross-protection was also reported as an effective approach to increase the acid stress resistance of LAB. For example, L. plantarum pre-exposed to sublethal heat treatment displayed enhanced growth at pH 5.0 (De Angelis et al. 2004). Generally, pre-exposure to mild acidic condition induced an ATR, which protected cells against multiple-environmental stresses. Pre-exposure of L. lactis subsp. cremoris to sublethal acid treatment displayed enhanced tolerance to acid, heat, NaCl, H2O2, and ethanol stresses (O'Sullivan and Condon 1997). In conclusion, pre-adaptation and cross-protection led to significant improvement of LAB to acid stress. However, the exact molecular mechanisms involved in pre-adaptation and cross-protection are not fully understood, and further exploration is also needed.
Exogenous addition of protectants
Exogenous addition of protectants is a relatively straightforward way to protect cells against acid stress or improve acid tolerance of LAB. Recently, numerous protectants have been developed to protect LAB against acid stress including amino acids, fatty acids, and saccharides (Table 3). For example, the addition of arginine increased the survival of L. casei Zhang at low pH (Zhang et al. 2012b). Physiological analysis showed that the exogenous arginine improved the viability of cells during acid stress by increasing the H+-ATPase activity and intracellular ATP level (Zhang et al. 2012b). Generally, regulation of ADI system is a widely reported ATR in a variety of bacteria including LAB, E. coli, S. mutans, Listeria monocytogenes, and Bacillus spp. (Zhao and Houry 2010; Senouci-Rezkallah et al. 2011; Matsui and Cvitkovitch 2010; Ryan et al. 2009). In another study with L. casei, aspartate was supplemented into the MRS media, and this led to the increment of the biomass and survival during acid stress (Wu et al. 2013a). The subsequent analysis of the intracellular microenvironment revealed that higher concentrations of intermediates involved in glycolysis and tricarboxylic acid cycle were observed, and L. casei shifted the metabolic pathway by increasing the flux from aspartate to arginine (ADI system) and decreasing the flux from aspartate to asparagine (Wu et al. 2012a, 2013a). Yet another example revealed that the addition of Tween-80 to the growth medium of L. rhamnosus resulted in 1,000-fold higher survival during exposure to gastric juice (Corcoran et al. 2007). Analysis of the fatty acids composition of L. rhamnosus revealed a 55-fold higher oleic acid content and a significantly higher unsaturated/saturated fatty acids ratio in the membrane of cells in the presence of Tween-80 (Corcoran et al. 2007). These results suggest that exogenous addition of protectants could be a feasible strategy to improve acid stress resistance of LAB.
Concluding remarks
Acid stress is a common environmental challenge to LAB, and improving the acid stress resistance is crucial to the application of LAB as probiotic. The advent of genome sequencing has increase our understanding of the molecular biology of LAB, and based on this, many post-genomic approaches (such as omics method) have accelerated the identification of genes/proteins involved in stress response and tolerance. This knowledge contributes to the design of rational approaches to engineering LAB with increased robustness. Moreover, specific fermentation conditions may be employed on the basis of the understanding of characterization of LAB to increase the stress tolerance. In conclusion, these systems biology approaches combined with the molecular techniques have provided us more opportunities to engineering LAB with improved robustness and industrial functionality.
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Sugimoto S, Al-Mahin A, Higashi C, Matsumoto S, Sonomoto K (2010) Improvement of multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 under conditions of thermal stress by heterologous expression of Escherichia coli dnaK. Appl Environ Microb 76(13):4277–4285
Berezina OV, Zakharova NV, Brandt A, Yarotsky SV, Schwarz WH, Zverlov VV (2010) Reconstructing the clostridial n-butanol metabolic pathway in Lactobacillus brevis. Appl Microbiol Biotechnol 87(2):635–646
Beshkova D, Frengova G (2012) Bacteriocins from lactic acid bacteria: microorganisms of potential biotechnological importance for the dairy industry. Eng Life Sci 12(4):419–432
Bongers RS, Hoefnagel MH, Kleerebezem M (2005) High-level acetaldehyde production in Lactococcus lactis by metabolic engineering. Appl Environ Microbiol 71(2):1109–1113
Broadbent JR, Larsen RL, Deibel V, Steele JL (2010) Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J Bacteriol 192:2445–2458
Burgess C, O'Connell-Motherway M, Sybesma W, Hugenholtz J, Van Sinderen D (2004) Riboflavin production in Lactococcus lactis: potential for in situ production of vitamin-enriched foods. Appl Environ Microbiol 70(10):5769–5777
Burne R, Marquis R (2000) Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193(1):1–6
Cardoso FS, Gaspar P, Hugenholtz J, Ramos A, Santos H (2004) Enhancement of trehalose production in dairy propionibacteria through manipulation of environmental conditions. Int J Food Microbiol 91(2):195–204
Carvalho AL, Cardoso FS, Bohn A, Neves AR, Santos H (2011) Engineering trehalose synthesis in Lactococcus lactis for improved stress tolerance. Appl Environ Microbiol 77:4189–4199
Chang YY, Cronan JE (1999) Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol 33(2):249–259
Cho SY, Park MJ, Kim KM, Ryu J-H, Park HJ (2011) Production of high γ-aminobutyric acid (GABA) sour kimchi using lactic acid bacteria isolated from Mukeunjee kimchi. Food Sci Biotechnol 20(2):403–408
Claudia S (2008) Contribution of citrate metabolism to the growth of Lactococcus lactis CRL264 at low pH. Appl Environ Microbiol 74(4):1136
Corcoran B, Stanton C, Fitzgerald G, Ross R (2005) Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol 71(6):3060–3067
Corcoran B, Stanton C, Fitzgerald G, Ross R (2007) Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 153(1):291–299
Cui F, Li Y, Wan C (2011) Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresource Technol 102(2):1831–1836
De Angelis M, Bini L, Pallini V, Cocconcelli PS, Gobbetti M (2001) The acid-stress response in Lactobacillus sanfranciscensis CB1. Microbiology 147:1863–1873
De Angelis M, Di Cagno R, Huet C, Crecchio C, Fox P, Gobbetti M (2004) Heat shock response in Lactobacillus plantarum. Appl Environ Microbiol 70(3):1336–1346
De Boeck R, Sarmiento-Rubiano LA, Nadal I, Monedero V, Pérez-Martínez G, Yebra MJ (2010) Sorbitol production from lactose by engineered Lactobacillus casei deficient in sorbitol transport system and mannitol-1-phosphate dehydrogenase. Appl Microbiol Biotechnol 85(6):1915–1922
Desmond C, Ross R, O'Callaghan E, Fitzgerald G, Stanton C (2002) Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J Appl Microbiol 93(6):1003–1011
Fong SS, Joyce AR, Palsson BO (2005) Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res 15(10):1365–1372. doi:10.1101/gr.3832305
Fozo EM, Quivey RG (2004) The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J Bacteriol 186(13):4152–4158. doi:10.1128/jb.186.13.4152-4158.2004
Fu RY, Bongers RS, Van Swam II, Chen J, Molenaar D, Kleerebezem M, Hugenholtz J, Li Y (2006) Introducing glutathione biosynthetic capability into Lactococcus lactis subsp. cremoris NZ9000 improves the oxidative-stress resistance of the host. Metab Eng 8(6):662–671
Fulde M, Willenborg J, de Greeff A, Benga L, Smith HE, Valentin-Weigand P, Goethe R (2011) ArgR is an essential local transcriptional regulator of the arcABC operon in Streptococcus suis and is crucial for biological fitness in an acidic environment. Microbiol 157(2):572–582
Ganesan B, Weimer BC (2004) Role of aminotransferase IlvE in production of branched-chain fatty acids by Lactococcus lactis subsp. lactis. Appl Environ Microbiol 70(1):638–641
Gaspar P, Carvalho AL, Vinga S, Santos H, Neves AR (2013) From physiology to systems metabolic engineering for the production of biochemicals by lactic acid bacteria. Biotechnol Adv 31(6):764–788
Gaspar P, Neves AR, Gasson MJ, Shearman CA, Santos H (2011) High yields of 2, 3-butanediol and mannitol in Lactococcus lactis through engineering of NAD+ cofactor recycling. Appl Environ Microbiol 77(19):6826–6835
Guo T, Kong J, Zhang L, Zhang C, Hu S (2012) Fine tuning of the lactate and diacetyl production through promoter engineering in Lactococcus lactis. PLoS One 7(4):e36296
Hartke A, Bouche S, Giard JC, Benachour A, Boutibonnes P, Auffray Y (1996) The lactic acid stress response of Lactococcus lactis subsp. lactis. Curr Microbiol 33(3):194–199
Higuchi T, Hayashi HU, Abe K (1997) Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J Bacteriol 179(10):3362–3364
Hu Y, Lu P, Zhang Y, Li L, Chen S (2010) Characterization of an aspartate-dependent acid survival system in Yersinia pseudotuberculosis. FEBS Lett 584(11):2311–2314
Kim JE, Eom H-J, Kim Y, Ahn JE, Kim JH, Han NS (2012) Enhancing acid tolerance of Leuconostoc mesenteroides with glutathione. Biotechnol Lett 34(4):683–687
Ladero V, Ramos A, Wiersma A, Goffin P, Schanck A, Kleerebezem M, Hugenholtz J, Smid EJ, Hols P (2007) High-level production of the low-calorie sugar sorbitol by Lactobacillus plantarum through metabolic engineering. Appl Environ Microbiol 73(6):1864–1872
Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol R 72(4):728–764
Li Y, Hugenholtz J, Abee T, Molenaar D (2003) Glutathione protects Lactococcus lactis against oxidative stress. Appl Environ Microbiol 69(10):5739–5745
Liu S, Nichols NN, Dien BS, Cotta MA (2006) Metabolic engineering of a Lactobacillus plantarum double ldh knockout strain for enhanced ethanol production. J Ind Microbiol Biotechnol 33(1):1–7
Martínez-Cuesta M, Gasson M, Narbad A (2005) Heterologous expression of the plant coumarate: CoA ligase in Lactococcus lactis. Lett Appl Microbiol 40(1):44–49
Matsui R, Cvitkovitch D (2010) Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol 5(3):403–417
Mills DA, Rawsthorne H, Parker C, Tamir D, Makarova K (2005) Genomic analysis of Oenococcus oeni PSU-1 and its relevance to winemaking. FEMS Microbiol Rev 29(3):465–475
Monedero V, Pérez-Martínez G, Yebra MJ (2010) Perspectives of engineering lactic acid bacteria for biotechnological polyol production. Appl Microbiol Biotechnol 86(4):1003–1015
Mykytczuk NCS, Trevors JT, Leduc LG, Ferroni GD (2007) Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog Biophys Mol Bio 95(1–3):60–82
Nyyssölä A, Pihlajaniemi A, Palva A, Von Weymarn N, Leisola M (2005) Production of xylitol from d-xylose by recombinant Lactococcus lactis. J Biotechnol 118(1):55–66
O'Sullivan E, Condon S (1997) Intracellular pH is a major factor in the induction of tolerance to acid and other stresses in Lactococcus lactis. Appl Environ Microbiol 63(11):4210–4215
Parvez S, Malik KA, Kang SA, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100(6):1171–1185. doi:10.1111/j.1365-2672.2006.02963.x
Patel S, Majumder A, Goyal A (2012) Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol 52(1):3–12
Portnoy VA, Bezdan D, Zengler K (2011) Adaptive laboratory evolution—harnessing the power of biology for metabolic engineering. Curr Opin Biotech 22:590–594
Prasad SB, Ramachandran K, Jayaraman G (2012) Transcription analysis of hyaluronan biosynthesis genes in Streptococcus zooepidemicus and metabolically engineered Lactococcus lactis. Appl Microbiol Biotechnol 94(6):1593–1607
Ryan S, Begley M, Gahan CGM, Hill C (2009) Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol 11(2):432–445
Sánchez B, Champomier-Vergès MC, Collado MC, Anglade P, Baraige F, Sanz Y, de los Reyes-Gavilan CG, Margolles A, Zagorec M (2007) Low-pH adaptation and the acid tolerance response of Bifidobacterium longum Biotype longum. Appl Environ Microbiol 73(20):6450–6459
Santiago B, MacGilvray M, Faustoferri RC, Quivey RG Jr (2012) The branched-chain amino acid aminotransferase encoded by ilvE is involved in acid tolerance in Streptococcus mutans. J Bacteriol 194:2010–2019
Santos F, Vera JL, van der Heijden R, Valdez G, de Vos WM, Sesma F, Hugenholtz J (2008) The complete coenzyme B12 biosynthesis gene cluster of Lactobacillus reuteri CRL1098. Microbiol 154(1):81–93
Senouci-Rezkallah K, Schmitt P, Jobin M (2011) Amino acids improve acid tolerance and internal pH maintenance in Bacillus cereus ATCC14579 strain. Food Microbiol 28:364–372
Serrazanetti D, Guerzoni M, Corsetti A, Vogel R (2009) Metabolic impact and potential exploitation of the stress reactions in Lactobacilli. Food Microbiol 26:700–711
Sheehan V, Sleator R, Hill C, Fitzgerald G (2007) Improving gastric transit, gastrointestinal persistence and therapeutic efficacy of the probiotic strain Bifidobacterium breve UCC2003. Microbiol 153(10):3563–3571
Song AAL, Abdullah JO, Abdullah MP, Shafee N, Rahim RA (2012) Functional expression of an orchid fragrance gene in Lactococcus lactis. Int J Mol Sci 13(2):1582–1597
Song SH, Vieille C (2009) Recent advances in the biological production of mannitol. Appl Microbiol Biotechnol 84(1):55–62
Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, de Vos WM, Hugenholtz J (2003) Increased production of folate by metabolic engineering of Lactococcus lactis. Appl Environ Microbiol 69(6):3069–3076
Tanaka Y, Watanabe J, Mogi Y (2012) Monitoring of the microbial communities involved in the soy sauce manufacturing process by PCR-denaturing gradient gel electrophoresis. Food Microbiol 31(1):100–106
Teusink B, Wiersma A, Jacobs L, Notebaart RA, Smid EJ (2009) Understanding the adaptive growth strategy of Lactobacillus plantarum by in silico optimisation. Plos Comput Biol 5(6):e1000410
Tian H, Tan J, Zhang L, Gu X, Xu W, Guo X, Luo Y (2012) Increase of stress resistance in Lactococcus lactis via a novel food-grade vector expressing a shsp gene from Streptococcus thermophilus. Braz J Microbiol 43(3):1157–1164
To TMH, Grandvalet C, Tourdot-Maréchal R (2011) Cyclopropanation of membrane unsaturated fatty acids is not essential to the acid stress response of Lactococcus lactis subsp. cremoris. Appl Environ Microbiol 77(10):3327–3334
Trip H, Mulder NL, Lolkema JS (2012) Improved acid stress survival of Lactococcus lactis expressing the histidine decarboxylation pathway of Streptococcus thermophilus CHCC1524. J Biol Chem 287:11195–11204
Tsuji A, Okada S, Hols P, Satoh E (2013) Metabolic engineering of Lactobacillus plantarum for succinic acid production through activation of the reductive branch of the tricarboxylic acid cycle. Enzyme Microbiol Technol 53:97–103
Vaidyanathan H, Kandasamy V, Ramakrishnan GG, Ramachandran K, Jayaraman G, Ramalingam S (2011) Glycerol conversion to 1, 3-Propanediol is enhanced by the expression of a heterologous alcohol dehydrogenase gene in Lactobacillus reuteri. AMB Express 1(1):1–8
Wang Y, Manow R, Finan C, Wang J, Garza E, Zhou S (2011) Adaptive evolution of nontransgenic Escherichia coli KC01 for improved ethanol tolerance and homoethanol fermentation from xylose. J Ind Microbiol Biot 38:1371–1377
Warnecke T, Gill RT (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microbiol Cell Fact 4(1):25–32
Wu C, Zhang J, Chen W, Wang M, Du G, Chen J (2012a) A combined physiological and proteomic approach to reveal lactic-acid-induced alterations in Lactobacillus casei Zhang and its mutant with enhanced lactic acid tolerance. Appl Microbiol Biotechnol 93:707–722
Wu C, Zhang J, Du G, Chen J (2013a) Aspartate protects Lactobacillus casei against acid stress. Appl Microbiol Biotechnol 97:4083–4093
Wu C, Zhang J, Du G, Chen J (2013b) Heterologous expression of Lactobacillus casei RecO improved the multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 during salt stress. Bioresource Technol 143:238–241
Wu C, Zhang J, Wang M, Du G, Chen J (2012b) Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J Ind Microbiol Biotechnol 39:1031–1039
Wu R, Zhang W, Sun T, Wu J, Yue X, Meng H, Zhang H (2011) Proteomic analysis of responses of a new probiotic bacterium Lactobacillus casei Zhang to low acid stress. Int J Food Microbiol 147(3):181–187
Xiong T, Guan Q, Song S, Hao M, Xie M (2012) Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control 26(1):178–181
Xu W, Huang Z, Zhang X, Li Q, Lu Z, Shi J, Xu Z, Ma Y (2011) Monitoring the microbial community during solid-state acetic acid fermentation of Zhenjiang aromatic vinegar. Food Microbiol 28(6):1175–1181
Yan Y-z, Qian Y-l, Ji F-d, Chen J-y, Han B-z (2013) Microbial composition during Chinese soy sauce koji-making based on culture dependent and independent methods. Food Microbiol 34(1):189–195
Ye W, Huo G, Chen J, Liu F, Yin J, Yang L, Ma X (2010) Heterologous expression of the Bacillus subtilis (natto) alanine dehydrogenase in Escherichia coli and Lactococcus lactis. Microbiol Res 165(4):268–275
Zhang J, Du GC, Zhang Y, Liao XY, Wang M, Li Y, Chen J (2010a) Glutathione protects Lactobacillus sanfranciscensis against freeze–thawing, freeze-drying, and cold treatment. Appl Environ Microbiol 76(9):2989–2996
Zhang J, Fu R-Y, Hugenholtz J, Li Y, Chen J (2007) Glutathione protects Lactococcus lactis against acid stress. Appl Environ Microbiol 73(16):5268–5275
Zhang J, Li Y, Chen W, Du GC, Chen J (2012a) Glutathione improves the cold resistance of Lactobacillus sanfranciscensis by physiological regulation. Food Microbiol 31(2):285–292
Zhang J, Wu C, Du G, Chen J (2012b) Enhanced acid tolerance in Lactobacillus casei by adaptive evolution and compared stress response during acid stress. Biotechnol Bioproc E 17(2):283–289
Zhang Y, Zhang Y, Zhu Y, Mao S, Li Y (2010b) Proteomic analyses to reveal the protective role of glutathione in resistance of Lactococcus lactis to osmotic stress. Appl Environ Microbiol 76(10):3177–3186
Zhao B, Houry WA (2010) Acid stress response in enteropathogenic gammaproteobacteria: an aptitude for survival. Biochem Cell Biol 88(2):301–314
Zheng J, Liang R, Zhang L, Wu C, Zhou R, Liao X (2013) Characterization of microbial communities in strong aromatic liquor fermentation pit muds of different ages assessed by combined DGGE and PLFA analyses. Food Res Int 54(1):660–666
Zhu L, Zhu Y, Zhang Y, Li Y (2012) Engineering the robustness of industrial microbes through synthetic biology. Trends Microbiol 20(2):94–101
Zhu Y, Zhang Y, Li Y (2009) Understanding the industrial application potential of lactic acid bacteria through genomics. Appl Microbiol Biotechnol 83(4):597–610
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31171742, 31301546).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, C., Huang, J. & Zhou, R. Progress in engineering acid stress resistance of lactic acid bacteria. Appl Microbiol Biotechnol 98, 1055–1063 (2014). https://doi.org/10.1007/s00253-013-5435-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5435-3