Abstract
UDP-hexose 4-epimerases are important enzymes that play key roles in various biological pathways, including lipopolysaccharide biosynthesis, galactose metabolism through the Leloir pathway, and biofilm formation. Unfortunately, the determinants of their substrate specificity are not yet fully understood. They can be classified into three groups, with groups 1 and 3 preferring non-acetylated and acetylated UDP-hexoses, respectively, whereas members of group 2 are equally active on both types of substrates. In this study, the UDP-Glc(NAc) 4-epimerase from Marinithermus hydrothermalis (mGalE) was functionally expressed in Escherichia coli and thoroughly characterized. The enzyme was found to be thermostable, displaying its highest activity at 70 °C and having a half-life of 23 min at 60 °C. Activity could be detected on both acetylated and non-acetylated UDP-hexoses, meaning that this epimerase belongs to group 2. This observation correlates well with the identity of the so-called “gatekeeper” residue (Ser279), which has previously been suggested to influence substrate specificity (Schulz et al., J Biol Chem 279:32796–32803, 2004). Furthermore, substituting this serine to a tyrosine brings about a significant preference for non-acetylated sugars, thereby demonstrating that a single residue can determine substrate specificity among type 1 and type 2 epimerases. In addition, two consecutive glycine residues (Gly118 and Gly119) were identified as a unique feature of GalE enzymes from Thermus species, and their importance for activity as well as affinity was confirmed by mutagenesis. Finally, homology modeling and mutational analysis has revealed that the enzyme’s catalytic triad contains a threonine residue (Thr117) instead of the usual serine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
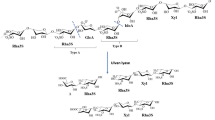
UDP-hexose 4-epimerases belong to the superfamily of short-chain dehydrogenase/reductase (SDR), which typically show a two-domain structure. The N-terminal domain forms a modified Rossmann fold and is involved in binding of the cofactor NAD+, whereas the smaller C-terminal domain is responsible for substrate binding (Holden et al. 2003). The mechanistical aspects of these epimerases have thoroughly been studied and described earlier (Liu et al. 1997; Thoden et al. 1996a, b; Wee and Frey 1973; Wee et al. 1972). Shortly summarized, a proton is abstracted from the 4′-hydroxyl group by a tyrosine, which is acidified by a nearby lysine. A serine facilitates this abstraction by creating a low-barrier hydrogen bond and so completes the catalytic triad (SxnYx3K). Subsequent hydride transfer to the NAD+ cofactor generates a keto intermediate, which is then flipped over inside the catalytic cleft. The final re-addition of the proton and hydride thus proceeds at the opposite face of the sugar, resulting in an overall C4-epimerization of the substrate.
UDP-hexose 4-epimerases are important in numerous biological pathways such as lipopolysaccharide (LPS) biosynthesis (Bhatt et al. 2011b; Ishiyama et al. 2004), galactose metabolism through the Leloir pathway (Holden et al. 2003; Leloir 1951), and biofilm formation (Niou et al. 2009). Defects in UDP-hexose 4-epimerase activity in humans leads to epimerase-deficient galactosemia (Bhatt et al. 2011b). Furthermore, these epimerases are important targets for drug development since LPS is implicated in different facets of host–pathogen interactions (Creuzenet et al. 2000). To do this in an efficient way, however, it is necessary to understand the enzymes’ determinants for substrate affinity and specificity. Three groups of UDP-hexose 4-epimerases have been described, namely group 1 that mainly accepts non-acetylated UDP-hexoses, group 2 that displays similar activity on both non-acetylated and acetylated substrates, and group 3 that prefers acetylated UDP-hexoses (Creuzenet et al. 2000; Ishiyama et al. 2004).
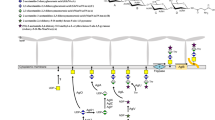
To account for these differences, a model has been proposed that depicts the active site as a hexagonal box (Demendi et al. 2005; Ishiyama et al. 2004). The bottom of the box is formed by the NAD+ cofactor, three of the six walls consist of conserved residues, whereas the other three are believed to be key determinants for substrate specificity (Fig. 1). In particular, a so-called gatekeeper residue is present on one of these walls and the type of amino acid found at this position roughly defines whether the epimerase discriminates substrates based on the presence of an N-acetyl group (Schulz et al. 2004). Indeed, a large residue such as Tyr results in a narrow substrate pocket, in which acetylated substrates cannot bind. Smaller residue (Ser, Cys), in contrast, allow acetylated UDP-hexoses to enter the catalytic site (Ishiyama et al. 2004). In addition, a specific stretch of hydrophobic amino acids (the “297–308 belt”) in group 3 enzymes is believed to form tight interactions with the carbohydrate’s N-acetyl group, which could explain their preference for UDP-GalNAc over UDP-Gal (Bhatt et al. 2011a, b).
Homology model of mGalE. The position of S116 and T117 indicates that the latter is most likely the catalytic residue. The NAD+ forms the bottom of the hexagonal box, whereas T117 (yellow), Y143 (blue), and N172 (orange) are the three conserved walls. The gatekeeper S279 (green) and its tyrosine mutant are both shown to emphasize their impact on the size of the active site. Of the two other variable walls, only A77 (red) is shown, since Q178-V186 (purple in Online Resource 2) would be blocking the view
Interestingly, the UDP-hexose 4-epimerase from Escherichia coli has also been found to convert free monosaccharides, albeit at a much lower rate and with a high K m value (Kim et al. 2011). Epimerases, thus, also hold significant promise for the production of rare sugars (Beerens et al. 2012), although considerable optimization will be required to become industrially viable. Since thermostable enzymes are typically preferred for industrial applications (Haki and Rakshit 2003; Turner et al. 2007), we have decided to examine the putative UDP-Glc(NAc) 4-epimerase from the thermophilic organism Marinithermus hydrothermalis (mGalE) (Sako et al. 2003). In this article, we describe the heterologous expression and characterization of the enzyme, including its thermal properties and substrate specificity. Furthermore, the determinants of its specificity have been analyzed by means of sequence alignments, homology modeling, and site-directed mutagenesis.
Materials and methods
Enzyme expression and purification
A codon optimized sequence encoding mGalE (NCBI YP_004368664.1) with a His6-tag attached to its N-terminus (Online Resource 1) was ordered from GenScript (USA). The gene was cut from the supplied plasmid using 10 U of both NcoI and SpeI restriction enzymes (New England Biolabs, USA) and subsequently cloned into a likewise treated empty pTRC99A plasmid (Pharmacia Biotech Inc., USA) containing the same His-tag with linker and multiple cloning site as the consecutive expression vector pCXhPxx reported earlier (Aerts et al. 2011), using a 3:1 insert/vector ratio and three Weiss units of T4 DNA ligase (New England Biolabs) at 22 °C for 1 h. After electroporation in E. coli BL21 (DE3), the culture was grown at 37 °C and 200 rpm in Luria broth containing 100 μg/ml ampicillin. For the production of enzyme, 10 mL of an overnight culture was inoculated in 500-mL fresh medium, which was then grown until OD600 ≈ 0.6. Subsequently, IPTG was added to a final concentration of 150 μM to induce enzyme expression. After six more hours of incubation, biomass was harvested by centrifugation for 20 min at 5,000 × g at 4 °C, and the cell pellet was stored at −20 °C.
For enzyme purification, frozen pellets were thawed on ice for 15 min and subsequently dissolved in lysis buffer containing 300 mM NaCl, 50 mM NaH2PO4, 10 mM imidazole, 0.1 mM PMSF, 1 mg/mL lysozyme, and 6 U/L Benzonase® nuclease (Merck, Germany). Lysis was achieved by 30 min of incubation on ice followed by sonication with a Branson sonifier 250 (three times for 2.5 min at level 3 and 50 % duty cycle). Cell debris was pelleted by centrifugation (30 min, 13,000 × g, 4 °C), and the His-tagged protein was purified from the lysate by Ni-NTA chromatography according to the supplier’s protocol (ThermoScientific, Belgium), with wash and elution buffers containing 35 and 250 mM imidazole, respectively. After elution, exchange of the buffer to 10 mM Tris–HCl at pH 7.5 was performed using Centricon YM-30 (Millipore, USA). The protein concentration of the samples was analyzed with the Pierce® BCA Protein assay kit (ThermoScientific).
Site-directed mutagenesis
Mutations were introduced according to the protocol described by Sanchis et al. (2008). Briefly summarized, reaction mixtures contained 50–100 ng of wild-type plasmid as template, 5 pmol of mutagenic primer, and a non-mutagenic reverse primer (Table 1), 0.2 mM dNTP mix, 2.5 U of PfuUltraTM High-Fidelity DNA polymerase, and the supplied buffer (Stratagene, The Netherlands) in a final volume of 50 μL. The PCR program consisted of the following steps: initial denaturation for 3 min at 95 °C, followed by 5 cycles of denaturation for 30 s at 95 °C, annealing for 45 s at 53 °C, and extension for 1 min/kb according to the megaprimer size at 72 °C. The second stage consisted of whole plasmid amplification starting from the created megaprimer by 20–25 cycles of 30 s at 95 °C and extension at 2 min/kb of template at 68 °C. The program was ended by a final extension of 2 min/kb of template at 68 °C. PCR mixtures where then digested with DpnI to remove template DNA, purified using the QIAquick PCR purification kit (Qiagen, The Netherlands) and transformed into electrocompetent BL21 cells as described above. Plasmids were then extracted using the QIAprep spin miniprep kit (Qiagen), and the mutations were checked by sequencing (Agowa, Germany).
Activity measurements
The activity of mGalE on UDP-Gal was determined using the colorimetric GOD-POD assay described earlier (Moreno et al. 1981). This assay determines the amount of glucose which is released after acid hydrolysis of the formed UDP-glucose. The formation of UDP-Gal from UDP-Glc was measured in a similar fashion, using the commercially available Lactose/d-Galactose Assay Kit (Megazyme, Ireland). In general, epimerase reaction mixtures consisted of 50 μg/mL enzyme and 2 mM substrate in 5 mM Tris–HCl at pH 7.5 and 45 °C. Samples (25 μL) were taken at different time points and adjusted to pH 2.0 by addition of 5 μL of 0.1 N HCl. After boiling for 6 min, neutralization was achieved with equimolar amounts of 0.1 N NaOH. Free monosaccharides were then measured by the addition of 200–250 μl of the colorimetric reagents. Standards (0.05–1.00 mM of UDP-Glc or UDP-Gal) were treated in the same way.
The temperature optimum was determined in 5 mM Tris–HCl at pH 8.6, whereas the pH optimum was determined at 45 °C in 5 mM of Tris–HCl (pH 2), acetate (pH 4–5), citrate (pH 6), GlyGly (pH 7.6), Tris–HCl (pH 7–9), or glycine (pH 8.6–10.6) buffers. Kinetic studies were performed with eight different substrate concentrations (0.3–3 mM), and the Michaelis–Menten parameters were calculated from a Hanes–Woolf linearization (Copeland 2000). Thermostability studies were performed by incubating purified enzyme at 45 or 60 °C for different time periods before initiating the reaction by the addition of substrate.
Conversion of the N-acetylated UDP-sugars was performed under the same conditions as for non-acetylated UDP-sugars. However, samples were immediately inactivated by boiling without prior acidification. Analysis was then performed using a HPAEC-PAD system (ICS-3000 system, Dionex, Belgium) with a CarboPac PA20 column (Dionex) at 30 °C. To that end, sodium acetate (NaOAc) gradient from 0.50 to 0.68 M was applied over a time span of 12 min. The NaOAc concentration was afterwards brought back to 0.50 M in 1 min, and the column was re-equilibrated during 1 min. The flow rate and NaOH concentration were kept constant during analysis and re-equilibration, at 0.5 mL/min and 100 mM, respectively. A calibration curve was established with 1–100 μM of N-acetylated UDP-sugar.
The wild-type mGalE and the S279Y mutant were also tested for their ability to epimerize free monosaccharides and α-Glc-1-P. The reaction was performed in 5 mM Tris at pH 7.5 and at 45 °C to allow prolonged incubation times (24 h). High enzyme concentrations (up to 1 mg/mL) were achieved by concentration of the purified enzyme solutions with a Centricon YM-30 (Millipore) before adding them to the substrate (500 mM). Samples were analyzed using the previously mentioned HPAEC-PAD system. Free monosaccharides (Fru, Tag, Glc, and Gal) were separated using an isocratic flow of 10 mM NaOH during 25 min on a PA20 column at 30 °C (0.5 mL/min). A gradient was applied for the separation of the phosphorylated sugars, α-Gal-1-P, and α-Glc-1-P. While NaOH concentration was kept constant at 100 mM, the NaOAc concentration gradually increased from 100 to 175 mM in 7.5 min at a 0.5 mL/min flow rate.
Sequence analysis and homology modeling
The identification of conserved residues was performed by sequence alignment, using ClustalW2 (Larkin et al. 2007) as well as the NCBI BLAST tool (Altschul et al. 1990). Homology models of the wild-type and variant enzymes were constructed with YASARA (Krieger et al. 2002), using the default settings and the crystal structures of UDP-Glc 4-epimerase from E. coli (Thoden et al. 1996a, 1997, 2002) as template. The structures were visualized with PyMOL (DeLano 2002).
Results
Sequence analysis and homology modeling
A multiple sequence alignment of GalE enzymes (Online Resource 2) has revealed that the UDP-hexose 4-epimerase from M. hydrothermalis shares 30 % amino acid identity with E. coli GalE, 27 % with human GalE, and 30 % with the WbpP enzyme from Pseudomonas aeruginosa, which are typical representatives of the three different types of substrate specificities (Demendi et al. 2005; Ishiyama et al. 2004). A higher identity of 66 % was found with the UDP-hexose 4-epimerases from Thermus thermophilis and Thermus aquaticus (tGalE), which are active on UDP-GalNAc as well as UDP-Gal (Niou et al. 2009). More importantly, a serine residue (S279) is observed at the gatekeeper position in both mGalE and tGalE, pointing towards a similar substrate preference. One notable difference between GalE from Thermus species and other UDP-hexose 4-epimerases, however, is the presence of two glycine residues (G118 and G119) close to the catalytic triad, which are replaced by a single alanine or serine in the other members. Because of this insertion, the identity of the catalytic residue that is involved in the low-barrier hydrogen bond is difficult to ascertain. Indeed, S116 has previously been postulated to have this function in tGalE (Niou et al. 2009), but that residue could correspond to either S116 or T117 in our alignment. However, the construction of a homology model has revealed that T117 is positioned close to the substrate’s C4-OH, whereas S116 is pointing away from the active site (Fig. 1).
Enzyme production and characterization
The gene coding for mGalE was chemically synthesized with a codon usage that is optimal for expression in E. coli. Furthermore, a His6-tag was added to the enzyme’s N-terminus, which allowed its convenient purification by affinity chromatography. In that way, about 2 mg of enzyme could be obtained from a culture of 1 L. The purified enzyme was subsequently used for activity tests. The highest activity on UDP-Gal could be observed at 70 °C, which corresponds to the optimal growth temperature of M. hydrothermalis (Sako et al. 2003). At temperatures above 75 °C, however, fast inactivation of the epimerase was observed (<1–2 min). The optimal pH for activity was found to be 7–7.5, with more than 50 % activity being retained between pH 6 and 9.
The enzyme’s thermostability was then examined in more detail by following its inactivation at 60 °C, i.e., the temperature at which most carbohydrate conversions are performed in industry (Haki and Rakshit 2003; Turner et al. 2007). Its half-life (t50) was found to be 23 min, with the activity being all but gone after about 2 h (Online Resource 3). Because longer incubation times might be necessary for measurements with alternative substrates or mutant enzymes, the kinetic stability was also determined at lower temperatures. At 45 °C, the t50 was found to have increased to about 13.5 h. Therefore, it was decided to perform all other activity tests at this temperature, so as to avoid the risk of protein denaturation during further characterization.
The mGalE was found to display activity on acetylated as well as non-acetylated UDP-hexoses and can, thus, be classified as type 2 epimerase. However, a small preference for the latter substrates could be detected, with a specific activity on UDP-Gal and UDP-Glc of 3.0 and 6.7 U/mg, respectively, compared to 1.2 and 0.3 U/mg on their respective acetylated counterparts. The determination of kinetic parameters confirmed this observed difference in activity (k cat), whereas the enzyme’s affinity (K m) is much less affected by the presence of an N-acetyl group (Table 2).
Mutational analysis
Since both S116 and T117 carry a hydroxyl group that could be part of the catalytic triad, both residues have been mutated to alanine. Variant S116A was found to be almost as active as the wild-type enzyme on UDP-Gal, whereas no activity could be detected with variant T117A (Fig. 2). Interestingly, the activity of the latter could be restored by a substitution with serine (T117S), confirming that both types of residues can function as catalytic amino acid in GalE enzymes. Both S116A and T117S were also tested on UDP-GlcNAc and found to exhibit a slightly higher activity than the wild-type enzyme. Interestingly, the determination of kinetic parameters has revealed that the catalytic threonine’s methyl group is involved in a specific interaction with the substrate’s N-acetyl group (Table 2). Indeed, the affinity of T117S for UDP-GlcNAc has significantly decreased, but that is much less the case for UDP-Gal.
As previously mentioned, mGalE and the other GalE enzymes from Thermus species (Niou et al. 2009) exhibit the unique feature of having two consecutive glycine residues directly next to the threonine from the catalytic triad, whereas the others possess a single alanine or serine. Introducing the corresponding mutations in mGalE reduces the specific activity on UDP-Gal to approximately 10–30 % of that of the wild-type enzyme (Fig. 2). The activity of both mutants on UDP-GlcNAc was reduced even more, to about 3–7 % of the wild-type activity. These effects were found to involve changes in k cat as well as in K m (Table 2). Indeed, the affinity of both mutants for UDP-Gal has clearly increased, whereas the turnover number has significantly decreased.
The serine at position 279 constitutes the so-called gatekeeper residue in the hexagonal box model (Ishiyama et al. 2004). Depending on the type of residue found here, the epimerase does or does not have activity on N-acetylated UDP-sugars. In agreement with the proposed model, variant S279Y was found to lose nearly all activity on UDP-GlcNAc, whereas nearly 40 % of its activity on UDP-Gal is retained (Fig. 2). More specifically, only a small decrease in K m value for the latter substrate can be observed, whereas the k cat shows a much larger decrease (Table 2).
Activity on free and phosphorylated monosaccharides
In contrast to previous observations with the GalE from E. coli (Kim et al. 2011), no activity on free monosaccharides (Glc, Fru) could be detected with mGalE. In order to trigger the activating effect of the nucleotide group (Samuel and Tanner 2002), either uridine, UMP, or UDP was added to the reaction, but activity could still not be detected. Since eGalE is a type 1 epimerase that harbors a small active site and large gatekeeper, the S279Y mutant of mGalE was also checked for activity on free monosaccharides, but that was not the case. Finally, α-glucose 1-phosphate was tried as substrate, since this compound resembles UDP-Glc more closely than a free monosaccharide. However, activity could not be detected again, neither with wild-type mGalE nor with its S279Y variant.
Discussion
Because UDP-hexose 4-epimerases play crucial roles in several biological pathways, they have become important targets for drug development (Creuzenet et al. 2000). In addition, epimerases hold significant potential for the biochemical production of rare sugars (Beerens et al. 2012; Kim et al. 2011). For both applications, understanding the determinants that contribute to the enzymes’ substrate affinity and specificity is a major benefit. In order to obtain more insights in these determinants, we have characterized a new representative, namely the putative UDP-hexose 4-epimerase from the thermophilic bacterium M. hydrothermalis and performed mutational analysis of the enzyme.
The mGalE was found to be active in a broad pH range (pH 6–9), which is rather typical for this enzyme class. However, the highest activity was measured at pH 7–7.5, whereas the optimum for the related GalE enzymes from T. thermophilus and T. aquaticus was reported to be pH 8.6 (Niou et al. 2009). As expected for an enzyme from a thermophilic organism, the optimal reaction temperature is rather high, at about 70 °C. This value is higher than that of its homologues from Thermus species (Niou et al. 2009) but 10 °C lower than that of the GalE from the hyperthermophilic archaeon Pyrobaculum calidifontis (Sakuraba et al. 2011). The Marinithermus enzyme, however, does not display the same thermostability as these counterparts, since it is quickly inactivated at temperatures above 75 °C. In contrast, the Thermus epimerases retain most of their activity after 10 min of incubation at 80 °C (Niou et al. 2009), whereas the P. calidifontis epimerase even remains fully active after 10 min at 90 °C (Sakuraba et al. 2011).
The mGalE can be classified as a type 2 UDP-hexose 4-epimerases since it displays activity on both non-acetylated and acetylated UDP-hexoses, albeit with a slight preference for the former. However, mutating its so-called gatekeeper residue S279 to a larger tyrosine made the enzyme specific for non-acetylated substrates. A similar observation has been reported for the C307Y and C297Y variants of human GalE (Holden et al. 2003) and Yersinia enterocolitica Gne (Bengoechea et al. 2002), respectively. Similarly, the reverse mutation in the E. coli enzyme (Y299C) lowered the activity on UDP-Gal almost fivefold, while the activity on UDP-GalNAc increased more than 230-fold (Guo et al. 2006). As such, it can be stated that the gatekeeper is indeed a prime determinant for substrate specificity in UDP-hexose 4-epimerases, especially in type 1 and type 2 enzymes. However, their possible conversion into type 3 epimerases is complicated by the need for a hydrophobic “297–308 belt” in the latter enzymes (Bhatt et al. 2011b). Furthermore, introducing a mutation in the active site can also affect other parameters of the protein. For example, the substitution of serine by tyrosine in mGalE resulted in complete inactivation after an overnight storage at 4 °C, whereas the wild-type retained most of its activity under those conditions.
Mutational analysis also revealed that the Marinithermus enzyme makes use of a TxnYx3K catalytic triad rather than the usual SxnYx3K triad. Indeed, replacing T117 by alanine almost completely abolished activity, whereas a similar replacement of the S116 only had a minor effect. It is known that the catalytic serine is replaced by threonine in some members of the SDR family (Blankenfeldt et al. 2002; Gerratana et al. 2001; Mulichak et al. 1999), but this is the first time that such a switch is demonstrated for GalE enzymes. However, the neighboring serine seems to be important for the enzyme’s stability, since variant S116A lost about 30 % of its activity after an overnight storage at 4 °C. This lowered stability could be the result of the loss of hydrogen bonds between the serine’s hydroxyl group and the main chain of the nearby residues Leu168, Phe114, and Arg169.
In GalE enzymes from Thermus species, two consecutive glycine residues can be observed next to the catalytic threonine. Exchanging these by a single alanine or serine influenced the activity on acetylated UDP-hexoses more than on the non-acetylated counterparts, thus pointing to a role in determining substrate specificity. A possible explanation might be found in the homology models of these variants, which suggest the formation of a 310-helix between residues 118 and 121 instead of the coil or loop in the wild-type enzyme (Fig. 3). As a consequence, A120 is pushed into the active site, thereby decreasing the cavity size and limiting the rotational freedom of acetylated substrates. On the other hand, a smaller cavity could improve the interactions with non-acetylated substrates, which is observed in the form of an increased affinity. In turn, the 310-helix could explain the variants’ decreased turnover number as this more stable structure would limit the flexibility of the catalytic threonine residue.
Although very low activity on free monosaccharides has been reported for the E. coli epimerase (Kim et al. 2011), a similar observation could not be made with mGalE even when high enzyme concentrations and long incubation times were used. Furthermore, it is well known that the presence of the UDP-group is required for tight binding of the substrate as well as to increase the reactivity of the enzyme-bound NAD+ cofactor (Wee and Frey 1973; Wong and Frey 1977). As such, GalE would only be able to oxidize free sugars to the C4-ketose intermediate, leaving behind an inactivated enzyme in its NADH-bound form (Bertland et al. 1966; Blackburn and Ferdinand 1976). Although this enzyme is in itself not active towards free monosaccharides, it can be incorporated in multienzyme processes for the production of rare sugars like N-acetylgalactosamine (Inoue et al. 2011), lacto-N-biose, and galacto-N-biose (Nishimoto and Kitaoka 2009). Furthermore, the Marinithermus epimerase can be used as stable biocatalyst for the production of UDP-Gal and UDP-GalNAc from their cheaper glucose-containing counterparts, thus providing valuable donor substrates for glycan synthesis with glycosyl transferases (Desmet and Soetaert 2011; Kwan and Withers 2011).
In conclusion, a novel UDP-Glc(NAc) 4-epimerase from M. hydrothermalis is described in this work. The enzyme was recombinantly expressed in E. coli and thoroughly characterized with respect to its substrate specificity and thermal behavior. Furthermore, analysis of its sequence and structure generated new insights in the mechanism of GalE enzymes, which were confirmed by site-directed mutagenesis.
References
Aerts D, Verhaeghe T, De Mey M, Desmet T, Soetaert W (2011) A constitutive expression system for high-throughput screening. Eng Life Sci 11:10–19
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Beerens K, Desmet T, Soetaert W (2012) Enzymes for the biocatalytic production of rare sugars. J Ind Microbiol Biotechnol 39:823–834
Bengoechea JA, Pinta E, Salminen T, Oertelt C, Holst O, Radziejewska-Lebrecht J, Piotrowska-Seget Z, Venho R, Skurnik M (2002) Functional characterization of gne (UDP-N-acetylglucosamine-4-epimerase), Wzz (chain length determinant), and Wzy (O-antigen polymerase) of Yersinia enterocolitica serotype O: 8. J Bacteriol 184:4277–4287
Bertland A, Bugge B, Kalckar HM (1966) Fluorescence enhancement of uridine diphosphogalactose 4-epimerase induced by specific sugars. Arch Biochem Biophys 116:280–283
Bhatt VS, Guan W, Xue M, Yuan H, Wang PG (2011a) Insights into role of the hydrogen bond networks in substrate recognition by UDP-GaINAc 4-epimerases. Biochem Biophys Res Commun 412:232–237
Bhatt VS, C-y G, Guan W, Zhao G, Yi W, Z-j L, Wang PG (2011b) Altered architecture of substrate binding region defines the unique specificity of UDP-GalNAc 4-epimerases. Protein Sci 20:856–866
Blackburn P, Ferdinand W (1976) The concerted inactivation of Escherichia coli uridine diphosphate galactose 4-epimerase by sugar nucleotide together with a free sugar. Biochem J 155:225–229
Blankenfeldt W, Kerr ID, Giraud MF, McMiken HJ, Leonard G, Whitfield C, Messner P, Graninger M, Naismith JH (2002) Variation on a theme of SDR: dTDP-6-deoxy-l-lyxo-4-hexulose reductase (RmID) shows a new Mg2 + −dependent dimerization mode. Structure 10:773–786
Copeland RA (2000) Enzymes: a practical introduction to structure, mechanism, and data analysis. Wiley, USA
Creuzenet C, Belanger M, Wakarchuk WW, Lam JS (2000) Expression, purification, and biochemical characterization of WbpP, a new UDP-GlcNAc C4 epimerase from Pseudomonas aeruginosa serotype O6. J Biol Chem 275:19060–19067
DeLano WL (2002) The PyMOL molecular graphics system. http://www.pymol.org. Accessed 20 September 2012
Demendi M, Ishiyama N, Lam JS, Berghuis AM, Creuzenet C (2005) Towards a better understanding of the substrate specificity of the UDP-N-acetylglucosamine C4 epimerase WbpP. Biochem J 389:173–180
Desmet T, Soetaert W (2011) Enzymatic glycosyl transfer: mechanisms and applications. Biocatal Biotransform 29:1–18
Gerratana B, Cleland WW, Frey PA (2001) Mechanistic roles of Thr134, Tyr160, and Lys 164 in the reaction catalyzed by dTDP-glucose 4,6-dehydratase. Biochemistry 40:9187–9195
Guo HJ, Li L, Wang PG (2006) Biochemical characterization of UDP-GlcNAc/Glc 4-epimerase from Escherichia coli O86: B7. Biochemistry 45:13760–13768
Haki GD, Rakshit SK (2003) Developments in industrially important thermostable enzymes: a review. Bioresour Technol 89:17–34
Holden HM, Rayment I, Thoden JB (2003) Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem 278:43885–43888
Inoue K, Nishimoto M, Kitaoka M (2011) One-pot enzymatic production of 2-acetamido-2-deoxy-d-galactose (GalNAc) from 2-acetamido-2-deoxy-d-glucose (GlcNAc). Carbohydr Res 346:2432–2436
Ishiyama N, Creuzenet C, Lam JS, Berghuis AM (2004) Crystal structure of WbpP, a genuine UDP-N-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa—Substrate specificity in UDP-hexose 4-epimerases. J Biol Chem 279:22635–22642
Kim HJ, Kang SY, Park JJ, Kim P (2011) Novel activity of UDP-galactose-4-epimerase for free monosaccharide and activity improvement by active site-saturation mutagenesis. Appl Biochem Biotechnol 163:444–451
Krieger E, Koraimann G, Vriend G (2002) Increasing the precision of comparative models with YASARA NOVA—a self-parameterizing force field. Proteins-Structure Function and Genetics 47:393–402
Kwan DH, Withers SG (2011) Toward efficient enzymatic glycan synthesis: directed evolution and enzyme engineering. J Carbohydr Chem 30:181–205
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Leloir LF (1951) The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem Biophys 33:186–190
Liu YJ, Thoden JB, Kim J, Berger E, Gulick AM, Ruzicka FJ, Holden HM, Frey PA (1997) Mechanistic roles of tyrosine 149 and serine 124 in UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 36:10675–10684
Moreno F, Rodicio R, Herrero P (1981) A new colorimetric assay for UDP-glucose 4-epimerase activity. Cell Mol Biol 27:589–592
Mulichak AM, Theisen MJ, Essigmann B, Benning C, Garavito RM (1999) Crystal structure of SQD1, an enzyme involved in the biosynthesis of the plant sulfolipid headgroup donor UDP-sulfoquinovose. Proc Natl Acad Sci U S A 96:13097–13102
Niou Y-K, Wu W-L, Lin L-C, Yu M-S, Shu H-Y, Yang H-H, Lin G-H (2009) Role of galE on biofilm formation by Thermus spp. Biochem Biophys Res Commun 390:313–318
Nishimoto M, Kitaoka M (2009) One-pot enzymatic production of beta-d-galactopyranosyl-(1- > 3)-2-acetamido-2-deoxy-d-galactose (galacto-N-biose) from sucrose and 2-acetamido-2-deoxy-D-galactose (N-acetylgalactosamine). Carbohydr Res 344:2573–2576
Sako Y, Nakagawa S, Takai K, Horikoshi K (2003) Marinithermus hydrothermalis gen. nov., sp nov., a strictly aerobic, thermophilic bacterium from a deep-sea hydrothermal vent chimney. Int J Syst Evol Microbiol 53:59–65
Sakuraba H, Kawai T, Yoneda K, Ohshima T (2011) Crystal structure of UDP-galactose 4-epimerase from the hyperthermophilic archaeon Pyrobaculum calidifontis. Arch Biochem Biophys 512:126–134
Samuel J, Tanner ME (2002) Mechanistic aspects of enzymatic carbohydrate epimerization. Nat Prod Rep 19:261–277
Sanchis J, Fernandez L, Carballeira J, Drone J, Gumulya Y, Hobenreich H, Kahakeaw D, Kille S, Lohmer R, Peyralans J, Podtetenieff J, Prasad S, Soni P, Taglieber A, Wu S, Zilly F, Reetz M (2008) Improved PCR method for the creation of saturation mutagenesis libraries in directed evolution: application to difficult-to-amplify templates. Appl Microbiol Biotechnol 81:387–397
Schulz JM, Watson AL, Sanders R, Ross KL, Thoden JB, Holden HM, Fridovich-Keil JL (2004) Determinants of function and substrate specificity in human UDP-galactose 4′-epimerase. J Biol Chem 279:32796–32803
Thoden JB, Frey PA, Holden HM (1996a) Molecular structure of the NADH/UDP-glucose abortive complex of UDP-galactose 4-epimerase from Escherichia coli: implications for the catalytic mechanism. Biochemistry 35:5137–5144
Thoden JB, Frey PA, Holden HM (1996b) High-resolution X-ray structure of UDP-galactose 4-epimerase complexed with UDP-phenol. Protein Sci 5:2149–2161
Thoden JB, Hegeman AD, Wesenberg G, Chapeau MC, Frey PA, Holden HM (1997) Structural analysis of UDP-sugar binding to UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 36:6294–6304
Thoden JB, Henderson JM, Fridovich-Keil JL, Holden HM (2002) Structural analysis of the Y299C mutant of Escherichia coli UDP-galactose 4-epimerase—teaching an old dog new tricks. J Biol Chem 277:27528–27534
Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact. doi:10.1186/1475-2859-6-9
Wee TG, Frey PA, Davis J (1972) Studies on mechanism of action of uridine diphosphate-galactose-4-epimerase. 1. Ambiguity in chemical trapping of a proposed keto-intermediate by NaB3H4. J Biol Chem 247:1339–1342
Wee TG, Frey PA (1973) Studies on mechanism of action of uridine diphosphate-galactose-4-epimerase. 2. Substrate-dependent reduction by sodium-borohydride. J Biol Chem 248:33–40
Wong SS, Frey PA (1977) Fluorescence and nucleotide binding properties of Escherichia coli uridine diphosphate galactose 4-epimerase: support for a model for nonstereospecific action. Biochemistry 16:298–305
Acknowledgments
This research project was funded by grants SB81309 and SB83309 from the Agency for Innovation by Science and Technology in Flanders (IWT-Vlaanderen) and by the Special Research Fund (BOF) of Ghent University (MRP Ghent Bio-Economy).
Conflict of interests
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 320 kb)
Rights and permissions
About this article
Cite this article
Beerens, K., Soetaert, W. & Desmet, T. Characterization and mutational analysis of the UDP-Glc(NAc) 4-epimerase from Marinithermus hydrothermalis . Appl Microbiol Biotechnol 97, 7733–7740 (2013). https://doi.org/10.1007/s00253-012-4635-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4635-6