Abstract
This study is focused on the characterization of bacterial cellulose (BC) produced by Komagataeibacter xylinus strains DSM 2325, DSM 2004, and DSM 46604 from styrene/glucose mixtures. Styrene, the aromatic monomer of petrochemical plastics such as polystyrene, served as a co-substrate for bacterial cultivation, being assimilated by all strains, although with differing efficiency for BC biosynthesis. The best performing strain was K. xylinus DSM 2325 with a BC production of 2.70 ± 0.4 g/L. Interestingly, K. xylinus DSM 2004 produced BC from styrene as the sole carbon source, yielding 0.32 ± 0.02 g/L BC. The presence of styrene in the cultivation media had a minor influence on the produced BC chemical structure, thermal degradation temperature (318–337 °C), and morphology, where compact fibers of diameters ranging from 31 to 47 nm were observed. The crystallinity index of the samples was obtained through X-ray diffraction and showed that values varied according to the medium used (41–33%). However, the membranes synthesized in the presence of styrene were thinner (3–22 μm) than those produced from glucose (12–44 μm) and had low gas permeability. K. xylinus DSM 2325 and DSM 2004 membranes had also low permeability for O2 (1.1–2.5 barrer) and CO2 (2.5–5.8 barrer), while those produced by K. xylinus DSM 46604 had a higher permeability to CO2 (42.3 barrer) together with low permeability to O2 (2.5 barrer). Moreover, BC produced by K. xylinus DSM 2325 with styrene as an additive showed the highest crystallinity among all strains and mediums (46%). These results show the feasibility of using styrene as an effective co-substrate in a sustainable approach for its valorisation into a value-added biopolymer, with the advantage of tuning BC properties according to the envisaged application, by selecting the appropriate producing strain and culture medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC) is a biopolymer composed of covalently linked glucose residues held together by hydrogen bonds between carbon atoms 1 and 4, β-(1,4) linkages (Cacicedo et al. 2016). Production of BC is carried out through oxidative fermentation by bacteria from the genera Komagataeibacter, Sarcina, and Agrobacterium (Aswini et al. 2020), offering the advantage over plant cellulose of not being associated with lignin or hemicelluloses, as their removal is pricy and polluting (Naomi et al. 2020). BC comprises a fibrous network nanostructure with a large surface area and considerable porosity (Mishra et al. 2022) standing out among other biomaterials due to its unique characteristics, such as a high degree of polymerization (Mishra et al. 2022), biocompatibility (Portela et al. 2019), biodegradability (Torgbo and Sukyai 2020), high crystallinity (35–96%) (Andritsou et al. 2018; Gorgieva and Trček 2019), and excelling mechanical performance, exhibiting tensile strength values in the range of 200–300 MPa and Young’s modulus up to 15–35 GPa (Cacicedo et al. 2016). Moreover, it presents superior water absorption and retaining abilities (around 99%) (Rebelo et al. 2018) due to its native hydrogel-like structure. These properties grant BC the versatility of being suitable for a wide range of applications, including cosmetics, food, textile, and biomedicine (Cacicedo et al. 2016; Zhong 2020; Mishra et al. 2022).
BC production is mainly carried out by either static or agitated cultivation, each strategy resulting in different material properties and morphology (Pang et al. 2020). Under static conditions, a gelatinous membrane is formed at the air–liquid interface of the culture medium and the process usually has higher BC production yields (Chen et al. 2018). Agitated cultivation generates small irregular BC pellet suspensions, and, although being a more scalable process, it shows lower yields due to the induction of non-cellulose producing mutants (Zhong 2020). Regardless of cultivation mode, the choice of culture medium is crucial to ensure bacterial cell growth and BC production, severely impacting polymer structure and physical properties. Glucose, sucrose, fructose, mannitol, arabitol, and molasses are common carbon sources reported for BC production, while yeast extract and peptone are preferred nitrogenous sources (Buldum et al. 2018; Singhsa et al. 2018; Andriani et al. 2020). Moreover, some agricultural and/or industrial wastes and by-products have been proposed as cost-effective alternative feedstocks to the refined carbon sources for BC production costs reduction, including but not limited to, wheat straw acid hydrolysate (Hong et al. 2011), whey (Revin et al. 2018), and industrial wastewater (Li et al. 2015). Other substrates and co-substrates have also been tested for BC production, e.g., aromatic compounds such as terephthalic acid (Esmail et al. 2022) and naphthalene (Marín et al. 2019), as well as alcohols (Lu et al. 2011) and organic acids (Lee et al. 2011). Although some improvement in BC biosynthesis has been achieved by using non-synthetic carbon sources, there is still a need to find other more efficient and scalable options.
Styrene is a mono-aromatic compound commonly used in the synthesis of petrochemical plastics, e.g., polystyrene (PS), acrylonitrile butadiene styrene (ABS), and styrene acrylonitrile (SAN) (Aliabadi et al. 2012). Although those materials are highly versatile and cheap to produce, and therefore used on a large scale, they are non-biodegradable, accumulating in landfills and marine ecosystems when not properly discarded, posing a threat to our environment, as well as our health, since styrene has been associated with the depression of the central nervous system, damage to the liver, hormonal disruptions, and cancer (Tan et al. 2015; Hwang et al. 2020). Thus, it is paramount to develop strategies that allow the elimination of styrene-laden effluents and styrene-based solid wastes from our environment. A promising prospect is its bioconversion into less harmful compounds, where microorganisms are employed as biocatalysts. Some efforts have been made to isolate bacterial strains that can feed on styrene and produce value-added compounds, such as poly(hydroxyalkanoate) (PHA) (Tan et al. 2015; Alonso-Campos et al. 2022) and phenylacetic acid (PAA) (Ebciba et al. 2020). However, to our knowledge, no studies focused on the biosynthesis of BC from styrene.
In this work, three bacterial strains, namely Komagataeibacter xylinus DSM 2325, DSM 2004, and DSM 46604, were cultivated using styrene as a co-substrate in styrene/glucose mixtures to assess their ability to biosynthesize BC and the impact of such cultivation conditions on the physical–chemical properties of the produced biopolymer. The resulting BC membranes were characterized in terms of their morphology, chemical structure, crystallinity, thermal and mechanical properties, and gas permeability.
Materials and methods
Bacterial cellulose production
Microorganisms and media
Komagataeibacter xylinus strains DSM 2325, DSM 2004, and DSM 46604 were used for BC production. Hestrin-Schramm (HS) medium (Hestrin and Schramm 1954) (per litre: glucose, 20 g; peptone, 5 g; yeast extract, 5 g; citric acid, 1.15 g; disodium hydrogen phosphate, 2.7 g; pH = 7) was used for all experiments. HS medium was supplemented with styrene (0.5 g/L) (synthesis grade, Sigma-Aldrich) as a co-substrate. Experiments were also conducted with HS medium devoid of glucose and with styrene as the sole carbon source.
Production assays
The inocula for the experiments were prepared by inoculation of 1 mL of the cryopreserved cultures in 100 mL of HS medium (20 g/L glucose) and incubation in an orbital shaker, at 30 ℃ and 150 rpm, for 24 h. The assays were conducted under static conditions, in T-75 flasks (BIOFIL) containing 30 mL medium, at 30 ℃, for 18 days. A 20% (v/v) inoculum prepared as described above was used in each assay. At the end of the assays, the BC membranes were collected from the flasks and treated with 0.1 N NaOH, at 80 ℃, for 20 min. After washing with deionized water in an orbital shaker for 48 h (200 rpm, at room temperature) (Esmail et al. 2022), the membranes were lyophilized (ScanVac CoolSafeTM, LaboGene) at −110 °C for 48 h. Wet BC pellicles were weighed after alkaline treatment, as well as after lyophilization, for BC gravimetric quantification.
Analytical techniques
Broth samples (10 mL) were collected at the beginning and the end of the assays, centrifuged (10,956 g, 15 min, 4 ℃), and used for glucose and styrene quantification. For glucose quantification, the cell-free supernatant was diluted in H2SO4 0.01 N and filtered with modified nylon centrifugal filters (0.2 μm, VWR), at 10,000g for 10 min. Glucose concentration was determined by HPLC with a VARIAN Metacarb column (BioRad) coupled to a refractive index (RI) detector, as described by Rebocho et al. (2019). The analyses were performed at 50 °C, with H2SO4 0.01 N as eluent at a flow rate of 0.6 mL/min. Glucose standards (analytical reagent grade, Fischer Chemical) were used at concentrations in the range of 0.01–1.0 g/L. Styrene quantification was carried out by UV spectroscopy, as described by Mustarichie et al. (2018), using a Camspec M509T UV spectrometer. The cell-free supernatant was diluted in deionized water and the absorbance was measured at 246 nm. Styrene standards (synthesis grade, Sigma-Aldrich) were used at concentrations in the range of 0.005–0.1 g/L.
BC characterization
Fourier transform infrared spectroscopy (FTIR)
FTIR analysis was executed with a Perkin-Elmer Spectrum two spectrometer. The dried polymer samples were directly analyzed on the FTIR cells. The spectra were recorded between 400 and 4000 cm−1 resolutions with 10 scans, at 20 °C.
Scanning electron microscopy (SEM)
To evaluate the nanostructure of BC, the lyophilized samples were mounted for SEM observation using double-sided carbon tape and aluminium stubs and sputter-coated with a thin layer of iridium (150 T ES, Quorum, UK). The analysis was performed in a scanning electron microscope (Hitachi, Regulus 8220) using an acceleration voltage of 3 kV. The obtained SEM images were processed by public domain imaging software ImageJ (NIH image).
X-ray diffraction
The structural analysis of the dried samples was carried out by X-ray diffraction (XRD) using an X-ray diffractometer (PANalytical X′Pert PRO MRD) with a Cu Kα radiation source (45 kV and 40 mA) and wavelength of 1.540598 Å and equipped with an 1D X’Celerator detector. The XRD measurements were performed in the range of 10° to 65° (2θ) in the Bragg–Brentano configuration, with a scanning step size of 0.033° in 2θ. Samples were mounted on a Si-0 background holder. The crystallinity index (CI) was estimated by the XRD deconvolution method as described by Park et al. (2010) using the X'Pert HighScore Plus software (Malvern Panalytical). Briefly, the crystalline and amorphous contributions were extracted by a Peak profile fit process using the diffraction intensity profiles. The analysis was restrained in the 2Theta range between 10° and 32°. Each peak was assumed a pseudo-Voigt function including a very broad peak near 21° assigned to the amorphous contribution. Furthermore, the background counts of the Si-0 background holder was also included in the analysis.
Thermogravimetric analysis
Thermogravimetric (TG) measurements were performed with a Simultaneous Thermal Analyser STA 449 F3 Jupiter from NETZSCH Thermal analysis (Wittelsbacherstraße, Germany), under a nitrogen atmosphere and loading 5 mg of each material into a covered aluminium crucible. The polymers were heated up to 500 °C at 10 K/min. The thermal degradation temperature (Tdeg, °C) corresponds to the temperature value obtained for the maximum decreasing peak of the sample mass.
Mechanical properties
Puncture tests were performed at ambient temperature (22 °C) using a texture analyzer (Food Technology Corporation, Kent, UK), equipped with a 10 N load cell. The wet BC pellicles were fixed on a film support rig and submitted to perforation by a needle moving at 10 mm/s until rupture. The force at perforation (N) was calculated as the maximum puncture force until the breaking point. The deformation at perforation (mm) was determined as the maximum distance of deformation at the breaking point. Five replicas were analyzed for each membrane.
Permeability to O2 and CO2
The pure gas permeability of the BC membranes for O2 and CO2 was determined as described by Neves et al. (2010). For the measurements, a stainless-steel cell rig with two identical compartments separated by the supported BC membrane with an effective membrane area of 1 cm2 was employed. Each gas permeability was assessed by pressurizing both compartments (feed and permeate) with a single gas and opening the permeate outlet until a driving force of ± 0.7 bar between compartments was established. The pressure change in both compartments was observed over time using two pressure transducers (Druck, PDCR 910 models 99,166 and 991,675, England). All assays were performed at a constant temperature, of 30 ºC, using a thermostatic bath (Julabo, Model EH, Germany).
Results and discussion
BC production
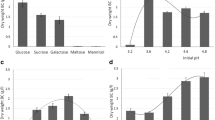
BC production by K. xylinus DSM 2325, DSM 2004, and DSM 46604 assays were conducted in styrene-supplemented HS medium, in which styrene was present at a low concentration (0.5 g/L) due to its low water solubility (Yeh et al. 2022), acting as a co-substrate. Higher concentrations of styrene resulted in a heterogeneous medium due to phase separation and/or were inhibitory for cell growth. Experiments with HS medium devoid of glucose were conducted to account for any BC production from the peptone and yeast extract available in the medium. Styrene was also tested as the sole carbon source. As shown in Table 1, all strains were able to grow in HS medium supplemented with styrene, although different BC production was observed.
K. xylinus DSM 2325 showed the highest BC production (2.70 ± 0.40 g/L) from the styrene/glucose mixtures (Table 1), with a product yield on a substrate basis of 0.25 gBC/gS. These values are higher than those found for the same strain grown with only glucose (2.37 ± 0.26 g/L and 0.24 gBC/gS, respectively), which demonstrates the utilization of styrene as a co-substrate to favor BC synthesis. These values fall within the range of those reported for other K. xylinus strains cultivated on glucose as the sole carbon source (0.24–6.23 g/L) (Yang et al. 2016; Andriani et al. 2020; Nascimento et al. 2021; Ogrizek et al. 2021). Other co-substrates have been successfully employed to enhance BC production, e.g. addition of 1% ethanol to the basal medium increased cellulose production by K. hansenii by 1.7 times (Park et al. 2003). Also, Ha et al. (2011) reported that medium supplementation with 1% glucuronic acid resulted in increased BC production by K. hansenii (from 7.4 to 10.5 g/L), as well as improvement in fiber diameter, along with higher tensile strength and water absorption capacity. K. xylinus PTCC 1734 also had improved BC production upon medium supplementation with low-quality date syrup (Moosavi-Nasab and Yousefi 2011). Therefore, despite K. xylinus DSM 2325 inability to use styrene as a sole carbon source, it was able to utilize it as a co-substrate with improved BC production.
Concerning K. xylinus DSM 46604, it presented the lowest BC production among all three tested strains, with a production of 0.53 ± 0.04 g/L (Table 1) on glucose, although among the range described in the literature (0.24–6.23 g/L) (Cavka et al. 2013; Yang et al. 2016; Ogrizek et al. 2021). Upon medium supplementation with styrene, BC synthesis was comparable to when using glucose as a sole carbon source 0.56 ± 0.06 g/L (Table 1). Similarly, to K. xylinus DSM 2325, K. xylinus DSM 46604 also did not yield any BC production from styrene as the sole carbon source. K. xylinus DSM 46604 was reported to synthesize BC from terephthalic acid, a plastic monomer from PET, both as the sole carbon source and as a co-substrate, reaching a BC production of 0.16 g/L and 0.70 g/L, respectively (Esmail et al. 2022).
BC production by K. xylinus DSM 2004 in styrene/glucose mixture assay (1.57 ± 0.06 g/L) was lower than in the glucose assay (2.05 ± 0.17 g/L) (Table 1), thus suggesting that the presence of styrene was detrimental to BC synthesis. Nonetheless, it was the only strain able to produce BC from styrene as the sole carbon source (0.32 ± 0.02 g/L), slightly higher than the production attained in the non-supplemented HS medium (0.29 ± 0.02 g/L) (Table 1). Cultivation on styrene as the sole carbon source also provided the highest product yield on a substrate basis (0.64 gBC/gS) when compared to glucose (0.20 gBC/gS), as well as the styrene/glucose mixture (0.15 gBC/gS). This bacterial strain has been previously reported to be able to use other synthetic plastics’ monomers, such as, for example, terephthalic acid and ethylene glycol, as sole carbon sources, reaching BC productions of 0.81 g/L and 0.64 g/L, respectively (Esmail et al. 2022). Production of BC using aromatic compounds, namely naphthalene, has also previously been reported for Starkeya sp. strain N1B (Marín et al. 2019).
Overall, the obtained results show that all strains were able to synthesize BC upon cultivation in styrene supplemented media, with K. xylinus DSM 2325 and DSM 46604 achieving higher BC production. Interestingly, although with a low production, K. xylinus DSM 2004 was able to utilize styrene as the sole substrate.
BC characterization
Morphology of BC pellicles
The produced BC pellicles exhibited an orange coloration, except those synthesized in the media devoid of glucose, namely, in the non-supplemented HS medium and in the experiment run with styrene as the sole carbon source, in which they displayed a light-yellow tint (Fig. 1). After the alkaline treatment, all pellicles became translucent and displayed minor shrinkage, indicative of purification and removal of bacterial cell debris and medium remnants (Fig. 1).
The membranes’ thickness was highly impacted by the cultivation conditions, besides also varying with the producing strain (Table 2). The thicker membranes were those produced by K. xylinus DSM 46604 from glucose as the sole carbon source (44 ± 2.2 µm) and in the glucose/styrene mixtures (22 ± 1.1 µm). These values are among those reported for BC membranes (20–200 µm) (Peres et al. 2016; Lee et al. 2017; Vázquez et al. 2021). However, considerably lower thickness values were observed for K. xylinus DSM 2325 and K. xylinus DSM 2004 BC produced in the glucose/styrene mixtures (6 ± 0.3 and 7 ± 0.4 µm, respectively), while even lower values were displayed by the BC membranes produced by K. xylinus DSM 2004 grown on styrene as sole carbon source (3 ± 0.2 µm) (Table 2), which is concomitant with the lower BC production observed in these conditions.
The SEM images (Fig. 1) revealed a nanostructure in line with that reported for BC membranes, specifically a porous structure with a three-dimensional network of ultrafine fibers on the nanometric scale (Kadier et al. 2021; Kim et al. 2021). Although some differences were observed for the BC synthesized by each strain and in the different tested media, fiber diameter varied from 29 to 47 nm for all samples (Table 2), which is in line with values reported for BC fiber networks (20–100 nm) (Choi and Shin 2020; Zhong 2020; Janeni and Adassooriya 2021).
The BC membranes produced by K. xylinus DSM 2325 were characterized by thinner fibers, with similar average diameters for the biopolymer synthesized from glucose (31 ± 5 nm) and from the styrene/glucose mixtures (33 ± 4 nm). Despite the presence of styrene in the cultivation medium having no impact on the fiber diameter, the pellicles produced from glucose alone displayed a more compact nanostructure (Fig. 1c) than those synthesized in the presence of styrene that were more porous (Fig. 1f). The same trend was observed for the BC produced by K. xylinus DSM 46604, for which the pellicles synthesized from glucose alone were composed of a denser nanostructure (Fig. 1i). Nevertheless, the fibers synthesized in the presence of styrene were characterized by a higher average diameter (44 ± 9 nm) (Table 2).
K. xylinus DSM 2004 produced BC fibers with 47 ± 13 nm and 47 ± 11 nm in the non-supplemented HS medium and the HS medium with glucose, respectively (Table 2). Additionally, the pellicles synthesized from glucose displayed a less densely packed network (Fig. 1r). However, in contrast to strains K. xylinus DSM 2325 and DSM 46604, a significant decrease in the average fiber diameter was observed for the BC produced by K. xylinus DSM 2004 in the presence of styrene (36 ± 6 nm in the styrene/glucose mixture, and 29 ± 4 nm in the medium containing only styrene) (Table 2). These values are within the range reported for BC produced by K. xylinus DSM 2004 using other synthetic plastic monomers as carbon sources, namely terephthalic acid (33–68 nm) and ethylene glycol (41–74 nm) (Esmail et al. 2022). Nonetheless, Starkeya sp. strain N1B was described to synthesize BC from naphthalene with higher fiber diameters (60 nm) (Marín et al. 2019).
These results show that each strain was affected differently by the presence of styrene in the cultivation medium, resulting in BC membranes with different morphology, namely, variable thickness, fiber diameter, and porosity.
Fourier-transform infrared spectroscopy
Although some differences can be observed, the FTIR spectra of the BC produced by all K. xylinus strains under the tested cultivation conditions show the characteristic bonds described for the biopolymer (Gea et al. 2011). In particular, the stretching of OH groups present in BC is evidenced by the peak at 3346 cm−1 (Choi et al. 2004), while at 2896 cm−1 there is a band exposing the asymmetric stretching for CH2 (Oh et al. 2005). Bending of OCH and HCH was revealed by a peak at 1427 cm−1 (Szymańska-Chargot et al. 2011) and the C-H bonds are defined by peaks at 1360–1315 cm−1 (Gea et al. 2011). Additionally, C–O–C asymmetric stretching and CH deformation were detected at 1162 cm−1, as well as stretching of C–C rings in polysaccharides, related to the peak at 1109 cm−1 (Movasaghi et al. 2008). The bands at 1652 and 1544 cm−1 indicates the presence of amide bonds which may correspond to proteins from the culture medium or residual bacterial biomass that were not completely removed during sample purification (Akintunde et al. 2022).
Regarding the BC produced by K. xylinus DSM 2004, the FTIR spectra were similar for all tested media (Fig. 2a), indicating that for this strain the carbon source had no significant impact on the biopolymer’s chemical structure, except for a slight decrease in the intensity of the peaks appearing at 800 and 1300 cm−1. Similarly, for strains K. xylinus DSM 2325 and DSM 46604 (Fig. 2b), the spectra of the synthesized BC also appear to not be significantly influenced by the choice of medium, despite a slight decrease in the peaks’ intensity in the presence of styrene for K. xylinus DSM 2325. Also, for the BC produced by K. xylinus DSM 46604, there was a decreased intensity of the peaks at 1300 and 1800 cm−1 (corresponding to the bending of OCH and HCH). These differences in peak intensity can be correlated to microstructural changes that can also impact the crystalline content of the samples (Riaz and Ashraf 2015), which will be further discussed. Also, the amide bond peaks were more evident in the case of BC grown in only glucose for K. xylinus DSM 46604, suggesting a need for further purification.
Crystallinity degree
The diffractograms of the BC produced by each strain from the different substrates, as well as the crystallinity index (CI) values are presented in Fig. 3 and Table 2, respectively. Most samples show the main reflections of the Iα X-ray diffraction pattern of crystalline BC, particularly, three narrow humps of varying intensity positioned at 2θ = 15, 17, and 23°, linked to the (100), (010), and (110) crystal planes (Wada and Okano 2001).
Regarding CI (deconvolution work shown in supplementary information), the highest values were observed for the BC synthesized by K. xylinus DSM 2325 grown in medium supplemented with glucose and styrene (46%) (Table 2), which is higher than the BC produced in the medium supplemented with only glucose (41%) (Table 2). For the BC produced by K. xylinus DSM 2004, the highest crystallinity was observed for non-supplemented medium (41%), followed by the BC cultivated in glucose and styrene mixtures (36%) while the mediums containing only glucose or styrene displayed the lowest CI (34 and 33%, respectively). K. xylinus DSM 2004 has been previously reported to produce BC with CI values of 57% and 40% from terephthalic acid and ethylene glycol as sole carbon sources, respectively (Esmail et al. 2022), which corroborates that different carbon sources impact polymer crystallinity. Concerning the BC synthetized by K. xylinus DSM 46604, it presented the lowest CI value overall, namely 19% when cultivated with a glucose and styrene mixture, compared to when only glucose was used for cultivation (44% (Table 2)). These results demonstrate that the CI differed depending on the producing strain and the cultivation medium. These variables have been described to influence CI, alongside the rate of BC synthesis, pH, and oxygen delivery (Pourramezan et al. 2009).
These results allow to compare the crystallinity of the samples and between samples. Similar analysis using the Segal methodology achieves much higher crystallinity Index values, but displaying the same evolution between samples. Although the Peak deconvolution methods may not be the more accurate to define cellulose crystallinity it takes into account an amorphous and a crystalline contribution and allows for a better description the observed diffraction patterns.
Thermogravimetric analysis
The thermograms of the produced BC pellicles (Fig. 4) show that all samples exhibited similar weight-loss behaviour, comprising three main degradation steps, which is concordant with what is reported for BC (Gayathri and Srinikethan 2019; Zhang et al. 2021). The first mass loss event (3–8%) occurred between 30–100 ºC and can be attributed to the loss of water from the samples (Zhang et al. 2021). The second and most prominent weight loss is relative to the main degradation of the polymer (Akintunde et al. 2022), and was observed between 225 and 375 ºC, with the lowest mass loss being for K. xylinus DSM 2325 BC produced from glucose (44%), and the highest for the biopolymer synthesized by K. xylinus DSM 2004 cultivated in the non-supplemented medium and in from styrene as the sole carbon source (67%). Between 380 and 500 ºC, the last degradation step was detected (weight loss of 7–14%) and can be assigned to the degradation of proteins or microorganisms that were not successfully removed during the purification procedure, as was evidenced by the amide bonds detected by FTIR analysis (Zhang et al. 2021). There was no significant variability among most of the samples’ char yield (19–27%), except for the BC produced by K. xylinus DSM 2325 from glucose as the sole carbon source (40%).
The thermal degradation temperatures (Tdeg) for the BC produced by K. xylinus DSM 2325 and DSM 46604 showed little disparity between the tested medium, presenting values of 329 and 328 °C for the biopolymer synthesized from glucose (Table 2), and of 324 and 327 °C for those produced from glucose/styrene mixtures, respectively (Table 2). On the other hand, K. xylinus DSM 2004 BC demonstrated a higher variation in values depending on the tested medium, showing degradation temperatures ranging from 318 °C (glucose/styrene medium) to 237 °C (non-supplemented HS medium) (Table 2). Moreover, the BC produced from glucose or styrene as sole carbon sources both presented an in-between value of 327 °C (Table 2). Nonetheless, the Tdeg for all samples fall among the range reported for BC (310–399 °C) (Costa et al. 2017; Jia et al. 2017; Akintunde et al. 2022; Thongwai et al. 2022), including those synthesized from other aromatic compounds such as terephthalic acid (315–334 °C) (Esmail et al. 2022).
Mechanical properties
Puncture tests were performed to assess the produced BC membranes’ mechanical properties. K. xylinus DSM 2325 wet BC membranes were clearly the ones with the highest resistance to perforation by the needle, with forces at perforation of 0.19 ± 0.08 N for the biopolymers produced from glucose, and of 0.27 ± 0.13 N (Table 2) for those produced from the styrene/glucose mixtures. On the other hand, the membranes differed in their flexibility, with the membrane produced from glucose presenting a higher perforation value (31 ± 3 mm) than that synthesized in the presence of styrene (23 ± 3 mm). These results indicate that the presence of styrene in the cultivation medium has apparently induced higher mechanical resistance to K. xylinus DSM 2325 BC membranes, concomitant with higher flexibility. The obtained values are lower than those reported for BC (0.6 N) produced by K. xylinus CECT using glucose as a carbon source (Cazón et al. 2019; Vázquez et al. 2021), which can probably be linked to a higher membrane thickness (20 µm) than in this study (6 µm). Interestingly, the deformation at perforation values obtained for K. xylinus DSM 2325 membranes are higher than that reported for BC (0.39 mm) (Cazón et al. 2019; Vázquez et al. 2021), as well as high-density polyethylene (HDPE) (16 mm) (Yang et al. 2012) or polypropylene (PP) (1.3 mm) (Yang et al. 2012) and close to the values described for PET (29 ± 8 mm) (Patti and Acierno 2020), revealing a high ability to be deformed before bursting.
The BC produced by K. xylinus DSM 46604 also displayed a higher deformation at perforation for the biopolymer produced from glucose (34 ± 4 mm) compared to that synthesized from the styrene/glucose mixture (28 ± 5 mm) (Table 2). However, K. xylinus DSM 46604 membranes had considerably lower mechanical resistance, as shown by their very low force at perforation values (0.07 ± 0.03 and 0.05 ± 0.004 N, respectively). Concerning K. xylinus DSM 2004 BC membranes, they also showed low forces at perforation, similar to DSM 46604 BC, varying from 0.03 ± 0.003 N to 0.09 ± 0.02 N (Table 2), with the highest value being attributed to the biopolymer produced in the non-supplemented medium and the lowest for the one produced from glucose. The corresponding deformation at perforation values were also similar and varied from 23 ± 4 N to 28 ± 2 N, mainly depending on the membrane’s thickness, where higher deformation at perforation can be observed for higher membrane thickness. These results show that the BC synthesized by K. xylinus DSM 2004 and DSM 46604 were flexible but presented low mechanical resistance.
Permeability to O2 and CO2
The permeability of O2 and CO2 for the wet BC membranes was investigated to evaluate their barrier properties. In general, the membranes’ permeability to CO2 (0.1–227.7 barrer) was either similar to or higher than the permeability to O2 (0.1–62.2 barrer) (Table 2). This is expected since the membranes were in their wet form and the permeability followed the same trend of each gas in water, where CO2 has higher permeability than O2 (1923 vs 91.0 barrer) (Lide 2008). Water molecules adsorb into the polymer matrix and may increase the fractional free volume of the membranes, playing a key role in enhanced gas transport (Tomé et al. 2010).
The BC membranes produced by K. xylinus DSM 2325 from the styrene/glucose mixtures showed lower O2 and CO2 permeability values (1.1 ± 0.1 and 5.8 ± 0.3 barrer, respectively) than the membranes synthesized from glucose (10.2 ± 0.5 and 10.3 ± 0.5 barrer, respectively) (Table 2). The same trend was observed for the BC produced by K. xylinus DSM 2004, though considerably higher values were attained for the membranes produced from glucose (62.2 ± 3.1 and 227.7 ± 11.4 barrer, respectively). These values differ from those reported by Tomé et al. (2010) for BC produced under similar conditions by the same strain, namely, an O2 permeability of 10 ± 0.5 barrer and a CO2, permeability 187 ± 9.4 barrer. The higher permeability observed for both gases can be explained by the higher porosity of the matrix, which can be observed in Fig. 1.
For K. xylinus DSM 46604, the presence of styrene in the cultivation medium has apparently had little effect on the membranes in terms of O2 permeability, as similar values were attained for both samples (3.4 ± 0.2 barrer for the BC produced from glucose and 2.5 ± 0.1 barrer for that produced from the styrene/glucose mixture). For CO2, on the other hand, the permeability of the membranes was much higher for the BC produced in the presence of styrene (42.3 ± 2.1 barrer) compared to that synthesized from glucose (3.4 ± 0.2 barrer).
Overall, these results indicate that it is possible to tune BC membrane’s permeability to CO2 and O2 by a selection of the appropriate strain and cultivation media, which is valuable when there are specific barrier property needs for different purposes such as packaging, where lower gas permeability is required (0.21–7.3 barrer for O2 and 1.2–90 barrer for CO2 (Bao et al. 2006; Semsarzadeh et al. 2008; Paunonen 2013; Michiels et al. 2017)), or biomedical applications, where higher gas permeability is essential (8.4–140 barrer for O2 and 172–950 barrer for CO2 (Roy et al. 2010; Eusébio et al. 2020; Tran et al. 2020)).
Overall assessment of the produced BC membranes properties
The BC membranes produced in this study showed differing properties depending on the producing strain and the culture medium. The BC produced by K. xylinus DSM 2325 grown in HS medium supplemented with glucose and styrene presented the highest crystallinity among all strains and media (CI = 46%), average gas permeability (10.2 ± 0.5 barrer for O2 and 10.3 ± 0.5 barrer for CO2) and force at perforation (0.19 ± 0.08N). Moreover, when the medium was co-supplemented with styrene, an increased resistance at perforation (0.27 ± 0.13 N) was observed, together with lower permeability to both O2 (1.1 barrer) and CO2 (5.8 barrer). These features render these produced membranes of interest for the development of packaging or coatings applications. K. xylinus DSM 2004 cultivated in a non-supplemented HS medium provided a more crystalline polymer in comparison to other media (CI = 41%) with low permeability (0.1 ± 0.01 barrer) to both O2 and CO2, as well as a higher force at perforation among all membranes from this strain (0.09 ± 0.02 N), making it fit for packaging purposes. Still, the biopolymer produced from glucose had a lower crystallinity (CI = 34%) but its permeability increased to 62.2 ± 3.1 barrer for O2 and to 227.7 ± 11.4 barrer for CO2, rendering such membranes suitable for biomedical applications. For products that require a thicker membrane (44 µm), with lower crystallinity (CI = 19%), the BC produced by K. xylinus DSM 46604 from HS medium with glucose and styrene is a promising option.
Conclusions
Komagataeibacter xylinus strains DSM 2325, DSM 2004, and DSM 46604 produced BC in styrene containing media. All strains grew on glucose/styrene mixtures, but only K. xylinus DSM 2004 was able to utilize styrene as the sole carbon source. While the BC produced in all tested media had comparable physico-chemical properties in terms of structure and thermal degradation behaviour, the produced biopolymers differed in their microstructure, mechanical properties and permeability to gases. Therefore, this study opens up the possibility of producing BC with varying properties according to specific applications needs, while contributing to reduce the burden of plastic pollution by using styrene as a co-substrate in the medium.
Availability of data and materials
Data and materials will be provided on request.
References
Akintunde MO, Adebayo-Tayo BC, Ishola MM et al (2022) Bacterial cellulose production from agricultural residues by two Komagataeibacter sp. Strains Bioengineered 13:10010–10025. https://doi.org/10.1080/21655979.2022.2062970
Aliabadi M, Aroujalian A, Raisi A (2012) Removal of styrene from petrochemical wastewater using pervaporation process. Desalination 284:116–121. https://doi.org/10.1016/j.desal.2011.08.044
Alonso-Campos V, Covarrubias-García I, Arriaga S (2022) Styrene bioconversion by Pseudomonas putida utilizing a non-aqueous phase for polyhydroxyalkanoate production. J Chem Technol Biotechnol 97:1424–1435. https://doi.org/10.1002/jctb.6935
Andriani D, Apriyana AY, Karina M (2020) The optimization of bacterial cellulose production and its applications: a review. Cellulose 27:6747–6766. https://doi.org/10.1007/s10570-020-03273-9
Andritsou V, De Melo EM, Tsouko E et al (2018) Synthesis and characterization of bacterial cellulose from citrus-based sustainable resources. ACS Omega 3:10365–10373. https://doi.org/10.1021/acsomega.8b01315
Aswini K, Gopal NO, Uthandi S (2020) Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol 20:1–16. https://doi.org/10.1186/s12896-020-00639-6
Bao L, Dorgan JR, Knauss D et al (2006) Gas permeation properties of poly(lactic acid) revisited. J Memb Sci 285:166–172. https://doi.org/10.1016/j.memsci.2006.08.021
Buldum G, Bismarck A, Mantalaris A (2018) Recombinant biosynthesis of bacterial cellulose in genetically modified Escherichia coli. Bioprocess Biosyst Eng 41:265–279. https://doi.org/10.1007/s00449-017-1864-1
Cacicedo ML, Castro MC, Servetas I et al (2016) Progress in bacterial cellulose matrices for biotechnological applications. Bioresour Technol 213:172–180. https://doi.org/10.1016/j.biortech.2016.02.071
Cavka A, Guo X, Tang SJ et al (2013) Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol Biofuels 6:1–10. https://doi.org/10.1186/1754-6834-6-25
Cazón P, Vázquez M, Velazquez G (2019) Composite films with UV-barrier properties based on bacterial cellulose combined with chitosan and Poly(vinyl alcohol): study of puncture and water interaction properties. Biomacromol 20(5):2084–2095
Chen W, Yu H, Lee SY et al (2018) Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem Soc Rev 47:2837–2872. https://doi.org/10.1039/c7cs00790f
Choi SM, Shin EJ (2020) The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 10:1–24. https://doi.org/10.3390/nano10030406
Choi YJ, Ahn Y, Kang MS et al (2004) Preparation and characterization of acrylic acid-treated bacterial cellulose cation-exchange membrane. J Chem Technol Biotechnol 79:79–84. https://doi.org/10.1002/jctb.942
Costa AFS, Almeida FCG, Vinhas GM, Sarubbo LA (2017) Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front Microbiol 8:2027. https://doi.org/10.3389/fmicb.2017.02027
Ebciba C, Pavithra N, Chris Felshia F, Gnanamani A (2020) Exploring the styrene metabolism by aerobic bacterial isolates for the effective management of leachates in an aqueous system. RSC Adv 10:26535–26545. https://doi.org/10.1039/d0ra03822a
Esmail A, Rebocho AT, Marques AC et al (2022) Bioconversion of terephthalic acid and ethylene glycol into bacterial cellulose by Komagataeibacter xylinus DSM 2004 and DSM 46604. Front Bioeng Biotechnol 10:1–11. https://doi.org/10.3389/fbioe.2022.853322
Eusébio TM, Martins AR, Pon G et al (2020) Sorption/diffusion contributions to the gas permeation properties of bi-soft segment polyurethane/polycaprolactone membranes for membrane blood oxygenators. Membranes (basel). https://doi.org/10.3390/membranes10010008
Gayathri G, Srinikethan G (2019) Bacterial cellulose production by K. saccharivorans BC1 strain using crude distillery effluent as cheap and cost effective nutrient medium. Int J Biol Macromol 138:950–957. https://doi.org/10.1016/j.ijbiomac.2019.07.159
Gea S, Reynolds CT, Roohpour N et al (2011) Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour Technol 102:9105–9110. https://doi.org/10.1016/j.biortech.2011.04.077
Gorgieva S, Trček J (2019) Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials 9:1–20. https://doi.org/10.3390/nano9101352
Ha JH, Shah N, Ul-Islam M et al (2011) Bacterial cellulose production from a single sugar α-linked glucuronic acid-based oligosaccharide. Process Biochem 46:1717–1723. https://doi.org/10.1016/j.procbio.2011.05.024
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. II. Biochem J, Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. https://doi.org/10.1042/bj0580345
Hong F, Zhu YX, Yang G, Yang XX (2011) Wheat straw acid hydrolysate as a potential cost-effective feedstock for production of bacterial cellulose. J Chem Technol Biotechnol 86:675–680. https://doi.org/10.1002/jctb.2567
Hwang J, Choi D, Han S et al (2020) Potential toxicity of polystyrene microplastic particles. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-64464-9
Janeni J, Adassooriya NM (2021) Nanocellulose biopolymer-based biofilms: applications and challenges. In: Biopolymer-based nano films: applications in food packaging and wound healing. pp 43–62
Jia Y, Wang X, Huo M et al (2017) Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater Nanotechnol 7:1–8. https://doi.org/10.1177/1847980417707172
Kadier A, Ilyas RA, Huzaifah MRM et al (2021) Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (BC) production: mechanism, advances, and future perspectives. Polymers (basel). https://doi.org/10.3390/polym13193365
Kim H, Son J, Lee J et al (2021) Improved production of bacterial cellulose through investigation of effects of inhibitory compounds from lignocellulosic hydrolysates. GCB Bioenergy 13:436–444. https://doi.org/10.1111/gcbb.12800
Lee KY, Quero F, Blaker JJ et al (2011) Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 18:595–605. https://doi.org/10.1007/s10570-011-9525-z
Lee YJ, An SJ, Bin BE et al (2017) The effect of thickness of resorbable bacterial cellulose membrane on guided bone regeneration. Materials (basel). https://doi.org/10.3390/ma10030320
Li Z, Wang L, Hua J et al (2015) Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr Polym 120:115–119. https://doi.org/10.1016/j.carbpol.2014.11.061
Lide D (2008) CRC handbook of chemistry and physics online
Lu Z, Zhang Y, Chi Y et al (2011) Effects of alcohols on bacterial cellulose production by Acetobacter xylinum 186. World J Microbiol Biotechnol 27:2281–2285. https://doi.org/10.1007/s11274-011-0692-8
Marín P, Martirani-Von Abercron SM, Urbina L et al (2019) Bacterial nanocellulose production from naphthalene. Microb Biotechnol 12:662–676. https://doi.org/10.1111/1751-7915.13399
Michiels Y, Van Puyvelde P, Sels B (2017) Barriers and chemistry in a bottle: mechanisms in today’s oxygen barriers for tomorrow’s materials. Appl Sci. https://doi.org/10.3390/app7070665
Mishra S, Singh PK, Pattnaik R et al (2022) Biochemistry, synthesis, and applications of bacterial cellulose: a review. Front Bioeng Biotechnol 10:1–12. https://doi.org/10.3389/fbioe.2022.780409
Moosavi-Nasab M, Yousefi A (2011) Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran J Biotechnol 9:94–101
Movasaghi Z, Rehman S, ur Rehman DI (2008) Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev 43:134–179. https://doi.org/10.1080/0570492070182904
Mustarichie R, Tahid T, Ramdhani D (2018) Comparison of residual styrene monomer determination of pharmaceutical materials packed with polystyrene using gas chromatography and ultraviolet/visible spectrophotometer. Natl J Physiol Pharm Pharmacol 8:1. https://doi.org/10.5455/njppp.2018.8.0207501032018
Naomi R, Idrus RBH, Fauzi MB (2020) Plant-vs. Bacterial-derived cellulose for wound healing: A review. Int J Environ Res Public Health 17:1–25. https://doi.org/10.3390/ijerph17186803
Nascimento FX, Torres CAV, Freitas F et al (2021) Functional and genomic characterization of Komagataeibacter uvaceti FXV3, a multiple stress resistant bacterium producing increased levels of cellulose. Biotechnol Reports 30:e00606. https://doi.org/10.1016/j.btre.2021.e00606
Neves LA, Crespo JG, Coelhoso IM (2010) Gas permeation studies in supported ionic liquid membranes. J Memb Sci 357:160–170. https://doi.org/10.1016/j.memsci.2010.04.016
Ogrizek L, Lamovšek J, Čuš F et al (2021) Properties of bacterial cellulose produced using white and red grape bagasse as a nutrient source. Processes. https://doi.org/10.3390/pr9071088
Oh SY, Dong IY, Shin Y et al (2005) Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr Res 340:2376–2391. https://doi.org/10.1016/j.carres.2005.08.007
Pang M, Huang Y, Meng F et al (2020) Application of bacterial cellulose in skin and bone tissue engineering. Eur Polym J 122:109365. https://doi.org/10.1016/j.eurpolymj.2019.109365
Park JK, Jung JY, Park YH (2003) Cellulose production by Gluconacetobacter hansenii in a medium containing ethanol. Biotechnol Lett 25:2055–2059. https://doi.org/10.1023/B:BILE.0000007065.63682.18
Park S, Baker JO, Himmel ME et al (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10. https://doi.org/10.1186/1754-6834-3-10
Patti A, Acierno D (2020) The puncture and water resistance of polyurethane-impregnated fabrics after UV weathering. Polymers (basel) 12:1–14. https://doi.org/10.3390/polym12010015
Paunonen S (2013) Strength and barrier enhancements of cellophane and cellulose derivative films: a review. BioResources 8:3098–3121. https://doi.org/10.15376/biores.8.2.3098-3121
Peres MFS, Nigoghossian K, Primo FL et al (2016) Bacterial cellulose membranes as a potential drug delivery system for photodynamic therapy of skin cancer. J Braz Chem Soc 27:1949–1959. https://doi.org/10.5935/0103-5053.20160080
Portela R, Leal CR, Almeida PL, Sobral RG (2019) Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol 12:586–610. https://doi.org/10.1111/1751-7915.13392
Pourramezan GZ, Roayaei AM, Qezelbash QR (2009) Optimization of culture conditions for bacterial cellulose production by Acetobacter sp. 4B–2. Biotechnology 8:150–154. https://doi.org/10.3923/biotech.2009.150.154
Rebelo AR, Archer AJ, Chen X et al (2018) Dehydration of bacterial cellulose and the water content effects on its viscoelastic and electrochemical properties. Sci Technol Adv Mater 19:203–211. https://doi.org/10.1080/14686996.2018.1430981
Rebocho AT, Pereira JR, Freitas F et al (2019) Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in apple pulp waste. Appl Food Biotechnol 6:71–82. https://doi.org/10.22037/afb.v6i1.21793
Revin V, Liyaskina E, Nazarkina M et al (2018) Cost-effective production of bacterial cellulose using acidic food industry by-products. Br J Microbiol 49:151–159. https://doi.org/10.1016/j.bjm.2017.12.012
Riaz U, Ashraf SM (2015) Characterization of polymer blends with FTIR spectroscopy. In: Characterization of polymer blends: miscibility, morphology and interfaces. pp 625–678
Roy N, Saha N, Humpolicek P, Saha P (2010) Permeability and biocompatibility of novel medicated hydrogel wound dressings. Soft Mater 8:338–357. https://doi.org/10.1080/1539445X.2010.502955
Semsarzadeh MA, Sadcghi M, Barikani M (2008) Effect of chain extender length on gas permeation properties of polyurethane membranes. Iran Polym J (english Ed) 17:431–440
Singhsa P, Narain R, Manuspiya H (2018) Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 25:1571–1581. https://doi.org/10.1007/s10570-018-1699-1
Szymańska-Chargot M, Cybulska J, Zdunek A (2011) Sensing the structural differences in cellulose from apple and bacterial cell wall materials by Raman and FT-IR spectroscopy. Sensors 11:5543–5560. https://doi.org/10.3390/s110605543
Tan GYA, Chen CL, Ge L et al (2015) Bioconversion of styrene to poly(hydroxyalkanoate) (PHA) by the new bacterial strain Pseudomonas putida NBUS12. Microbes Environ 30:76–85. https://doi.org/10.1264/jsme2.ME14138
Thongwai N, Futui W, Ladpala N et al (2022) Characterization of bacterial cellulose produced by Komagataeibacter maltaceti P285 isolated from contaminated honey wine. Microorganisms 10:528. https://doi.org/10.3390/microorganisms10030528
Tomé LC, Brandão L, Mendes AM et al (2010) Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties. Cellulose 17:1203–1211. https://doi.org/10.1007/s10570-010-9457-z
Torgbo S, Sukyai P (2020) Biodegradation and thermal stability of bacterial cellulose as biomaterial: the relevance in biomedical applications. Polym Degrad Stab 179:109232. https://doi.org/10.1016/j.polymdegradstab.2020.109232
Tran NPD, Ting CC, Lin CH, Yang MC (2020) A novel approach to increase the oxygen permeability of soft contact lenses by incorporating silica sol. Polymers (basel). https://doi.org/10.3390/POLYM12092087
Vázquez M, Velazquez G, Cazón P (2021) UV-shielding films of bacterial cellulose with glycerol and chitosan. Part 1: equilibrium moisture content and mechanical properties. CYTA J Food 19:105–114. https://doi.org/10.1080/19476337.2020.1870566
Wada M, Okano T (2001) Localization of Iα and Iβ phases in algal cellulose revealed by acid treatments. Cellulose 8:183–188. https://doi.org/10.1023/A:1013196220602
Yang Y, Ponting M, Thompson G et al (2012) Puncture deformation and fracture mechanism of oriented polymers. J Appl Polym Sci 124:2524–2536. https://doi.org/10.1002/app.34109
Yang XY, Huang C, Guo HJ et al (2016) Bacterial cellulose production from the litchi extract by Gluconacetobacter xylinus. Prep Biochem Biotechnol 46:39–43. https://doi.org/10.1080/10826068.2014.958163
Yeh V, Goode A, Johnson D et al (2022) The role of lipid chains as determinants of membrane stability in the presence of styrene. Langmuir 38:1348–1359. https://doi.org/10.1021/acs.langmuir.1c02332
Zhang Y, Chen Y, Cao G et al (2021) Bacterial cellulose production from terylene ammonia hydrolysate by Taonella mepensis WT-6. Int J Biol Macromol 166:251–258. https://doi.org/10.1016/j.ijbiomac.2020.10.172
Zhong C (2020) Industrial-scale production and applications of bacterial cellulose. Front Bioeng Biotechnol 8:1425. https://doi.org/10.3389/fbioe.2020.605374
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (Grant Numbers: Institute of Microbiology, Chinese Academy of Sciences: 31961133016; Beijing Institute of Technology: 31961133015; Shandong University: 31961133014) for support of the Project Bio Innovation of a Circular Economy for Plastics (BioICEP).
Funding
This work was financed by national funds from FCT/MCTES—Fundação para a Ciência e a Tecnologia, I.P., Ministério da Ciência, Tecnologia e Ensino Superior, in the scope of the projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO, project LA/P/0140/202019 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB, projects UIDB/50006/2020 and UIDP/50006/2020 of the Associate Laboratory for Green Chemistry—LAQV, and projects LA/P/0037/2020, UIDP/50025/2020 and UIDB/50025/2020 of the Associate Laboratory Institute of Nanostructures, Nanomodelling and Nanofabrication—i3N, and by the European Union’s Horizon 2020 research and innovation programme through Project Bio Innovation of a Circular Economy for Plastics (BioICEP), under grant agreement No 870292. Asiyah Esmail and Paloma Ortiz-Albo acknowledge FCT I.P. for PhD Grants 2021.05014.BD and SFRH/BD/139389/2018, respectively.
Author information
Authors and Affiliations
Contributions
Conceptualization: AE, CAVT and FF; Methodology: AE, POA, ACM, JVP and AG; Formal analysis and investigation: AE, JVP and LAN; Writing—original draft preparation: AE; Writing—review and editing: CAVT, LAN, MAMR and FF; Funding acquisition: MAMR and FF; Resources: LAN, MAMR and FF; Supervision: CAVT and FF.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esmail, A., Torres, C.A.V., Ortiz-Albo, P. et al. Characterization of bacterial cellulose produced by Komagataeibacter xylinus strains grown in styrene/glucose mixtures. Cellulose 30, 10811–10824 (2023). https://doi.org/10.1007/s10570-023-05559-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05559-0