Abstract
Metabolic flux analysis was used to reveal the metabolic distributions in Gluconacetobacter xylinus (CGMCC no. 2955) cultured on different carbon sources. Compared with other sources, glucose, fructose, and glycerol could achieve much higher bacterial cellulose (BC) yields from G. xylinus (CGMCC no. 2955). The glycerol led to the highest BC production with a metabolic yield of 14.7 g/mol C, which was approximately 1.69-fold and 2.38-fold greater than that produced using fructose and glucose medium, respectively. The highest BC productivity from G. xylinus CGMCC 2955 was 5.97 g BC/L (dry weight) when using glycerol as the sole carbon source. Metabolic flux analysis for the central carbon metabolism revealed that about 47.96 % of glycerol was transformed into BC, while only 19.05 % of glucose and 24.78 % of fructose were transformed into BC. Instead, when glucose was used as the sole carbon source, 40.03 % of glucose was turned into the by-product gluconic acid. Compared with BC from glucose and fructose, BC from the glycerol medium showed the highest tensile strength at 83.5 MPa, with thinner fibers and lower porosity. As a main byproduct of biodiesel production, glycerol holds great potential to produce BC with superior mechanical and microstructural characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant biopolymer in the world and is foundational to many industries including pulp and paper, textiles (Wan et al. 2006), bioenergy (Zhong et al. 2009a), and tissue engineering (Tamahkar et al. 2010). In addition to plants, various microorganisms (algae, fungi, and bacteria) are also able to produce cellulose, with notable bacterial cellulose (BC) producers including species of Acetobacter and Achromobacter (Klemm et al. 2005). BC is distinguished from plant-based cellulose by its high purity and does not contain residual hemicellulose or lignin (Klemm et al. 2006). Recently, BC has attracted more attention due to its high quality in mechanical strength, water-holding capacity, crystallinity, biodegradability, renewability, as well as biocompatibility (Zhu et al. 2011; Ha et al. 2011).
The substrates, especially the carbon source, govern the costs of microbial fermentation processes. So far with Acetobacter strains, only pure sugars (glucose, fructose, etc.) have been used as carbon sources to produce BC; however, this practice was found to be uneconomical (Keshk et al. 2006). Therefore, a number of investigations have been conducted to find economic feedstocks that are able to increase the economic efficiency of the fermentation process. Zeng et al. (2011) demonstrated that BC production with the addition of maple syrup was comparable to that using pure fructose as the carbon source. Fermentation efficiency has also been improved by addition of acetic acid to the medium, which is believed might counteract the pH decrease caused by gluconic acid formation, a major byproduct of cellulose production (Toda et al. 1997). Nguyen et al. (2008) reported the Gluconacetobacter xylinus strain isolated from kombucha gave a maximum cellulose production of 2.64 g/L with mannitol (20 g/L) and corn steep liquor (40 g/L) as the carbon sources. After 7 days of cultivation, the BC yield and crystallinity index were 380 % and 9 % higher when glycerol was used as the sole carbon source rather than glucose (Jung et al. 2010). A previous study indicated that several carbon sources could support the production of BC by G. xylinus strain ATCC 53524 (Mikkelsen et al. 2009). In that study, the cellulose productivity, cellulose purity, and structural features were compared across a large group of carbon sources including glucose, mannitol, glycerol, fructose, sucrose, and galactose. The various carbon sources led to differences in BC productivity and the molecular and microscopic structure of the cellulose. However, possible reasons for the observed differences still need be elucidated. Metabolic flux analysis was widely applied to estimate intracellular fluxes and improve production of desired products (Schaub et al. 2008; Huang et al. 2012; Ma et al. 2012). Up to now, there is limited knowledge regarding the metabolism of existing strains of Gluconacetobacter, which makes it difficult to understand the metabolic network and relate it to the production of interested products (such as acetic acid, cellulose, etc.) (Velasco-Bedran et al. 2007).
In this study, BC was produced using G. xylinus (CGMCC no. 2955) grown on various carbon sources, including glucose, inositol, lactose, sucrose, maltose, starch, ethanol, and fructose. The metabolic productivity in G. xylinus (CGMCC no. 2955), as well as mechanical properties and microstructure of BCs by various carbon sources were compared. Differences in flux distributions during fermentation on the various carbon sources were investigated using metabolic flux analysis.
Materials and methods
Microorganism and culture conditions
G. xylinus (CGMCC no. 2955) was used in this study as described previously (Zhu et al. 2011) and was isolated by our group and deposited in China General Microbiological Center Collection with the registered number, no. 2955. The components of the culture medium were as follows: carbon source (0.833 mol C/L), peptone (10 g/L), yeast extract (7.5 g/L), and disodium phosphate (10 g/L). Mixed carbon source medium contained the same components as the culture medium except the carbon source, which consisted of glucose, fructose, and glycerol with a ratio of 1:1:1 (w/w/w) to a final concentration of 25 g/L. G. xylinus (CGMCC no. 2955) was inoculated into 100 mL of inoculum culture medium (using glucose as the carbon source) in a 500 mL flask. The flask was incubated at 30 °C and 160 rpm for 24–30 h, after which the cell suspension was inoculated into culture medium of a different carbon source at a ratio of 8 % (v/v).
For the metabolic flux analysis, a chemically defined medium was prepared that contained the specified sugar (25 g/L), Na2HPO4 (3 g/L), KH2PO4 (1 g/L), (NH4)2SO4 (5 g/L), MgCl2 (0.02 g/L), CaCl2 (0.02 g/L), and para-aminobenzoic acid (0.0015 g/L). The initial pH of the medium was adjusted to 6.0. The pH of the medium was recorded every 24 h over the 96-h experimental period using a PHSJ-4A basic benchtop pH meter (Leici Corporation, China).
BC harvest and cell dry weight
Harvest of BC was described before with some modifications (Wan et al. 2006). After incubation, BC produced at the gas–liquid interface was harvested and rinsed with distilled water to remove excess media and organisms and then placed immediately in NaOH (0.1 mol/L) boiling bath for 20 min to inactivate the attached bacterial cells. After boiling, BC was purified by extensive washing in distilled water at room temperature until the pH of the water became neutral. The BC was then dried on a Teflon plate at 80 °C for 10 h until it reached a stable weight, and the dry weight was recorded for each pellicle at room temperature.
Cell dry weight was determined using 10-mL cell suspensions with five replicates. Samples were centrifuged at 10,000 rpm, washed twice with 30 mL of distilled water, and then dried at 80 °C for 10 h to reach a stable weight.
The consumption of carbon sources
Consumption of carbon sources was determined by measuring sugars remaining in the medium using an Agilent high-performance liquid chromatography (HPLC). The HPLC was run on an Aminex HPX-87H column (Biorad, CA, USA) with a differential refractometer detector at a flow rate of 0.6 mL/min at 50 °C, with 5 mM H2SO4 as the mobile phase.
Measurement of acetic acid
Acetic acid concentration in the media was analyzed using a Waters HPLC with a UV detector at 230 nm. A Shodex NH2P-50 column (150 × 4.6 mm, Showa Denko K.K., Japan) was mounted using 0.05 M KH2PO4 as the mobile phase with a flow rate of 1 mL/min. The injection volume is 20 μL. Samples were taken after fermentation and centrifuged at 10, 000 rpm for 5 min. The supernatant was diluted and filtered with 0.22-μm filter for further analysis.
Tensile strength
After incubation, BC at the gas–liquid interface was harvested and washed with distilled water, and then dried on a Teflon plate. The computer-controlled tensile tester (CMT4503, Shenzhen Xinsansi Material Determination Ltd. Co., China) was used to measure the tensile strengths of the BC. Rings of BC (10 mm in length, 1 mm in width) were cut and stretched radially. The measurements were made at a velocity of 5 mm/s. The thickness of the BC was measured at ten different positions with a thickness gauge (CH-1-ST, Shanghai, China), and the values were averaged.
Scanning electron microscopy
The BC was freeze-dried, and the prepared samples were coated with gold palladium to avoid electrical charging using SC7620 (FISON Corp., Britain), as described in previous studies (Tamahkar et al. 2010; Tang et al. 2010). Scanning electron microscopy (SEM) was conducted using a LEO 1530VP microscope (LEO Corp., Deutschland) at an accelerating voltage of 10 kV.
Fourier transformed infrared (FT-IR) spectroscopy
The BC was freeze-dried and crushed into powdered form, mixed with KBr, and pressed into a small tablet. The Fourier transformed infrared (FT-IR) spectrum of the BC was measured at wave numbers ranging from 4,000 to 400 cm−1 with a VECTOR 22 FTIR spectrometer (Bruker Corp., Deutschland) (Shezad et al. 2010).
X-ray diffractometry
X-ray measurements were carried out to analyze the change in crystallinity of the BC using a Bruker D8 Advance X-ray diffractometer (Bruker-AXS Corp, Deutschland ) with reflection geometry and CuKα radiation (λ = 0.154 nm), operated at 40 kV, and 30 mA. The scanning was made through 2θ = 10–30° with a scanning speed of 10°/min. The crystallinity was calculated from the diffracted intensity data using the method of Segal et al. (1959), where the crystallinity index, CrI = (I 020 − I am)/I 020; I 020 is the maximum intensity of the lattice diffraction, and I am is the intensity at 2θ = 18°.
Metabolic flux analysis
The reaction network of central metabolism was constructed as described by Ross et al. (1991) and Tonouchi et al. (2003). The accumulation rate of a metabolite is given by the summation of all reactions producing that metabolite minus the summation over all reactions consuming that metabolite—r i (t) = \( \sum\limits_j {{\alpha_j}{x_j}(t)} -\sum\limits_k {{\alpha_k}{x_k}(t)} \). Where x j (t) is the rate or flux through reaction j, α j is a stoichiometric coefficient, and r i (t) is the accumulation rate of metabolite i. The set of equations formed from such balances constructed for each metabolite in the network is represented in matrix notation by Ax(t) = r(t) (1). Where A is an m × n matrix of stoichiometric coefficient, x(t) an n-dimensional flux vector, and r(t) an m-dimensional metabolite accumulation rate vector. The biochemical reaction equations were shown in the Supplementary material 1. Here, we used LINGO to solve the equations and obtain the values of metabolic fluxes.
Results
Effect of carbon source on BC production
The effects of different carbon sources on BC yields and final pH are shown in Fig. 1. At the end of fermentation process, the highest production of BC was obtained at 5.97 g/L from glycerol, followed by 4.97 g/L from glucose and 3.99 g/L from fructose. The BC yields from the other carbon sources (inositol, lactose, sucrose, maltose, starch, and ethanol) were relatively low.
Final BC concentration and pH in G. xylinus (CGMCC no. 2955) fermentation using different carbon sources: Culture medium (grams per liter): carbon source 25.00, peptone 10.00, yeast extract 7.50, disodium phosphate 10.00. The initial pH of all media was 6.0. G. xylinus (CGMCC no. 2955) was inoculated into 100 mL of culture medium in a 500 mL flask with shaking at 160 rpm and cultured at 30 °C for 24–30 h. The cell suspension was inoculated into the culture medium at a ratio of 8 % (v/v), and 30 mL of inoculated media was poured into 90-mm (i.d.) Petri dishes, and static cultured at 30 °C for 5 days. This experiment was repeated three times, and data correspond to the mean values ± standard deviation
Considering the relatively high BC yields from G. xylinus (CGMCC no. 2955) grown in glucose, fructose, and glycerol, further investigations were focused on these three carbon sources. These carbon sources were added in the same amount in terms of C moles. The pH control for the culture medium was extremely critical for high BC productivity (Vandamme et al. 1998; Park et al. 2006). When glucose was used as the sole carbon source, the pH of the culture medium decreased sharply from 6.0 to 3.5 in the first 2 days, then increased slightly and kept consistent at 4.1 until harvest (Fig. 2). This decrease is attributed to the formation of gluconic acid as a byproduct in the medium (Toda et al. 1997). In contrast, the pH in the glycerol medium and fructose medium fluctuated between 6.0 and 7.0, during fermentation process. The highest yield of BC was obtained at 6.05 g/L by using glycerol as the sole carbon source (Fig. 2). It is also found that glucose as the sole carbon source gave a higher consumption (almost completely depleted after 4 days, in Fig. 3). However, only 54.7 % and 48.9 % of fructose and glycerol were utilized, respectively. In terms of metabolic yields (grams BC per mole C), the glycerol gave the highest metabolic yields of BC at 14.7 g/mol C, which was approximately 1.69-fold and 2.38-fold of that of the fructose and glucose medium, respectively (Table 1).
Changes in BC concentration and pH profiles during G. xylinus (CGMCC no. 2955) fermentation from different carbon sources: Culture medium (grams per liter): glucose 25.0/fructose 25.0/glycerol 25.6, peptone 10.0, yeast extract 7.5, disodium phosphate 10.0; the initial pH of all media was 6.0. G. xylinus (CGMCC no. 2955) was inoculated into 100 mL of culture medium in a 500-mL flask with shaking at 160 rpm and cultured at 30 °C for 24–30 h. The cell suspension was inoculated into the culture medium at a level of 8 % (v/v). The inoculated 30 ml culture media was incubated at 30 °C for 6 days under static conditions using 90 mm Petri dishes. Cellulose pellicles were harvested every 24 h over a 144 h fermentation process
The carbon source consumption and BC yield during fermentation by G. xylinus (CGMCC no. 2955) using different carbon sources. Culture medium (grams per liter), sole carbon source: glucose 25/fructose 25/glycerol 25.6; mixed carbon source: 24.99 (glucose/fructose/glycerol, 1:1:1 w/w/w), peptone 10.00, yeast extract 7.50, disodium phosphate 10.00; the initial pH was 6.0
When BC was produced using glycerol and fructose, the acidic compounds in the growth medium were utilized through tricarboxylic acid cycle (TCA) cycle to generate energy and promote cell growth (Jung et al. 2010). So the pH of the growth media increased after incubation. As shown in Fig. 3, the BC production (5.30 g/L) obtained in mixed carbon source medium was lower than that using glycerol as the sole carbon source. After 4 days of fermentation, the consumption percentages of glucose, fructose, and glycerol were 99 %, 10 %, and 85 %, respectively. Based on the metabolic yields of BC from different carbon sources (Table 1), together with their consumption percentage in mixed carbon source medium, we obtain a theoretical yield from mixed carbon source medium (5.31 g/L) by summing each of the other three carbon sources (Table 1), which was close to the actual cellulose production (5.30 g/L) in the mixed carbon source medium. This result suggested that BC could be produced efficiently by adding a combination of several different carbon sources (Table 1).
Metabolic flux analysis
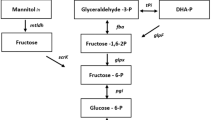
Table 2 lists the precursor requirements of G. xylinus (CGMCC no. 2955). The metabolic network of BC from glucose, fructose, and glycerol was shown in Fig. 4, phosphofructokinase is one of the key enzymes in the Embden Meyerhof pathway (EMP), but it is absent or presents low activity in Acetobacter xylinum (Schramm et al. 1957). So there is no pathway from fructose-6-phosphate to phosphoglyceraldehyde (GAP). The flux distributions of carbon sources such as glucose, fructose, and glycerol are shown in Fig. 4. It was indicated that 19.05 % of glucose was incorporated into BC, and 40.03 % of glucose was fluxed into the byproduct gluconic acid. In comparison, about 24.78 % and 47.96 % of fructose and glycerol were used for BC synthesis, respectively. The results of metabolite flux analysis matched well with the experimental results. Therefore, from the metabolic flux analysis, it is also shown that glycerol was the best carbon source for BC production with efficient conversion (47.96 %) to the desired product.
Metabolic network of G. xylinus (CGMCC no. 2955) cultured from different carbon sources (glucose, fructose, and glycerol). Network reaction of central metabolism was constructed as described by Ross and Tonouchi (Ross et al. 1991; Tonouchi et al. 2003). Metabolic flux analysis was carried out for the calculation of volumetric rates of formation of intracellular metabolites. Acronym definition: GLC glucose; FRU fructose, GLY glycerol, GLC6P glucose-6-phosphate, GLC1P glucose-1-phosphate, UDPG uridine diphosphoglucose, BC bacterial cellulose, GLCA gluconate, RIBU5P ribulose-5-phosphate XYL5P xylulose-5-phosphate, RIB5P ribose-5-phosphate, GAP glyceraldehyde-3-phosphate, SED7P sedoheptulose-7-phosphate E4P erythrose-4-phosphate, FRU6P fructose-6-phosphate, G3P 3-phosphoglycerate, PEP phosphoenol pyruvate, PYR pyruvate, AC acetate, ACP acetyl phosphate, ACCOA acetyl-coenzyme A, AKG α-keto-gluterate, SUCC succinate, OAA oxaloacetate, FAD flavin adenine dinucleotide, FADH reduced flavin adenine dinucleotide, NAD nicotinamide adenine dinucleotide, NADH reduced nicotinamide-adenine dinucleotide, NADP nicotinamide adenine dinucleotide phosphate, NADPH triphosphopyridine nucleotide
Effects of carbon sources on BC mechanical properties and microstructures
The tensile strength of the dry BC from the glycerol medium was 83.5 MPa, which is much higher than that from either glucose or fructose medium (shown in Fig. 5). However, the dry BC from the glucose medium had the highest elongation-to-break, followed by that from glycerol and then from fructose. It was shown by FT-IR spectroscopy (Fig. 6) that BC obtained from glycerol, fructose, and mixed carbon source medium had similar spectra to that from glucose. The samples elicited a band at around 2,895 cm−1 corresponding to the C–H bond of typical cellulose type I. The bands at around 1,060 cm−1 corresponded to the C–O–C and C–O–H stretching vibration of cellulose. The bands at around 3,350 cm−1 and 1,428 cm−1 corresponded to the O–H stretching frequencies and the C–H stretching vibration of cellulose, respectively. The band at 3,400 cm−1 is attributed to the intramolecular hydrogen bond for 3O…H-O5 (Oh et al. 2005; Jung et al. 2010) and is particularly useful for elucidating hydrogen-bonding patterns, because, in favorable cases, each distinct hydroxyl group gives a single stretching band at a frequency that decreases with increasing strength of hydrogen bonding (Sturcová et al. 2004). In this study, this band became broader in BC from the glycerol medium, indicating there were more hydrogen-bonding patterns in BC from the glycerol medium (Fig. 6).
The X-ray diffraction patterns were obtained to compare the microstructural changes in BC from the glucose, fructose, glycerol, and mixed carbon sources. The (1–10), (110), and (020) planes particular to cellulose I occur at 2θ—14.4°, 16.4°, and 22.7° (UI-Islam et al. 2012). Diffraction peaks at 14.4° and 22.7° are assigned to the cellulose I α and I β phases (Braud 2007). The crystallinity index values of BC produced in the glycerol and glucose media were 85.03 % and 80.50 %, respectively (Fig. 7a).
The micro-architecture of cellulose pellicles was investigated by SEM (Fig. 7b). Micrographs obtained from these samples revealed a densely packed network of cellulose fibers with a diameter of approximate 60–200 nm. Compared with other mediums, cellulose obtained from glycerol medium appeared to have slightly thinner fibers and lower porosity (Fig. 7b).
Discussion
Revealing the metabolic differences by different carbon sources
Synthesis of BC is a precisely and precisely regulated multi-step process, which includes two main operative amphibolic pathways (Ross et al. 1991; Schramm Gromet and Hestrin 1957): the pentose phosphate cycle (also known as hexose monophosphate pathway (HMP)) for the oxidation of carbohydrates and the Krebs cycle (also known as TCA) for the oxidation of organic acid with related compounds. It was reported by Mikkelsen et al. (2009) that relatively low concentrations of BC were produced by G. xylinus ATCC 53524 when sucrose was used as the sole carbon source. It is possibly due to the inability of the organism to transport sucrose through the cell membrane, which means it would need hydrolyzation in the periplasm to glucose and fructose (Velasco-Bedran et al. 2007). Matsuoka et al. (1996) suggested that lactose could stimulate the cell growth by diverting the flow of sugar within the metabolic pathway from BC production into the TCA cycle (Matsuoka et al. 1996; Jung et al. 2010). Lactate mostly functions as an energy source rather than a substrate for the BC biosynthesis (Naritomi et al. 1998). Ethanol is believed to generate reduced forms of nicotinamide adenine dinucleotide, which lowers the redox potential to levels necessary for BC production (Heo and Son. 2002). It has been also suggested that ethanol functions as an energy source for ATP generation during the early stage of the fermentation in the HMP pathway (Yunoki et al. 2004; Li et al. 2012). When ethanol was supplemented to the culture medium, it led to a reduction in glycerol production and stimulated the production of BC (Heo and Son 2002).
Glucose was easily transported through the cell membrane and incorporated into the cellulose biosynthetic pathway (Ross et al. 1991; Oikawa et al. 1997), so glucose was consumed rapidly in the first 4 days of fermentation (Fig. 3). The problem on using glucose as a carbon source is the formation of gluconic acid as a byproduct by a membrane-bound dehydrogenase, which will decrease the pH of the culture and ultimately lower BC production (Fig. 2) (Toda et al. 1997). By knocking out the glucose dehydrogenase gene, a mutant stain named GD-I produced 1.7 times more BC compared with the parent strain G. xylinus BPR 2001 (Shigematsu et al. 2005).
Pyruvate, dicarboxylic acids, and intermediates of the pentose phosphate cycle enter the Krebs cycle. On the other hand, gluconeogenesis takes place from pyruvate, via glyceraldehyde-3-phosphate to hexoses, and then to the synthesis of BC. For glycerol to be utilized for BC synthesis, which was reported to be the highest cellulose yield of 4.98 g/L in Hestrin and Shramm (HS) media by Acetobacter sp. V6 (Jung et al. 2010). Weinhouse et al. indicated that glycerol switched the pathway from the pentose cycle to the Krebs cycle, which used oxidation of triose phosphate and further produced BC without the formation of gluconic acid (Weinhouse and Benziman 1976; Ross et al. 1991). This could explain the high efficiency in BC production and smaller pH fluctuation (Fig. 2) when glycerol was used as the sole carbon source compared to glucose. Ruka et al. found that glycerol was consumed by G. xylinus ATCC 53524 to produce cellulose in the HS and Son media, but ineffective in Yamanak, Zhou, and CSL media (detailed information in the reference; Ruka et al. 2012). It was inferred that disodium hydrogen phosphate may activate or increase some enzyme activity in glycerol metabolism because it existed in both HS and Son media, rather than the other three. In a previous study, when ethanol or sodium citrate was added to the medium, the accumulated glycerol could enter the gluconeogenesis pathway and BC synthesis pathway in the later stage of fermentation (Li et al. 2012), which matched well with our result. It indicated from Fig. 4 that glycerol favored more metabolic flux into gluconeogenesis pathway, resulting in high productivity of BC.
Fructose is phosphorylated to fructose 6-phosphate by the phosphotransferase system, which uses phosphoenol pyruvate as the phosphate donor with the concomitant production of pyruvate. Gluconeogenesis can occur from oxaloacetate via pyruvate by means of the enzymes including oxaloacetate decarboxylase and pyruvate phosphate dikinase, which is similar to that of glycerol (Ross et al. 1991). Cannon et al. inferred that A. xylinum usually converted glycerol to pyruvate with an efficiency around 50 % (Cannon and Anderson 1991), which matched well with our results.
Comparison of metabolic flux analysis revealed that there are many differences in metabolic distributions in G. xylinus (CGMCC no. 2955) from glucose, fructose, and glycerol medium. It is indicated in Fig. 4 that most of glucose was fluxed into gluconic acid (r24, 92.85 %). Although more than half of it (r25, 52.83 %) was converted into glucose-6-phosphate (GLC6P in Fig. 4) by HMP pathway. The metabolic byproducts are widely branched from the HMP pathway, Embden-Meyerhof-Parnas pathway and TCA cycle (Ross et al. 1991). Therefore, only a small amount of carbon source (19.05 %) was fluxed into the desired product BC. It showed higher flux into BC from fructose medium (24.78 %) compared with that from glucose medium (19.05 %). However, BC productivity from fructose (4.03 g/L) was lower than that from glucose (5.01 g/L). This was attributed to lower percentage in fructose consumption. At the end of fermentation process, 54.70 % of fructose and 97.48 % of glucose were consumed, respectively. It is indicated from Table 1 that the production efficiency of BC from glucose medium was 1.41-fold of that from fructose medium. As for glycerol consumption, most of the carbon sources were distributed in HMP, EMP pathway, and BC synthesis. Metabolic flux into BC synthesis from glycerol medium was 2.52- and 1.94-fold of that from glucose and fructose medium, respectively. Meanwhile, consumption percentage of glycerol was 48.89 %, which was 0.5- and 0.89-fold of glucose and fructose, respectively. It can be inferred that the theoretical productivity of BC from glycerol was 1.26- and 1.73-fold of that from glucose and fructose, which matched well the experimental data (1.21- and 1.50-fold for glucose and fructose, respectively).
Characterization of bacterial cellulose membrane
It was shown by the X-ray diffraction patterns that the peak (110), although coincident in terms of position, was less distinguishable. It indicated that these BC samples were typical crystalline forms of cellulose I. On the other hand, the use of glycerol and glucose instead of mixed carbon source or fructose gave a crystal growth in both planes. Glycerol also had the highest crystallinity index value of all the BCs (85.03 %) (Fig. 7a). These results implied that the presence of glycerol favored the crystallization process and reduced the relative amorphous regions of the BC. However, the same peaks with no change in positions indicated the preservation of the same planar spacings in the crystalline regions. Tang et al. (2010) reported that, when glycerol was the sole carbon source for G. xylinus growth, the cellulose had the lowest porosity. This was attributed to lack of gluconic acid formation and pH stability, which was a favorable condition for BC synthesis. Under these conditions, the bacteria secreted larger, leading to a more compact structure. On the other hand, limited growth of bacteria in fructose medium and an unstable pH environment in glucose medium resulted in higher porosity. The composite BC pellicle appeared to be comprised of an aggregate of thinner and broader cellulose fibers, forming local web-like structures within the network (Fig. 7b). Our previous work demonstrated that the scaffold network structure retained significant quantities of water and favored nutrient transportation (Tang et al. 2010). A densely packed network of cellulose and thinner fibers indicated that BC from the glycerol medium (Fig. 7b) has more hydrogen-bonding patterns, a more compact network and a higher crystalline index than that from the glucose and fructose medium. Such microstructure could also explain the higher tensile strength of this BC.
Different carbon sources led to the variations in BC productivity as well as microstructure. Fructose cannot be used by G. xylinus (CGMCC no. 2955) rapidly, which made the BC tend to be thin. Glucose can be rapidly used by G. xylinus (CGMCC no. 2955), while most of glucose was fluxed into gluconic acid instead of cellulose biosynthetic pathway (Fig. 4). Glycerol cannot only be rapidly used by G. xylinus (CGMCC no. 2955) but also gave high cellulose biosynthesis flux, so the BC from this medium had compact microstructure. Tang et al. (2010) reported sucrose limited the growth of G. xylinus, resulting in lowest thickness and highest porosity of BC compared with glucose and glycerol, while massive microfibrils were secreted by large amounts of bacteria from glycerol medium, leading to a compact structure.
Applications of bacterial cellulose from glycerol medium
BC has a high tensile strength, high porosity, and tight microfibrillar structure that provide a moist wound healing environment, which is necessary for optimal healing. BC from glycerol medium has a dense microstructure, which could be preferable for biomedical applications since it results in a smooth surface that improves adhesion and proliferation of endothelial cells and thus enables introduction or transfer of medicines into the wound, serving as an efficient physical barrier against external infection (Bodin et al. 2006). Glycerol is the main byproduct of biodiesel production, which is typically generated by transesterification of glycerides with methanol. Energy security and environmental stress force the world to the continuous demand for alternatives of fossil energy, which has resulted in significant increases in biodiesel production (Zhong et al. 2009b). Moreover, glycerol is also produced equal to roughly 10 % of the mass of the oil (Saenge et al. 2011). How to deal with an excess of glycerol is a major concern, because biodiesel produced by transesterification need to compete with petroleum-based diesel production. In this study, we presented that BC could be produced with superior mechanical characteristics using glycerol as the sole carbon source. As mentioned above, the tensile strength of the dry BC from the glycerol medium was as high as 83.5 MPa (Fig. 5). Furthermore, it showed slightly thinner fibers with lower porosity. However, it has to be stated that different drying methods would affect the porosity of BC (Tang et al. 2010). Freeze-dried membranes had much higher porosity (92 %) than hot air-dried ones (65 %).
BC from glycerol medium has improved mechanical properties, which could be beneficial for use as a scaffold for tissue engineered blood vessels (Svensson et al. 2005). BC composites can be prepared by dipping a BC sheet into a certain polymer solution. BC from glycerol medium has a dense microstructure and more hydrogen bonding, which could favor applications such as heavy metal removal and enzyme immobilization (Zhu et al. 2011).
Our study mainly compared the metabolic flux distributions in G. xylinus CGMCC 2955 cultured on three different carbon sources (glucose, fructose, and glycerol). The glycerol was the best substrate for BC production with a metabolic yield of 14.7 g/mol C, which was approximately 1.69-fold and 2.38-fold that of the fructose and glucose medium, respectively. The metabolic flux distributions were investigated to elucidate the differences in metabolic yield and fermentation efficiency. BC could be produced with superior mechanical characteristics by glycerol, with slightly thinner fibers and lower porosity.
References
Bodin A, Backdahl H, Fink H, Gustafsson L, Risberg B, Gatenholm P (2006) Influence of cultivation conditions on mechanical and morphological properties of bacterial cellulose tubes. Biotechnol Bioeng 97:425–434
Braud HS (2007) Thermal characterization of bacterial cellulose-phosphate composite membranes. J Therm Anal Calorim 87:815–818
Cannon RE, Anderson SM (1991) Biogenesis of bacterial cellulose. Crit Rev Microbiol 17:435–447
Ha JH, Shah N, Ul-Islam M, Khan T, Park JK (2011) Bacterial cellulose production from a single sugar α-linked glucuronic acid-based oligosaccharide. Process Biochem 46:1717–1723
Heo MS, Son HJ (2002) Development of an optimized simple chemically defined medium for bacterial cellulose production by Acetobacter sp. A9 in shaking cultures. Biotechnol Appl Biochem 36:41–45
Huang D, Wen JP, Wang GY, Yu GH, Jia XQ, Chen YL (2012) In silico aided metabolic engineering of Streptomyces roseosporus for daptomycin yield improvement. Appl Microbiol Biotechnol 94:637–649
Li YJ, Tian CJ, Tian H, Zhang JL, He X, Ping WX, Lei H (2012) Improvement of bacterial cellulose production by manipulating the metabolic pathways in which ethanol and sodium citrate involved. Appl Microbiol Biotechnol 96:1479–1487
Jung HI, Jeong JH, Lee OM, Park GT, Kim KK, Park HC, Lee SM, Kim YG, Kim HG, Son HJ (2010) Influence of glycerol on production and structural–physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour Technol 101:3602–3608
Keshk S, Sameshima K (2006) The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl Microbiol Biotechnol 72:291–296
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Klemm D, Schumann D, Kramer F, Hessler N, Hornung M, Schmauder H, Marsch S (2006) Nanocelluloses as innovative polymers in research and application. Adv Polym Sci 205:49–96
Ma K, Zhao HX, Zhang C, Lu Y, Xing XH (2012) Impairment of NADH dehydrogenase for increased hydrogen production and its effect on metabolic flux redistribution in wild strain and mutants of Enterobacter aerogenes. Int J Hydrogen Energy. doi:10.1016/j.ijhydene. 2012.08.017
Matsuoka M, Tsuchida T, Matsushita K, Adachi O, Yoshinaga F (1996) A synthetic medium for bacterial cellulose production by Acetobacter xylinum subsp. sucrofermentans. Biosci Biotechnol Biochem 100:575–579
Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 107:576–583
Naritomi T, Kouda T, Yano H, Yoshinaga F (1998) Effect of lactate on bacterial cellulose production from fructose in continuous culture. J Ferm Bioeng 85:89–95
Nguyen VY, Flanagan B, Gidley MJ, Dykes GA (2008) Characterization of cellulose production by a Gluconacetobacter xylinus strain from kombucha. Curr Microbiol 57:449–453
Oh SY, Yoo DI, Shin Y, Kim HC (2005) Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr Res 340:2376–2391
Oikawa T, Nakai J, Tsukagawa Y, Soda K (1997) A novel type of d-mannitol dehydrogenase from Acetobacter xylinum occurrence purification and basic properties. Biosci Biotechnol Biochem 61:1778–1782
Park ST, Kim E, Kim YM (2006) Overproduction of cellulose in Acetobacter xylinum KCCM 10100 defective in GDP-mannosyltransferase. J Microbiol Biotechnol 16:961–964
Ross P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55:35–58
Ruka DR, Simon GP, Dean GM (2012) Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr Polym 89:613–622
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011) Potential use of oleaginous red yeast Rhodotcorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218
Schaub J, Mauch K, Reuss M (2008) Metabolic flux analysis in Escherichia coli by integrating isotopic dynamic and isotopic stationary 13C labeling data. Biotechnol Bioeng 99:1170–1185
Schramm M, Gromet Z, Hestrin S (1957) Role of hexose phosphate in synthesis of cellulose by Acetobacter xylinum. Nature 179:28–29
Segal L, Creely J, Martin A, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Shezad O, Khan S, Khan T, Park JK (2010) Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr Polym 82:173–180
Shigematsu T, Takamine K, Kitazato M, Morita T, Naritomi T, Morimura S, Kida K (2005) Cellulose production from glucose using a glucose dehydrogenase gene (gdh)-deficient mutant of Gluconacetobacter xylinus and its use for bioconversion of sweet potato pulp. J Biosci Bioeng 99:415–422
Sturcová A, His I, Apperley DC, Sugiyama J, Jarvis MC (2004) Structural details of crystalline cellulose from higher plants. Biomacromolecules 5:1333–1339
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M, Gatenholm P (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26:419–431
Tamahkar E, Babac C, Kutsl T, Piskin E, Denizli A (2010) Bacterial cellulose nanofibers for albumin depletion from human serum. Process Biochem 45:1713–1719
Tang WH, Jia SR, Jia YY, Yang HJ (2010) The influence of fermentation conditions and post-treatment methods on porosity of bacterial cellulose membrane. World J Microbiol Biotechnol 26:125–131
Toda K, Asakura T, Fukaya M, Entani E, Kawamrua Y (1997) Cellulose production by acetic acid-resistant Acetobacter xylinum. J Ferment Bioeng 84:228–231
Tonouchi N, Sugiyama M, Yokozeki K (2003) Coenzyme specificity of enzymes in the oxidative pentose phosphate pathway of Gluconobacter oxydans. Biosci Biotechnol Biochem 67:2648–2651
UI-Islam M, Khan T, Park JK (2012) Nanoreinforced bacterial cellulose–montmorillonite composites for biomedical applications. Carbohyd Polym 89:1189–1197
Vandamme EJ, De Baets S, Vanbaelen A, Joris K, De Wulf P (1998) Improved production of bacterial cellulose and its application potential. Polym Degrad Stab 59:93–99
Velasco-Bedran H, Lopez-Isunza F (2007) The unified metabolism of Gluconacetobacter entanii in continuous and batch processes. Proc Biochem 42:1180–1190
Wan YZ, Hong L, Jia SR, Huang Y, Zhu Y, Wang YL, Jiang HL (2006) Synthesis and characterization of hydroxyapatite-bacterial cellulose nanocomposites. Compos Sci Technol 66:1825–1832
Weinhouse H, Benziman M (1976) Phosphorylation of glycerol and dihydroxyacetone in Acetobacter xylinum and its possible regulatory role. J Bacteriol 127:747–754
Yunoki S, Osada Y, Kono H, Takai M (2004) Role of ethanol in improvement of bacterial cellulose production: analysis using 13C-labeled carbon sources. Food Sci Technol Res 10:307–313
Zeng XB, Small DP, Wan WK (2011) Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr Polym 86:1558–1564
Zhong C, Cao XY, Li BZ, Yuan YJ (2009a) Biofuels in China: past, present and future. Biofuels Bioprod Bioref 3:247–270
Zhong C, Lau MW, Balan V, Dale BE, Yuan YJ (2009b) Optimization of enzymatic hydrolysis and ethanol fermentation from AFEX-treated rice straw. Appl Microbiol Biotechnol 84:667–676
Zhu HX, Jia SR, Wan T, Jia YY, Yang HJ, Li J, Yan L, Zhong C (2011) Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr Polym 86:1558–1564
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (project no. 21106105, project no. 20976133), the Foundation of Tianjin Educational Committee (no. 20100602), and Changjiang Scholars and Innovative Research Team in University (no. IRT1166). We also gratefully acknowledge Rebecca G. Ong for her assistance in editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Cheng Zhong and Gui-Cai Zhang contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 46 kb)
Rights and permissions
About this article
Cite this article
Zhong, C., Zhang, GC., Liu, M. et al. Metabolic flux analysis of Gluconacetobacter xylinus for bacterial cellulose production. Appl Microbiol Biotechnol 97, 6189–6199 (2013). https://doi.org/10.1007/s00253-013-4908-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4908-8