Abstract
Biobased platform chemicals have attracted growing interest recently. Among them, 3-hydroxypropionic acid receives significant attention due to its applications in the synthesis of novel polymer materials and other derivatives. To establish a biotechnology route instead of the problematic chemical synthesis of 3-hydroxypropionic acid, biosynthetic pathway is required, and the strategies of how to engineer a microbe to produce this product should be considered. In the present review, we summarize and review all known pathways, which could be potentially constructed for 3-hydroxypropionic acid production. Mass and redox balances are discussed in detail. Thermodynamic favorability is evaluated by standard Gibbs free energy. The assembly of pathways and possible solutions are proposed. Several new techniques and future research needs are also covered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3-Hydroxypropionic acid (abbreviated as 3-HP; MF C3H6O3; CAS Registry Number 503-66-2; pK 4.51; MW 90.07884; density 1.08) is a three-carbon non-chiral organic molecule, the 3-hydroxy isomer of lactate. The carboxyl group and β hydroxyl group get 3-HP high reactivity, which is a promising property for using it in production of polymer materials and other chemical feedstocks.
3-HP is of special interest in view of that the biodegradable polymers of it can potentially replace lot kinds of traditional petrochemistry-based polymers and be used in some new fields such as surgical biocomposite material and drug release material. Poly(3-hydroxypropionic acid) [P(3-HP)] exhibits promising physical characteristics: a high glass transition temperature and a melting point of 170–184 ºC, which means unusual heat stability (Mochizuki and Hirami 1997). As an advantage in comparison with poly(l-lactide), poly(d-lactide), and poly(d,l-lactide), P(3-HP) does not have the side-chain methyl groups, which might weaken the intermolecular hydrogen-bonding interactions by steric hindrance and hence influence the strength of the materials (Zhu et al. 2004). Besides, many high-volume commercially intermediates traditionally produced from propylene can be made from 3-HP (see Table 1).
These significant market opportunities rank 3-HP in the list of top 20 value added chemicals from biomass released by the US Department of Energy (Werpy and Petersen 2004).
Several chemical synthesis routes have been described to produce 3-HP including: (1) oxidation from 1,3-propanediol (1,3-PD) via a palladium-containing supported catalyst (Behr et al. 1996), (2) oxidation from 3-hydroxypropionaldehyde (3-HPA) via a palladium-containing supported catalyst (Haas et al. 2000), (3) hydration from acrylic acid in the presence of liquid acid or solid acid catalyst (Meng et al. 2007). The costs of these chemical routes are too high and result in a limited application area and the absence of 3-HP as a bulk chemical. In addition, the conventional industry is based on platform chemicals derived mainly from fossil resources and hence is unsustainable and suffering from the limited source and unstable cost of petroleum. Bioconversion of biomass to chemical building blocks or platform intermediates is an important alternative to petrochemical industry. Compared with chemical routes, biological routes from renewable resources have four advantages: (1) relieving our dependence on ever more expensive petroleum and improving energy security, (2) less or no net contribution of carbon dioxide to the atmosphere, (3) less environmental pollution, (4) more mild operation conditions. A well-known example of platform intermediates produced biologically is lactic acid (2-hydroxypropionic acid). Lactic acid produced by certain bacteria has already proved itself in successful commercial applications such as biodegradable polymers and environmentally friendly solvents. As mentioned previously, 3-HP may have greater potential than lactic acid and offer equal or greater properties than conventional fossil-based products in many application fields. These provide a clear incentive for a biological production of 3-HP. Cargill (http://www.cargill.com) and the United States Department of Energy planned to invest over US $6 million to produce 3-HP from renewable resources.
This review focuses on the synthetic pathways toward 3-HP and their evaluation with respect of the redox balance, metabolic energy generation, and thermodynamic favorability. Two pathways are proposed as most promising ones.
Roles of 3-HP in microbial metabolism
-
1.
3-HP is a key intermediate in the 3-HP cycle, a pathway for autotrophic carbon dioxide fixation, first found in Chloroflexus aurantiacus (Holo 1989) and also reported to be operated in Acidianus brierleyi, Acidianus ambivalens, and Sulfolobus metallics. Sequence comparison suggests that the 3-HP cycle pathways have evolved convergently in the eubacterium and archaea (Ishii et al. 2004). In 2007, a new variant of 3-HP cycle was discovered in Metallosphaera sedula (Berg et al. 2007).
-
2.
A number of organisms such as Alcaligenes latus, Ralstonia eutropha, and Pseudomonas oleovorans can synthesize polyhydroxyalkanoates as storage polymers from exogenously supplied 3-HP with other hydroxyalkanoates (Hiramitsu and Doi 1993).
-
3.
3-HP was found in the degradation of dimethylsulfoniopropionate (DMSP) by the aerobic bacterium Alcaligines faecalis. In marine environments, DMSP comes from the decay of phytoplankton, macroalgae, and phototrophic prokaryotes. DMSP is degraded extracellularly to dimethylsulfide and acrylate by DMSP lyase. Then, the acrylate is metabolized to 3-HP (Ansede et al. 1999).
-
4.
Fungi Byssochlamys sp. (Takamizawa et al. 1993), Geotrichum sp., and Trichoderma sp. (Dave et al. 1996) were reported to be able to metabolize acrylate to 3-HP.
-
5.
3-HP is secreted as a nematicidal principle by endophytic fungi (Schwarz et al. 2004).
-
6.
3-HP is one of the final products of uracil degradation in yeast (Andersen et al. 2008).
Directing metabolic pathways to 3-HP
As mentioned above, several organisms are able to produce 3-HP from acrylic acid, 1,3-propanediol, or propionic acid (Hasegawa et al. 1982). However, these routes are commercially inefficient because the starting compounds required are even more valuable than 3-HP. No known organism produces 3-HP as a major metabolic end product. Genetically modified metabolic pathways are thus required.
Pathways starting with glucose
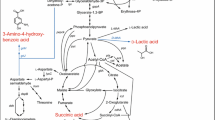
Seven synthetic pathways, via which 3-HP could be produced at a theoretical yield of 100% from glucose, have been patented by Cargill et al. (Gokarn et al. 2001; Liao et al. 2005; Liao et al. 2007; Marx et al. 2007). Figure 1 summarizes these pathways. Table 2 shows the enzymes involved and their sources.
Overview of seven fermentation pathways for the production of 3-HP from glucose. Enzymes involved: 1, phosphoenolpyruvate carboxylase; 2, pyruvate carboxylase; 3, aspartate aminotransferase; 4, aspartate decarboxylase; 5 (5a, 5b, and 5c), CoA transferase; 6, β-alanine-CoA ammonia lyase; 7, 3-HP-CoA dehydratase; 8, glutamate dehydrogenase; 9, 4-aminobutyrate aminotransferase; 10, 3-hydroxyisobutyrate dehydrogenase; 11, lactyl-CoA dehydratase; 12, OS17 enzyme (consists of three functional domains: 12a, CoA synthetase; 12b, dehydrogenase; 12c, 3-HP dehydratase); 13, acetyl-CoA carboxylase; 14, malonyl-CoA reductase; 15, 3-hydroxypropionyl-CoA hydrolase; 16, 3-hydroxyisobutryl-CoA hydrolase; 17, pyruvate-glutamate transaminase; 18, alanine 2,3-aminomutase; 19, β-alanine-2-oxoglutarate aminotransferase; 20, alanine dehydrogenase
In the synthetic pathway for the production of 3-HP from glucose via a lactate intermediate (pathway 1, see Fig. 1, enzymes used: 5a, 11, 7, 5b/15/16), the conversion of glucose to lactate produces one ATP per lactate. CoA transferase consumes no ATP. As a result, this pathway is redox neutral with one net ATP produced per 3-HP. However, if 3-hydroxypropionyl-CoA hydrolase or 3-hydroxyisobutryl-CoA hydrolase is used instead of CoA transferase, two ATPs will be required to recover the thioester link consumed by the CoA hydrolases, and this leads to a negative ATP production. There are majorly two problems with this pathway. (1) As 3-HP is an isomer of lactate, and the K eq for the equilibrium between lactate and 3-HP is estimated to be 0.4 (Herrmann et al. 2005), converting lactate into 3-HP is thermodynamically unfavorable, unless 3-HP is exported selectively while lactate is locked and accumulates inside the cell. Unfortunately, such mechanisms of 3-HP export are not known to date. (2) If lactate is secreted into the fermentation broth as well, the separation of lactate and 3-HP will be very difficult.
In the fermentation pathway with a malonyl-CoA intermediate (pathway 2, see Fig. 1, enzymes used: 13 and 14), the conversion of glucose into acetyl-CoA generates one ATP and two NADH per acetyl-CoA produced. The acetyl-CoA carboxylase consumes one ATP, and the reduction of malonyl-CoA to 3-HP consumes two NADPH per 3-HP (Hugler et al. 2002). As a result, this pathway is redox neutral but produces no net ATP. Besides the lack of metabolic energy production, the other major problem is that the malonyl-CoA reductase cannot accept NADH as a substrate. A similar problem appeared in the fermentation of xylose (Yablochkova et al. 2004). Possible solutions include (1) mutating malonyl-CoA reductase to expand its range of substrates and (2) introducing NAD(P) transhydrogenase (EC 1.6.1.1) into the host to maintain a balance of NADH and NADPH.
In the fermentation pathway with a β-alanine intermediate (pathway 3, see Fig. 1, enzymes used: 1, 2, 3, 8, 4, 5c, 6, 7, and 5b) and the similar pathway 4 (see Fig. 1, enzymes used: 1, 2, 3, 8, 4, 9, and 10), no matter whether PEP carboxylase or pyruvate carboxylase is used, the conversion of glucose into oxaloacetate produces one NADH and no ATP per oxaloacetate. The glutamate dehydrogenase or 3-hydroxyisobutyrate dehydrogenase consumes one NADH. The whole pathways are redox neutral but produce no ATP. To overcome the lack of energy production, two pathways (see Fig. 1, pathway 5, enzymes used: 17, 18, 19, 10 and pathway 6, enzymes used: 20, 18, 5c, 6, 7, 5b) were proposed. Compared with pathway 3 and 4, they bypass the ATP consuming carboxylation by introducing alanine 2,3-aminomutase. So, they are redox neutral with one net ATP produced per 3-HP. A naturally occurring alanine 2,3-aminomutase activity has not been described so far. Thus, a lysine 2,3-aminomutase (B12 dependent) was used as a starting point for mutation to get a enzyme with the desired activity. In addition to the ability of converting α-alanine to β-alanine, the substrate specificity should also be improved to reduce the deleterious effect to the host (Liao et al. 2005).
The fermentation pathway with a propionate intermediate (pathway 7, see Fig. 1, enzymes used: 12 and 15) is based on the succinic acid fermentation pathway, which exists in Actinobacillus sp. (Guettler et al. 1999) and has been constructed in Escherichia coli (Zeikus et al. 1999). The conversion of glucose into propionate can be theoretically carried out with a yield of two propionates, 3.34 ATP, and a consumption of four [H] per glucose (Emde and Schink 1990). The dehydrogenase recovers these [H]. The CoA synthetase requires two ATPs. This pathway is redox neutral with a net energy production of −0.33 ATP per 3-HP. In addition, in C. aurantiacus, OS17 enzyme is responsible for catalyzing the reductive conversion of 3-HP to propionyl-CoA, which is thought to be irreversible (Alber and Fuchs 2002). Thus, it is questionable whether OS17 enzyme can process reactions in desired direction under fermentation conditions.
Pathways starting with glycerol
Glycerol is a main by-product of biodiesel production. Owing to the growth of global biodiesel industry, the price of crude glycerol has dropped dramatically, almost tenfold, to $0.011/kg over the past few years (Yazdani and Gonzalez 2007). Besides, glycerol is non-toxic to many microorganisms even at high concentrations. It will be a competitive substrate for 3-HP fermentation (see Fig. 2).
Fermentation pathways for the production of 3-HP using glycerol as a substrate or intermediate. Enzymes involved: 1, glycerol dehydratase; 2, aldehyde dehydrogenase; 3, 1,3-propanediol oxidoreductase; 4, propionaldehyde dehydrogenase; 5, phosphotransacylase; 6, propionate kinase; 7, glycerol-3-phosphate dehydrogenase; 8, glycerol-3-phosphatase
In one approach (pathway 8, see Fig. 2, enzymes used: 1 and 2), glycerol dehydratase and aldehyde dehydrogenase were introduced into E. coli conferring upon that host the ability to produce 3-HP. The glycerol dehydratase encoding gene dhaB came from Klebsiella pneumoniae; the aldehyde dehydrogenase genes came from Saccharomyces cerevisiae or E. coli (Suthers and Cameron 2001; Jo et al. 2008). In this pathway, exogenous electron acceptors are needed, such as sulfate and CO2. Consequently, sulfide and methane are produced. Methane is an ideal by-product, as it can be readily separated. In coculture with Methanospirillum hungatei, Desulfovibrio carbinolicus produces 3-HP from glycerol almost at a 100% yield with the formation of methane from CO2 (Qatibi et al. 1998). A similar naturally occurring pathway (pathway 9, see Fig. 2, enzymes used: 1, 2, and 3) was found in a number of microorganisms, such as Desulfovibrio fructosovorans, D. carbinolicus, Lactobacillus reuteri, Lactobacillus collinoides, and Ilyobacter polytropus (Stieb and Schink 1984; Vollenweider and Lacroix 2004; Garai-Ibabe et al. 2008). The separation of 3-HP and 1,3-PD is expected to be not difficult due to the high difference between their structures and properties. Thus, an efficient heterofermentation should be possible. However, all of these microorganisms failed to grow with glycerol as the sole carbon source under anaerobic conditions, as this pathway produces no ATP for the cell. When fructose or glucose was added into the culture medium, 1,3-PD was the main end product to recover the NAD used during glycolysis. If an aerobic cofermentation of glycerol and glucose is carried out with the glycerol reoriented to the production of 3-HP and glucose used for cell growth and maintenance, a competitive route might be available. In 2006, an ATP generating pathway (pathway 10, see Fig. 2, enzymes used: 1, 3, 4, 5, and 6) improved from pathway 8 was proposed (Yasuda et al. 2006). The key enzyme propionaldehyde dehydrogenase was obtained from Salmonella enteric (Leal et al. 2003). By replacing 1,3-PD oxidoreductase with exogenous coenzyme-A-acylating propionaldehyde dehydrogenase, phosphotransacylase, and propionate kinase, one net ATP per 3-HP is generated.
As described above, several organisms ferment glycerol to 3-HP, and some other organisms ferment glucose to glycerol, but no known single natural organism can ferment glucose to 3-HP. Thus, it is possible to construct a single organism to produce 3-HP from sugars via a glycerol intermediate. A combination of the pathway 9 and the glycerol production pathway (pathway 11 see Fig. 2, enzymes used: 7, 8, 1, and 2) was mentioned in a patent to synthesize polyhydroxyalkanoate, but not included in the claims (Skraly and Peoples 2003). Glycerol production from glucose by fermentation route was first carried out on a large scale during World War I and has advanced a lot since then (Wang et al. 2001). All of the genes that needed to convert glucose to 3-HPA via glycerol have been cloned (Nakamura and Whited 2003). This combined pathway has a net metabolic energy production of −1ATP per 3-HP. A combination of the pathway 10 and the glycerol production pathway is another option. Because such a combination generates no positive metabolic energy either, its advantage over the pathway 11 should be questionable.
Pathways based on 3-HP cycle
The fifth CO2 fixation pathway, named 3-HP cycle mentioned previously was proposed in C. aurantiacus (Herter et al. 2002). It has a potential for production 3-HP from CO2 and H2O. Considering the costs of raw materials, mainly the carbon sources, usually account for a large part of organic acids manufacturing costs; in the long term, producing 3-HP autotrophically has significant advantages. This possibility is discussed here (pathway 12). Through 3-HP cycle, CO2 is fixed to produce pyruvate. Then, if the pyruvate is diverted back into the 3-HP cycle intermediate pool via succinate, acetyl-CoA, malonyl-CoA, or acryloyl-CoA, the accumulation and secretion of 3-HP will be possible. Wild C. aurantiacus produces low concentrations (1.5 μM) of 3-HP in autotrophically grown cultures, a higher levels (52 μM) are obtained when acetate and KCN are added into the cultures, and the highest concentration (350 μM) are observed when propionate is added. These increases in the 3-HP production could be explained by the entrance of acetate and propionate into the 3-HP cycle intermediate pool, which has been demonstrated by 13C labeling experiments (Holo 1989). To further improve its productivity, genetic modification is required. For example, in an engineered strain, the distribution of all the fixed CO2 is assumed to be directed to 3-HP production. Then, a maximum productivity of 9 nmol 3-HP min-1 (mg of cell protein)-1 could be reached based on the CO2 fixation rate (Herter et al. 2001; Hugler et al. 2002).
Selection of the most attractive routes
Preferably, the constructed pathway should keep a balance of reducing power and generate enough ATP for cell growth, maintenance, and product export, or else additional pathway is required for balancing the reducing power or generating the ATP. Such an additional pathway is thought to be detrimental as it would consume additional fermentation substrate and either require aeration or generate undesired by-product (Straathof et al. 2005). From this view point, pathways 1, 5, 6, and 10 are more attractive. It is worth mentioning that the lactic acid pathway also produces net ATP and is redox-balanced, which contribute to the competitive cost of biobased lactic acid production. However, if the pathways 1, 5, 6, and 10 turn out to be unfeasible in practice, pathways 2, 3, 4, 7, 8, 9, or 11 should be considered, in which no or even negative metabolic energy is produced. To overcome this obstacle, additional pathway is required for ATP generation under aerobic or anaerobic conditions. Additional pathways carried out under anaerobic conditions generally produce much less metabolic energy, and the end products of these pathways such as lactate and acetate are difficult to separate; in contrast, the aerobic strategy requires aeration equipments but is more desirable because it can generate much more ATPs by TCA cycle and oxidative phosphorylation, and the usual end product, carbon dioxide, is much easier to remove from the reactor. The aerobic strategy is supported by several successful examples in 1,3-PD microbial production (Chen et al. 2003; Cheng et al. 2006; Nakamura and Whited 2003).
The thermodynamics of these pathways were examined for further favorability assessment. According to the second law of thermodynamics, the changes in Gibbs free energy must be negative for enzyme reactions or overall pathways to proceed. Experimental thermodynamic data are not available for most reactions involved in the 3-HP production pathways, and the available data were measured under ununified temperature and pH conditions (Goldberg et al. 2004). Thus, group contribution method was utilized as an alternative to estimate the standard Gibbs free energy change of every reaction (Mavrovouniotis 1990; Mavrovouniotis 1991; Jankowski, et al. 2008). The standard state here for Gibbs free energy is a dilute aqueous solution at pH = 7, T = 25 °C, P = 1 atm, and concentrations of reactants and products (other than H+, OH−, and water) equal 1 M. Among the four ATP-producing pathways (Fig. 3), pathways 5 and 10 are thermodynamically favorable. Pathway 1 turns out to be unfavorable because the overall standard Gibbs free energy change is positive. This result is consistent with the previously described problem of the pathway 1 and the reference (Herrmann et al. 2005). In the case of pathway 6, although the standard Gibbs free energy change of the overall pathway is negative, this pathway is thermodynamically unfavorable because four points (standing for four intermediates) are below the end point (standing for 3-HP) in the landscape (e.g., the subpathways from these four points to the 3-HP point is unfavorable). Certain thermodynamically unfavorable steps, the enzyme 19 in pathway 5 and enzyme 5 in pathway 10, do not make the overall pathways unfavorable, but may let to thermodynamic bottlenecks by high substrates concentrations and low products concentrations (Mavrovouniotis 1996). Among the seven non-ATP-producing pathways (Fig. 4), only pathways 3 and 7 are thermodynamically unfavorable. Indeed, the landscape of pathway 7 very well consistent with the reference (Alber and Fuchs 2002).
Thermodynamic free energy landscape of four ATP-producing pathways. Reaction steps are named by the serial numbers of the corresponding enzymes, which are shown in Fig. 1 for pathway 1, 5, and 6 and Fig. 2 for pathway10. Note that the cumulative reaction Gibbs free energy values of the points (metabolites) from different figures cannot be compared, as they are all relative values
Thermodynamic free energy landscape of six non-ATP-producing pathways. Reaction steps are named by the serial numbers of the corresponding enzymes, which are shown in Fig. 1 for pathway 2, 3, 4, and 7 and Fig. 2 for pathway 7, 9, and 11. Pathway 8 is not shown individually, as it can be seen as a subpathway of pathway 11. In the pathway 7, the conversion catalyzed by enzyme 12 is decomposed to three steps assuming that reaction intermediates, propionyl-CoA and acryl-CoA, can be released into solution. Note that the cumulative reaction Gibbs free energy values of the points (metabolites) from different figures cannot be compared, as they are all relative values
In conclusion, the pathways 5 and 10 are most attractive. A new patent (Jessen et al. 2008) suggests that the pathway 5 was chosen by Cargill Company. In this patent, the concern is to increase the activity of the enzyme 19, which is a thermodynamically bottleneck of the pathway 5. Less attractive pathways, pathway 3, 4, 8, and 11, were also attempted by others (Marx et al. 2007; Raj et al. 2008; Burgard and Van Dien 2008). The pathway 12 may have interesting potential as it can produce 3-HP directly from water, carbon dioxide, and sunshine.
Recovery
Titer of 50–100 g/L in broth is expected for commercially competitive production of building block acids by fermentation. However, it is expected that more metabolic energy is needed for cell growth and maintenance with such a high concentration of 3-HP and a resulting low pH, which will reduce the yield and productivity. To maintain appropriate broth pH, large volumes of base titrant are traditionally required. To reduce the cost of the base titrant and simplify the recovery process, it is desirable to carry out the fermentation with high product concentration and low extracellular pH by host modification (Gill and Lynch 2008). For more details of the potential of this strategy, readers are recommended to refer to a review made especially for lactate and 3-HP fermentation (Maris et al. 2004). If the attempts to produce 3-HP in a low pH broth fail, an alternative option remains open for circumventing the use of expensive precipitation. The in situ product recovery (ISPR) can maintain a low product concentration and thus overcome the product inhibition in microbial production of organic acid. Examples of ISPR include reactive extraction (Pai et al. 2002), carbon-dioxide-aided extraction (Van Halsema et al. 1998), electrodialysis, ion exchange, membrane, etc. (Schügerl 2000).
Conclusions
Compared to conventional petrochemical production, biotechnological production of platform chemical has several advantages. Recently, lactic acid and succinic acid as chemical intermediates have been produced by fermentation. 3-HP has the potential to be the next one. Twelve synthetic pathways for producing 3-HP from sugars, glycerol, or carbon dioxide based on known enzyme activities have been proposed. Two of them that can keep a balance of reducing power, generate net metabolic energy, and are thermodynamically favorable that deserve preferential consideration as they are potential to be used for anaerobic fermentations, which are usually more economical than aerobic ones. In future work, the factors that may affect the efficiency of these pathways should be considered, such as the enzyme kinetics, activities of the non-native enzymes in recombined hosts, competing pathways, the growth ability, and the genetical stability of recombined hosts under fermentation conditions (Chotani et al. 2000; Burgard and Van Dien 2008). In order to simplify downstream processes, it is desired to engineer a host which is able to not only live but also export 3-HP efficiently in low pH medium.
Although the outlet of 3-HP is still limited according to existing literatures, commercialization of low cost fermentation routes will expand the current demand and open up new markets for 3-HP. Considering the enormous potential markets and the under-going advances in genetic engineering and metabolic engineering, the chances of a biotechnological production of 3-HP appear to be good.
References
Alber BE, Fuchs G (2002) Propionyl-coenzyme A synthase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J Biol Chem 277:12137–12143
Andersen G, Björnberg O, Polakova S, Pynyaha Y, Rasmussen A, Møller K, Hofer A, Moritz T, Sandrinl MPB, Merico AM, Compagno C, Åkerlund HE, Gojkovic Z, Piškur J (2008) A second pathway to degrade pyrimidine nucleic acid precursors in eukaryotes. J Mol Biol 380(4):656–666
Ansede JH, Pellechia PJ, Yoch DC (1999) Metabolism of acrylate to beta-hydroxypropionate and its role in dimethylsulfoniopropionate lyase induction by a salt marsh sediment bacterium, Alcaligenes faecalis M3A. Appl Environ Microbiol 65:5075–5081
Behr A, Botulinski A, Carduck F-j, Schneider M (1996) process for preparing 3-hydroxypropionic acid. Patent application no. EP0579617
Berg IA, Kockelkorn D, Buckel W, Fuchs G (2007) A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 318:1782–1786
Burgard AP, Van Dien SJ (2008) Methods and organisms for growth-coupled production of 3-hydroxypropionic acid. Patent application no. 20080199926
Chen X, Zhang DJ, Qi WT, Gao SJ, Xiu ZL, Xu P (2003) Microbial fed-batch production of 1, 3-propanediol by Klebsiella pneumoniae under micro-aerobic conditions. Appl Microbiol Biotechnol 63(2):143–146
Cheng KK, Zhang JA, Liu DH, Sun Y, Yang MD, Xu JM (2006) Production of 1, 3-propanediol by Klebsiella pneumoniae from glycerol broth. Biotechnol Lett 28:1817–1821
Chotani G, Dodge T, Hsu A, Kumar M, LaDuca R, Trimbur D, Weyler W, Sanford K (2000) The commercial production of chemicals using pathway engineering. Biochimica Biophysic Acta Protein Struct Mol Enzymol 1543:434–455
Dave H, Ramakrishna C, Desai JD (1996) Degradation of acrylic acid by fungi from petrochemical activated sludge. Biotechnol Lett 18:963–964
Emde R, Schink B (1990) Enhanced propionate formation by Propionibacterium freudenreichii subsp. freudenreichii in a three-electrode amperometric culture system. Appl Environ Microbiol 56:2771–2776
Garai-Ibabe G, Ibarburu I, Berregi I, Claisse O, Lonvaud-Funel A, Irastorza A, Dueñas MT (2008) Glycerol metabolism and bitterness producing lactic acid bacteria in cider making. Int. J Food Microbiol 121:253–261
Gill RT, Lynch MD (2008) Compositions and methods for enhancing tolerance for the production of organic chemicals produced by microorganisms. Patent application no. WO/2008/089102
Gokarn RR, Selifonova OV, Jessen HJ, Steven JG, Selmer T, Buckel W (2001) 3-hydroxypropionic acid and other organic compounds. Patent application no. PCT/US2001/043607
Goldberg RN, Tewari YB, Bhat TN (2004) Thermodynamics of enzyme-catalyzed reactions–a database for quantitative biochemistry. Bioinformatics 20:2874–2877
Guettler MV, Rumler D, Jain MK (1999) Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int J Syst Bacteriol 49:207–216
Haas T, Brossmer C, Meier M, Arntz D, Freund A (2000) Process for preparing 3-hydroxypropionic acid or its salt. Patent application no. EP0819670
Hasegawa J, Ogura M, Kanema H, Kawahara H, Watanabe K (1982) Production of beta-hydroxypropionic acid from propionic acid by a Candida rugosa mutant unable to assimilate propionic acid. J Ferment Technol 60:591–594
Herrmann G, Selmer T, Jessen HJ, Gokarn RR, Selifonova O, Gort SJ, Buckel W (2005) Two beta-alanyl-CoA:ammonia lyases in Clostridium propionicum. FEBS Journal 272:813–821
Herter S, Farfsing J, Gad’On N, Rieder C, Eisenreich W, Bacher A, Fuchs G (2001) Autotrophic CO2 Fixation by Chloroflexus aurantiacus: study of glyoxylate formation and assimilation via the 3-hydroxypropionate cycle. J Bacteriol 183:4305–4316
Herter S, Fuchs G, Bacher A, Eisenreich W (2002) A bicyclic autotrophic CO2 fixation pathway in Chloroflexus aurantiacus. J Biol Chem 277:20277–20283
Hiramitsu M, Doi Y (1993) Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxypropionate). Polymer 34:4782–4786
Holo H (1989) Chloroflexus aurantiacus secretes 3-hydroxypropionate, a possible intermediate in the assimilation of CO2 and acetate. Arch Microbiol 151:252–256
Hugler M, Menendez C, Schagger H, Fuchs G (2002) Malonyl-Coenzyme A reductase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J Bacteriol 184:2404–2410
Ishii M, Chuakrut S, Arai H, Igarashi Y (2004) Occurrence, biochemistry and possible biotechnological application of the 3-hydroxypropionate cycle. Appl Microbiol Biotechnol 64:605–610
Jankowski MD, Henry CS, Broadbelt LJ, Hatzimanikatis V (2008) Group contribution method for thermodynamic analysis of complex metabolic networks. Biophys J 95(3):1487–1499
Jessen HJ, Liao HH, Gort SJ, Selifonova OV (2008) Beta-alanine/alpha-ketoglutarate aminotransferase for 3-hydroxypropionic acid production. Patent application no. WO/2008/027742
Jo JE, Mohan Raj S, Rathnasingh C, Selvakumar E, Jung WC, Park S (2008) Cloning, expression, and characterization of an aldehyde dehydrogenase from Escherichia coli K-12 that utilizes 3-hydroxypropionaldehyde as a substrate. Appl Microbiol Biotechnol 81:51–60
Leal N, Havemann G, Bobik T (2003) PduP is a coenzyme-a-acylating propionaldehyde dehydrogenase associated with the polyhedral bodies involved in B 12-dependent 1, 2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. Arch Microbiol 180:353–361
Liao HH, Gokarn RR, Gort SJ, Jessen HJ, Selifonova OV (2005) Alanine 2,3-aminomutase. Patent application no. EP1575881
Liao HH, Gokarn RR, Gort SJ, Jessen HJ, Selifonova OV (2007). Production of 3-hydropropionic acid using beta-alanine/pyruvate aminotransferase. Patent application no. 20070107080
Maris AJAv, Konings WN, Dijken JPv, Pronk JT (2004) Microbial export of lactic and 3-hydroxypropanoic acid: Implications for industrial fermentation processes. Metab Eng 6:245–255
Marx A, Wendisch VF, Rittmann D, Buchholz S (2007) Microbiological production of 3-hydroxypropionic acid. Patent application no WO/2007/042494
Mavrovouniotis ML (1990) Group contributions for estimating standard gibbs energies of formation of biochemical compounds in aqueous solution. Biotechnol Bioeng 36:1070–1082
Mavrovouniotis ML (1991) Estimation of standard Gibbs energy changes of biotransformations. J Biol Chem 266:14440–14445
Mavrovouniotis ML (1996) Duality theory for thermodynamic bottlenecks in bioreaction pathways. Chem Eng Sci 51:1495–1507
Meng XS, Abraham T, Tsobanakis P (2007) Process for preparing 3-hydroxycarboxylic acids. Patent application no. 20070015936
Mochizuki M, Hirami M (1997) Structural effects on the biodegradation of aliphatic polyesters. Polymer Adv Tech 8:203–209
Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1, 3-propanediol. Curr Opin Biotechnol 14(5):454–459
Pai RA, Doherty MF, Malone MF (2002) Design of reactive extraction systems for bioproduct recovery. AICHE J 48:514–526
Qatibi AI, Bennisse R, Jana M, Garcia JL (1998) Anaerobic degradation of glycerol by Desulfovibrio fructosovorans and D. carbinolicus and evidence for glycerol-dependent utilization of 1, 2-propanediol. Curr Microbiol 36:283–290
Raj SM, Rathnasingh C, Jo JE, Park S (2008) Production of 3-hydroxypropionic acid from glycerol by a novel recombinant Escherichia coli BL21 strain. Process Biochem 43(12):1440–1446
Schügerl K (2000) Integrated processing of biotechnology products. Biotechnol Adv 18:581–599
Schwarz M, Köpcke B, Weber R, Sterner O, Anke H (2004) 3-Hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochem 65:2239–2245
Skraly FA, Peoples OP (2003) Polyhydroxyalkanoate production from polyols. Patent application no. 6576450
Stieb M, Schink B (1984) A new 3-hydroxybutyrate fermenting anaerobe, Ilyobacter polytropus, gen. nov. sp. nov., possessing various fermentation pathways. Arch Microbiol 140:139–146
Straathof AJJ, Sie S, Franco TT, van der Wielen LAM (2005) Feasibility of acrylic acid production by fermentation. Appl Microbiol Biotechnol 67(6):727–734
Suthers PF, Cameron DC (2001) Production of 3-hydroxypropionic acid in recombinant organisms. Patent application no. PCT WO 01-16346
Takamizawa K, Horitsu H, Ichikawa T, Kawai K, Suzuki T (1993) β-hydroxypropionic acid production by Byssochlamys sp. grown on acrylic acid. Appl Microbiol Biotechnol 40:196–200
Van Halsema FED, Van der Wielen LAM, Luyben KCAM (1998) The modeling of carbon dioxide-aided extraction of carboxylic acids from aqueous solutions. Ind Eng Chem Res 37:748–758
Vollenweider S, Lacroix C (2004) 3-Hydroxypropionaldehyde: applications and perspectives of biotechnological production. Appl Microbiol Biotechnol 64:16–27
Wang ZX, Zhuge J, Fang H, Prior BA (2001) Glycerol production by microbial fermentation: a review. Biotechnol Adv 19:201–223
Werpy T, Petersen G (2004) Top value added chemicals from biomass, vol 1: results of screening for potential candidates from sugars and synthesis gas. US Department of Energy http://www.osti.gov/bridge
Yablochkova EN, Bolotnikova OI, Mikhailova NP, Nemova NN, Ginak AI (2004) The activity of key enzymes in xylose-assimilating yeasts at different rates of oxygen transfer to the fermentation medium. Microbiology 73:129–133
Yasuda S, Mukoyama M, Horikawa H, Toraya T, Morita H (2006) Process for producing 1,3-propanediol and/or 3-hydroxypropionic acid. Patent application no. EP1731604
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18(3):213–219
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552
Zhu B, Li J, He Y, Yamane H, Kimura Y, Nishida H, Inoue Y (2004) Effect of steric hindrance on hydrogen-bonding interaction between polyesters and natural polyphenol catechin. J Appl Polym Sci 91:3565–3573
Acknowledgment
This work was financially supported by the CAS 100 Talents Program (KGCX2-YW-801).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, X., Meng, X. & Xian, M. Biosynthetic pathways for 3-hydroxypropionic acid production. Appl Microbiol Biotechnol 82, 995–1003 (2009). https://doi.org/10.1007/s00253-009-1898-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1898-7