Abstract
Archaea are ubiquitous single-cell microorganisms that have often adapted to harsh conditions and play important roles in biogeochemical cycles with potential applications in biotechnology. Methanococcus maripaludis, a methane-producing archaeon, is motile through multiple archaella on its cell surface. The major structural proteins (archaellins) of the archaellum are glycoproteins, modified with N-linked tetrasaccharides that are essential for the proper assembly and function of archaella. The aglW gene, encoding the putative 4-epimerase AglW, plays a key role in the synthesis of the tetrasaccharide. The goal of our work was to biochemically demonstrate the 4-epimerase activity of AglW, and to develop assays to determine its substrate specificity and properties. We carried out assays using UDP-Galactose, UDP-Glucose, UDP-N-acetylglucosamine, UDP-N-acetylgalactosamine and N-acetylglucosamine/N-acetylgalactosamine-diphosphate – lipid as substrates, coupled with specific glycosyltransferases. We showed that AglW has a broad specificity towards UDP-sugars and that Tyr151 within a conserved YxxxK sequon is essential for the 4-epimerase function of AglW. The glycosyltransferase-coupled assays are generally useful for the identification and specificity studies of novel 4-epimerases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Archaea are single-cell microorganisms that are ubiquitous in nature and have often adapted to growth under harsh conditions; cultivatable members include sulfur-dependent thermophiles, hyperthermophiles, extreme halophiles, methanogens and ammonia oxidizers [1, 2]. They play important roles in biogeochemical cycles and have potential applications in biotechnology [3,4,5]. Methanococcus maripaludis is a motile, mesophilic, methane-producing member of the archaea that was originally isolated from salt marsh sediments in South Carolina [6]. It serves as a model for genetic and structural studies of N-glycosylation [7, 8] due to its sequenced genome, efficient growth capability, and a wide variety of available genetic tools [9].
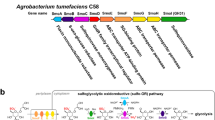
M. maripaludis has multiple motility structures (archaella) on its cell surface [10]. The major structural proteins of the archaellum are three archaellins, each carrying multiple Asn-linked tetrasaccharides (Fig. 1) [12, 13]. FlaB1 and FlaB2 are the two major archaellins and FlaB3 is a minor archaellin that comprises the curved hook-like region of the archaellum [1]. Disruptions in the N-glycosylation of the archaellins adversely affect their assembly into archaella and motility [14, 15].
Proposed biosynthetic pathway of N-glycan in Methanococcus maripaludis. The tetrasaccharide glycan (inset) is assembled on a lipid carrier Phospho-dolichol (P-Dol) by a series of GTs as indicated (AglO, AglA and AglL; the GT for the transfer of the first sugar (D) is currently unknown). AglO transfers the second sugar (C), 2,3-di-N-acetyl glucuronic acid, from UDP-2,3-di-N-acetyl glucuronic acid to GalNAc (sugar D) in β1–3 linkage. A number of enzymes (Agl20, Agl19, Agl18, Agl17) are involved in the synthesis of the corresponding nucleotide sugar donor substrate from UDP-GlcNAc. Agl21 and AglXYZ synthesize the nucleotide sugar required for the third sugar (B). AglA is the glycosyltransferase that transfers 2-N-acetyl-3-N-amidino-mannuronic acid (sugar B) from the UDP-sugar donor in β1–4 linkage to 2,3-di-N-acetyl glucuronic acid. The threonine residue is thought to be added to sugar B by AglU only after the terminal sugar (A) has been transferred by GT AglL in β1–4 linkage. AglW appears to be involved in the pathway for the terminal sugar while AglV is the O-methyl-transferase that transfers a methyl group to the last sugar in the unique 5-O-methyl linkage, likely at the nucleotide sugar stage. After the assembly of the N-glycan on the P-Dol lipid intermediate by transferases at the cytoplasmic face of the plasma membrane, the oligosaccharide is flipped across the membrane by an unidentified flippase. The membrane-bound oligosaccharyltransferase AglB then transfers the oligosaccharide to Asn residues of proteins within the Asn-X-Ser/Thr sequons. With the exception of 4-epimerase AglW, none of the enzymes involved have been biochemically characterized in M. maripaludis. AglW does not appear to affect GalNAc at the reducing end of the N-glycan but may have a possible role in the synthesis of the donor substrate for the addition of the terminal sugar A. Figure modified from Siu et al. [11]
In archaea, N-glycans are often highly complex and unusual in structure [7]. The N-glycan of archaellins in M. maripaludis is a tetrasaccharide with the structure 4-deoxy-5-O-Me-ManNAcβ1-4ManNAc3NAmA[Thr-6]β1-4GlcNAc3NAcAβ1-3GalNAcβ-Asn (Fig. 1) [13]. By using bioinformatic analyses and targeted gene deletions coupled with mass spectrometry analyses of purified archaella from the mutant strains, most of the genes involved in the biosynthesis of the N-glycan have been identified, although biochemical proof is still absent. Genes encoding glycosyltransferases (GTs) as well as enzymes that synthesize the donor substrates required for the assembly of the glycan on its dolichol-phosphate (P-Dol) carrier have been identified. N-glycosylation starts with the addition of sugar-phosphate to P-Dol by an unidentified sugar-1-phosphate transferase. This is followed by the sequential transfer of sugars by the putative GTs AglO, AglA and AglL (Agl, archaeal glycosylation) (Fig. 1). The P-Dol-linked oligosaccharide is then flipped to the outside of the plasma membrane and an oligosaccharyltransferase transfers the lipid-linked glycan to select asparagine residues within the conserved sequon N-x-S/T of target proteins [11, 14,15,16].
Many steps in the synthesis of the nucleotide sugar donor substrates for GTs have been identified in recent studies. It was shown that M. maripaludis synthesizes the second sugar (a GlcNAc derivative) and the third sugar (a Man derivative) of the tetrasaccharide by the same pathway used by Pseudomonas aeruginosa to make di-N-acetylated sugars [11, 16]. UDP-GlcNAc is converted to UDP-GlcNAc(3NAc)A through dehydrogenation (mediated by Agl20), oxidation (Agl19), amination (Agl18) and N-acetylation steps (Agl17). Epimerization by Agl21 of UDP-GlcNAc(3NAc)A yields UDP-ManNAc(3NAc)A which is converted to UDP-ManNAc3NAmA by the activities of AglXYZ to add the acetamidino group at position 3 [8, 17] (Fig. 1). The threonine addition by AglU to the third sugar appears to occur after the addition of the unique terminal sugar at the non-reducing end of the chain (4-deoxy-5-O-Me-ManNAc) by the putative GT AglL [8, 15]. The pathway to generate the donor substrate for AglL is much less understood. AglV likely transfers a methyl group from S-adenosylmethionine to the 5-position of the terminal sugar, either while it is bound to UDP [8] or, alternatively, after the transfer of the fourth sugar to the Dol-linked growing glycan. Mass spectrometry analysis has revealed that a mutant harboring a deletion of the gene aglW has a truncated N-glycan lacking the terminal sugar residue, as well as the threonine residue linked to the third sugar [12]. The absence of the Thr residue on the third sugar is not directly related to the activity of AglW as this transfer has been previously shown to be carried out by AglU [8]. This suggests that the function for AglW lies in the biosynthesis pathway of the donor substrate for AglL [12] although this role is not obvious.
Based on protein sequence similarity, AglW (MMP_RS05635; formerly MMP1090) has been suggested to be a 4-epimerase but this had not been shown biochemically. Most 4-epimerases reversibly convert UDP-hexoses and/or UDP-hexosamines in the presence of NAD+ and are classified based on their substrate specificity (Table 1). Group 1 4-epimerases act primarily on UDP-Glc/Gal and group 2 enzymes act on both UDP-hexoses and UDP-HexNAc [19, 24]. A third group acts primarily on UDP-GlcNAc and UDP-GalNAc as substrates. Another type of 4-epimerase (Gnu, group 4) encoded by the Z3206 gene from Escherichia coli O157 does not act on nucleotide sugars but instead can reversibly convert GlcNAcα-diphosphate-undecaprenol (GlcNAc-PP-Und) to GalNAcα-PP-Und [23]. In M. maripaludis, the sugar at the reducing end of the N-glycan is also GalNAc. It is unknown if archaea express a 4-epimerase that can interconvert GlcNAc-diphosphate-Dol (GlcNAc-PP-Dol) and GalNAc-PP-Dol. In addition to these enzymes, there are 4-epimerases that interconvert the uronic acid forms of UDP-hexose (UDP-N-acetylglucuronic acid and UDP-N-acetylgalacturonic acid) or UDP-pentoses (UDP-Xylose and UDP-Arabinose) [25].
The goal of this work was to provide biochemical evidence for the 4-epimerase activity of AglW, and to identify its substrate specificity and enzymatic properties. We developed highly sensitive and specific assays for the conversion of UDP-Gal, UDP-Glc, UDP-GlcNAc, UDP-GalNAc and GlcNAc/GalNAc-diphosphate – lipid by coupling 4-epimerase to GT reactions. Our work demonstrates that AglW from M. maripaludis is a 4-epimerase with broad specificity towards UDP-sugars. These assays should prove to be generally useful for specificity studies of 4-epimerases.
Materials and methods
Materials
All reagents and materials were purchased from Sigma unless otherwise stated. Radioactive nucleotide sugars were purchased from American Radiolabeled Chemicals (ARC). GlcNAcα-diphosphate-(CH2)11-O-phenyl (GlcNAc-PP-Phenylundecyl, GlcNAc-PP-PhU) and GalNAcα-diphosphate-(CH2)11-O-phenyl (GalNAc-PP-PhU) were synthesized as acceptor substrates for bacterial GTs as previously reported [26]. Manα1–6(Manα1–3)Manβ-octyl was purchased from Toronto Research Chemicals. UDP-Gal was donated by B. Ernst, University of Basel, Switzerland. Purified bovine milk β1,4-Gal-transferase was from Sigma. Purified recombinant bovine polypeptide GalNAc transferase-1 (ppGalNAc-T1) was a gift from A. Elhammer, Pharmacia and Upjohn, Kalamazoo MI. Human GlcNAc-transferase I expressed in insect cells was donated by H. Schachter, Hospital for Sick Children, Toronto, ON. The β1,3-Glc-transferase WbdN from E.coli O157 was produced and assayed as described previously [27]. The β1,4-Gal-transferase WfeD from Shigella boydii was produced as described before [28] and purified using Ni2 + -NTA Sepharose (Qiagen) as described [26].
Strains and growth conditions
Strains and plasmids used in this study are listed in Table S1. M. maripaludis S2 ∆hpt (Mm900 [9]) and the ∆aglW mutant isolated from it [12] were grown anaerobically in sealed serum bottles containing 10 ml Balch Medium 3 at 37 °C under an atmosphere of CO2/H2 (20:80) with shaking, as described previously [12]. Archaeal homogenates were prepared by sonication of M. maripaludis Mm900 in 50 mM sucrose as described [26]. Escherichia coli BL21 (DE3) cells used for cloning steps were grown in Luria-Bertani medium at 37 °C with 100 μg/ml ampicillin and 30 μg/ml chloramphenicol for selection when required.
Cloning and expression of AglW
The aglW gene from M. maripaludis was amplified by polymerase chain reaction (PCR) using the AglW-F forward and AglW-R reverse primers listed in Table S2 that contained either NdeI or XhoI restriction sites, respectively. The gene was cloned into Ndel/XhoI digested expression vector pET23a, creating a fusion of aglW with the C-terminal His6-tag-encoded plasmid sequence, resulting in plasmid pKJ919 which was transformed into E. coli BL21 (DE3) cells for protein expression. Bacteria were grown overnight at 37 °C in 5 ml LB broth containing 100 μg/ml ampicillin and 30 μg/ml chloramphenicol with shaking at 200 rpm. Five ml bacterial suspension were transferred to 100 ml LB broth containing ampicillin and chloramphenicol and incubated at 37 °C with constant shaking. When the suspension reached an absorbance at 600 nm of 0.8, it was cooled by placing it on ice for 15 min. After cooling, isopropyl-beta-thiogalactoside was added to a final concentration of 0.2 mM. Cells were grown for an additional 14–16 h at 16 °C, then harvested by centrifugation for 5 min at 12,000 x g. Pellets were resuspended in 4 ml binding buffer (5 mM imidazole, 0.5 M NaCl and 20 mM Tris-HCl, pH 8) and stored at −20 °C until required for use in purification of AglW protein.
Protein purification
AglW protein was purified using Ni2+-NTA Sepharose [26]. To obtain the total cell lysate, E. coli cells producing the His6-AglW fusion protein from pKJ919 were sonicated in binding buffer for 5 cycles of 2-s sonication and 2-s on ice. Sonicates were centrifuged at 12,000 x g for 40 min. The supernatant containing the fusion protein was loaded on a Ni2+-NTA Sepharose column. Bound proteins were eluted with 1 M imidazole buffer (20 mM Tris-HCl, pH 8, 0.5 M NaCl). Fractions were analyzed by SDS-PAGE (12% gels), and fractions containing AglW were pooled and dialyzed in dialysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl) at 4 °C overnight. Western blots were carried out using rabbit anti-His Tag (Santa Cruz Biotechnology) as the primary antibody (1:1000 in 5% non-fat milk) and HRP-linked goat anti-rabbit IgG (Cell Signaling Technology) as the secondary antibody (1:5000 in 5% non-fat milk). All antibodies were dissolved in 1× Tris-buffered saline (TBS), 0.1% Tween-20 (BioShop Canada). Bands were visualized using Clarity Western ECL substrate (Bio-Rad) and the Azure c600 imaging system.
Expression and purification of mutant versions of AglW
To determine the importance of the highly conserved Tyr and Lys residues of the central YxxxK motif, pET23a derivatives carrying versions of aglW with a mutation at either Y151 (Y155A; pKJ1111) or K155 (K155A; pKJ1112) were also used to transform the E. coli expression strain. For these constructions, plasmid pKJ919 was used as template in site-directed PCR with either primer pair Y151A-F and Y151A-R or primer pair K155A-F and K155A-R (Table S2). The genes encoding the AglW Y151A and K155A mutations were sequenced (ACGT Corporation) to confirm the codon change and to ensure that no errors were introduced. The expression and purification of theY151A and K155A mutant versions of AglW were monitored by SDS-PAGE and Western blots as described above.
Assay for 4-epimerase using Griffonia simplicifolia lectin I Sepharose
Assays for AglW activity were performed using affinity chromatography on Griffonia simplicifolia lectin I Sepharose (GSL 1, Vector Laboratories) that preferentially binds to α-Gal/GalNAc compounds [29]. In brief, reaction mixtures (20 μl total volume) contained: 0.1 M Tris-HCl buffer, pH 8.5, 0.1 mM UDP-[3H]GalNAc (or UDP-[3H]Gal, UDP-[3H]Glc, UDP-[3H]GlcNAc), 0.1 mM MgCl2, 0.02 mM NAD+ and 97 ng of purified AglW. Control assays lacked AglW. Mixtures were incubated at 37 °C for 10 min followed by boiling for 3 min. After the addition of 75 μl of ice-cold buffer (10 mM NaH2PO4, pH 7.2, 150 mM NaCl, 0.1 mM MgCl2, 0.1 mM CaCl2, 0.1 mM MnCl2), the assay mixture was passed through a column of 1 ml GSL 1 Sepharose equilibrated in GSL buffer (10 mM NaH2PO4, pH 7.2, 150 mM NaCl, 0.1 mM MnCl2) to separate UDP-[3H]Gal(NAc) from UDP-[3H]Glc(NAc). The column was washed with 1 ml GSL buffer (fraction A), and then with 2 ml GSL buffer to elute unbound UDP-[3H]GlcNAc (or UDP-[3H]Glc) (fraction B). UDP-[3H]GalNAc (or UDP-[3H]Gal) was eluted with 2 ml 2.5 mM Gal-α-methyl in GSL buffer (fraction C). The radioactivity of fractions was determined by scintillation counting.
Coupled 4-epimerase-glycosyltransferase assays
Coupled assays for AglW activity were carried out in two-step assays. The first step of the two-step coupled assays consisted of the 4-epimerase reaction using radioactive UDP-sugar substrate which was allowed to proceed at 37 °C for 10 min. In the second step, the AglW reaction product from the first step was used as a donor substrate for a GT reaction that was highly specific for the UDP-sugar. The activities of all GTs were verified prior to AglW assays. Assays were carried out in at least duplicate reactions that varied by <15%. Negative control assays lacked the specific GT acceptor substrate or AglW.
Assay 1. AglW – β1,4-Gal-transferase-coupled assay to measure the conversion of UDP-Glc to UDP-Gal
Bovine milk β1,4-Gal-transferase (β4GalT) was used to determine the amount of UDP-Gal formed from UDP-Glc [30]. The first step (AglW reaction) of the two-step reactions contained in a total volume of 20 μl: 0.15 M Tris Base, pH 8, 0.69 mM UDP-[3H]Glc (5550 cpm/nmol) and 5 μg of AglW. Mixtures were incubated for 10 min at 37 °C, followed by boiling for 3 min. The mixtures containing the reaction product UDP-Gal were used as the donor substrate for β4GalT. Assays for the second step (β4GalT reaction) contained in a total volume of 40 μl: 20 μl 4-epimerase reaction mixture, 0.125 M MES buffer, pH, 7, 2 mM GlcNAcβ-Bn acceptor substrate, 10 mM MnCl2 and 1.8 μg β4GalT. Reaction mixtures were incubated for 1 h at 37 °C. The reaction was quenched by the addition of 600 μl of ice-cold H2O and mixtures were passed through AG 1-X8 anion exchange columns. The reaction product was quantified by scintillation counting and using a standard curve for β4GalT.
Assay 2. AglW – WbdN-coupled assays to measure the conversion of UDP-Gal to UDP-Glc
The β1,3-Glc-transferase WbdN from E.coli O157 [27], specific for UDP-Glc as donor substrate, was used to determine the amount of UDP-Glc formed in 4-epimerase assays from UDP-Gal.
The first step contained 0.15 M Tris-HCl, pH 8, 1.44 mM UDP-[3H]Gal (2900 cpm/nmol) and 5 μg AglW in a total volume of 20 μl. Mixtures were incubated for 10 min at 37 °C followed by boiling for 3 min. The mixtures were then used as the donor substrate for WbdN activity assays. Assay mixtures contained in a total volume of 40 μl: 20 μl 4-epimerase product mixtures, 0.1 M MES buffer, pH 7, 0.01 M MnCl2, 0.2 mM GalNAc-PP-PhU as the WbdN acceptor, and WbdN-containing bacterial homogenate (140 μg protein). After incubation for 30 min at 37 °C, reactions were quenched with 700 μl cold H2O. Enzyme product was isolated using a C18 Sep-Pak column as described [27]. The radioactivity of the final reaction product was quantified by scintillation counting and using a standard curve.
Assay 3. AglW and GlcNAc-transferase 1 (GlcNAc-T1)-coupled assay to detect conversion of UDP-GalNAc to UDP-GlcNAc
GlcNAc-transferase 1 (GlcNAc-T1 [31] was used as a GT specific for UDP-GlcNAc as the donor substrate. For the first step, mixtures contained 0.25 M Tris Base, pH 8, 1.32 mM UDP-[3H]GalNAc (1140 cpm/nmol) and 5 μg AglW in a total volume of 20 μl. Mixtures were incubated at 37 °C for 10 min followed by boiling for 3 min and were used as the donor substrate for the second step, GlcNAc-T1 activity assays. The assay mixtures contained in a total volume of 40 μl: 20 μl 4-epimerase reaction mixture, 0.175 M MES buffer, pH 6, 1 mM Manα1–6(Manα1–3)Manβ-octyl acceptor substrate, 20 mM MnCl2 and GlcNAc-T I (60 μg protein), and were incubated for 1 h at 37 °C. The reaction was quenched by the addition of 600 μl of ice-cold H2O and mixtures were passed through AG-1X8 anion exchange columns. The final reaction product was quantified by scintillation counting and using a standard curve.
Assay 4. AglW - polypeptide GalNAc-transferase T1-coupled assay to detect conversion of UDP-GlcNAc to UDP-GalNAc
Polypeptide GalNAc-transferase T1 (ppGalNAc-T1) was used to specifically detect UDP-GalNAc formed by AglW from UDP-GlcNAc [32, 33]. Assay mixtures for the first step contained 0.25 M Tris Base, pH 8, 0.15 mM UDP-[3H]GlcNAc (4040 cpm/nmol), and 5 μg AglW in a total volume of 20 μl. Mixtures were incubated at 37 °C for 10 min, followed by boiling for 3 min and were then used as the donor substrate for ppGalNAc-T1 activity assays. Mixtures for the second step contained in a total volume of 40 μl: 20 μl 4-epimerase reaction mixture, 0.125 M Tris-HCl, pH 7.5, 2 mM AQPTPPP acceptor substrate, 10 mM MnCl2 and 0.033 μg of ppGalNAc-T1. The reaction proceeded for 1 h at 37 °C and was quenched by the addition of 600 μl of ice-cold H2O. Mixtures were then passed through AG 1-X8 anion exchange columns and the final reaction product was quantified by scintillation counting and using a standard curve.
Assay 5. AglW – WfeD-coupled assay for the conversion of GalNAc-PP-PhU to GlcNAc-PP-PhU.
The conversion of GalNAc-PP-PhU by 4-epimerase to GlcNAc-PP-PhU was monitored with β1,4-Gal-transferase WfeD from Shigella boydii [28] that is specific for GlcNAc-PP-PhU as the acceptor substrate and UDP-Gal as the donor substrate. The first step for AglW activity assays contained 0.25 M Tris Base pH 8, 2.5 mM GalNAc-PP-PhU and 5 μg of AglW in a total volume of 20 μl. Mixtures were incubated for 10 min at 37 °C followed by boiling for 3 min. The possible reaction product, GlcNAc-PP-PhU, was then used as an acceptor substrate for WfeD reaction. Assays for the second step contained in a total volume of 40 μl: 20 μl mixture from the AglW reaction, 0.15 M MES buffer, pH 7, 1.92 mM UDP-[3H]Gal (2900 cpm/nmol), 0.01 M MnCl2 and 0.075 μg purified WfeD. After incubation for 30 min at 37 °C, reactions were quenched with 700 μl cold H2O. Enzyme product was isolated using a C18 Sep-Pak column as described [28]. The radioactivity of the final reaction product was quantified by scintillation counting.
Assay 6. AglW – WbdN-coupled assay for the conversion of GlcNAc-PP-PhU to GalNAc-PP-PhU
To measure the conversion of GlcNAc-PP-PhU to GalNAc-PP-PhU, the β1,3-Glc-transferase WbdN was used as a GT specific for GalNAc-PP-PhU as the acceptor substrate [27]. The two-step coupled assay reaction mixtures for the first (AglW) step contained 0.25 M Tris Base pH 8, 2.5 mM GlcNAc-PP-PhU and 5 μg of AglW in a total volume of 20 μl. Mixtures were incubated for 10 min at 37 °C followed by boiling for 3 min. The reaction product was used as the acceptor substrate for WbdN activity assays. The second step contained in a total volume of 40 ul: 20 μl AglW reaction mixture, 0.1 M MES buffer, pH 7, 0.92 mM UDP-[3H]Glc (5550 cpm/nmol), 0.01 M MnCl2 and WbdN-containing bacterial homogenate (140 μg protein). After incubation for 30 min at 37 °C, WbdN assays were performed by C18 Sep-Pak assays [27]. The final reaction product was quantified by scintillation counting.
Results
Sequence analysis of AglW
An NCI-BLAST analysis revealed that AglW is a member of the short chain dehydrogenase/reductase superfamily [20] with high protein sequence identity to other predicted UDP-Glc 4-epimerases. For example, AglW (Accession number Q6LYA1) shares 89% protein sequence identity with a putative UDP-Glc 4-epimerase from the mesophilic relative Methanococcus vannielii, (A6UPA1), 71% to the predicted enzyme in the thermophilic Methanothermococcus thermolithotrophicus (WP_018154753) and 72% to the same enzyme in the hyperthermophilic relative Methanocaldococcus jannaschii. (WP_010869707). AglW is also 33% identical to a characterized UDP-glucose 4-epimerase in the hyperthermophile Pyrococcus horikoshii (O58762) [34] as well as 31% with characterized human 4-epimerase hGalE (Q14376) [19], and 29% with eGalE from the E.coli strain K-12 (P09147) [20]. AglW also shares 24.7% sequence identity with 4-epimerase Z3206 (Gnu, Q8X7P7) in E. coli O157 that is responsible for the conversion of GlcNAc-PP-Und to GalNAc-PP-Und. A BLAST search identified the presence of genes highly homologous to Z3206 in those E. coli strains that contain GalNAc at the reducing end of the O antigen repeating units (ECODAB). This suggests that this GalNAc residue is derived from 4-epimerization of GlcNAc-PP-Und by 4-epimerase Z3206. UniProt (InterPro) searches suggest that AglW has a 4-epimerase domain (amino acids 17–244) that includes a Rossmann-fold NAD(P)H/NAD(P)(+) binding domain at the amino terminus, as well as conserved motifs GxxGxxG (located within the Rossmann fold and involved in NAD+ binding) and YxxxK (containing the Tyr/Lys couple that plays a key role in catalysis) characteristic of 4-epimerases [25]. Thus, AglW is expected to be a 4-epimerase that tightly binds NAD+. The BLAST search of the M. maripaludis S2 non-redundant protein sequence database using AglW as query revealed no other significant protein sequences indicating AglW appears to be the only 4-epimerase in this organism.
Purification of AglW
Homogenates of M. maripaludis Mm900 cells prepared by sonication in 50 mM sucrose were assayed for 4-epimerase activity using the β4GalT-coupled assays 1 and WbdN-coupled assays 2 but activities were below detection levels. Therefore, purification of AglW was required for analysis of its enzymatic properties. Following induction of AglW expression in E. coli and subsequent purification of the His-tagged AglW via Ni-affinity chromatography, analysis of the purified AglW samples by SDS-PAGE showed an intense band at the predicted AglW molecular mass (34.4 kDa) (Fig. 2a). This band was also detected in western blots using anti-HisTag antibodies (not shown). AglW has a hydrophobic sequence at the N-terminus that may serve to associate the enzyme with the membrane. No protein aggregation or inclusion bodies were observed when the protein was expressed at 16 °C, 25 °C or 37 °C. The purified Y151A mutant version of AglW also appeared as a 34 kDa band on SDS-PAGE (Fig.2b). However, in spite of repeated expression experiments, SDS-PAGE showed that the K155A mutant form of AglW was not well expressed.
Purification of His-tagged AglW. SDS-PAGE is shown after purification of C-terminal His-tagged AglW by nickel affinity chromatography. a Molecular mass standards are shown on the right in kDa. AglW shows a dark band at 34 kDa. Lane 1, induced whole cell lysate; lane 2, lysed 12,000 x g pellet of sonicated cells; lane 3, supernatant of 12,000 x g sonicated cells; lane 4, flow-through fraction after loading supernatant from lane 3; lane 5, wash with 1X binding buffer; lane 6, wash with 1X wash buffer; lane 7, elution of bound protein with 1X elution buffer. b Comparison of AglW wild type and mutant Y151A. Lane 1, molecular mass standards; lane 2, AglW wild type; lane 3, Y151A mutant
Characterization of AglW activity
Using several different assay procedures, AglW was shown to be active with UDP-Gal, UDP-Glc, UDP-GalNAc and UDP-GlcNAc as substrates. The Glc-oxidase-coupled assays showed 4-epimerase activity of AglW using UDP-Gal as the substrate (data not shown) but were limited with respect to the use of different substrates, and only quantified the production of UDP-Glc. We therefore used the α-Gal/GalNAc-binding GSL 1 lectin and radioactive UDP-sugars to assay 4-epimerase activity. AglW activity was linear up to about 10 min incubation time at 37 °C. Using UDP-GalNAc as substrate, full activity was found in the presence of 5 mM Mg2+, Mn2+, Ca2+ ions or EDTA, with Co2+ reducing the activity by 50% (Fig. 3a). This shows that these divalent metal ions are not required for, or inhibitory to, epimerase activity. The optimal pH was between pH 8 and 9 (Fig. 3b). However, the GSL 1 assays were found to lack sensitivity. We therefore developed highly sensitive and specific coupled assays that reduced the reversible reaction of the epimerase and allowed the use of various substrates by GTs that are specific for one nucleotide sugar substrate.
GSL-1 lectin assay. Griffonia simplicifolia lectin I (GSLI) Sepharose was used to assess the percent substrate conversion of UDP-Glc (unbound) to UDP-Gal (bound). a Assays were carried out as described in the Methods section using 5 mM concentrations of CoCl2, MgCl2, MnCl2, CaCl2 or EDTA in the assays. Negative, no metal ions or EDTA were added. None of the metal ions were found to stimulate the 4-epimerase activity. b Assays were carried out in the presence of buffers at pH values between 5 and 9.5 (pH). MES buffer was used at pH 5 to 7, and Tris-Base was used at pH 7 to 9.5. The optimal pH was between 8 and 9. Error bars represent the difference between duplicate determinations
The two-pot β4GalT-coupled assays demonstrated that AglW activity does not require the addition of NAD+, suggesting that this cofactor was tightly bound to the enzyme throughout the purification procedure. In these assays, the 4-epimerase was again found to be fully active in the presence of EDTA. AglW activity was found to be maximal over a broad pH range of pH 7.5 to 9 and stable in an incubation temperature range of 30–50 °C.
When the first step (4-epimerase reaction) was omitted before carrying out the GT reaction (i.e. in one-step assays), activities near Vmax values determined by two-step assays were observed. Thus, the continuous removal of the 4-epimerase product by a GT made the reaction highly efficient.
Substrate specificity of AglW
With the highly sensitive GT-coupled assays we were able to show that AglW had a broad specificity for UDP-Gal/Glc and UDP-GalNAc/GlcNAc (Table 2). The β4GalT- and WbdN-coupled assays were used for the determination of kinetics parameters for UDP-Glc and UDP-Gal. The apparent KM for UDP-Glc for purified AglW was 0.30 mM and the apparent Vmax was 464 nmol/h/mg (Fig. S1A). For UDP-Gal, the apparent KM was 1.36 mM with a Vmax of 2694 nmol/h/mg (Fig. S1B) in WbdN-coupled assays. The apparent KM and Vmax values for UDP-GlcNAc using ppGalNAc-T1-coupled assays were 0.12 mM and 1219 nmol/h/mg, respectively (Fig. S1C). The GlcNAc-T1-coupled assay yielded an apparent KM for UDP-GalNAc of 0.05 mM and an apparent Vmax of 2745 nmol/h/mg (Fig. S1D). Thus, all of these UDP-sugars were effective substrates.
GlcNAc/GalNAc-PP-PhU are analogs of the natural P-Und-linked intermediates in the biosynthesis of bacterial O antigens that were shown to be highly effective as acceptor substrates for bacterial GTs [26,27,28]. In archaea, the biosynthetic intermediates for N-glycosylation are Dol-linked with fewer isoprene units compared to mammalian Dol-linked phospholipids. However, mammalian GTs have been shown to be active using simple phosphate-lipid substrates as analogs of natural Dol-bound substrates [35]. This suggests that the lipid moieties may not be directly recognized by enzymes and Dol can be replaced by smaller synthetic lipids. We therefore tested GlcNAc-PP-PhU and GalNAc-PP-PhU as 4-epimerase substrates in 4-epimerase-coupled assays but no activity of AglW was detected. AglW thus appears to differ in specificity from that of the 4-epimerase Z3206 from E.coli O157 that acts on GlcNAc-PP-Und [23].
Activities of mutated forms of AglW
A YxxxK motif is characteristic of 4-epimerases and involved in catalysis [25]. AglW has three such YxxxK motifs with the central one (YGLSK, amino acids 151–155) aligning with the known active site determined in other 4-epimerases (Fig. S2). To assess the possible roles in enzyme activity, each of the two key residues, Y151 and K155, was mutated to alanine. Expression and purification of the Y151A version of the His Tagged AglW was successful. Like the wild type AglW, the purified Y151A mutant version of AglW also appeared as a 34 kDa band on SDS-PAGE and was detected in western blots using anti-Histag antibodies. Purified Y151A AglW was inactive with all UDP-Hex/HexNAc compounds as well as with GlcNAc/GalNAc-PP-PhU as substrates, indicating that Tyr151 is essential for enzyme activity. Repeated attempts to express and purify the K115A mutant form of the enzyme failed, implicating a role for Lys155 in proper protein folding or stability of AglW, in addition to a likely role in catalysis.
Inhibition of AglW
In order to develop 4-epimerase inhibitors of AglW that could be useful in studying the biological role of AglW, substrate analogs were tested as inhibitors in β4GalT-coupled assays (Fig. 4). None of the 4-epimerase inhibitors inhibited β4GalT activity. The reaction intermediate NADH at 0.5 to 2 mM concentration in the assays inhibited the AglW activity up to 62%. Effective inhibitors at 2 mM concentrations were UDP and UDP-Xylose that reduced 4-epimerase activity by 57 and 67%, respectively. Other nucleotide mono- and diphosphates showed inhibition between 14 and 46%. Interestingly, in contrast to other UDP compounds, UDP-GlcA inhibited AglW activity by only 15%. Thus, the 6-carboxyl group of UDP-GlcA may have interfered with binding to the enzyme, either because of its size or its charge.
Inhibition of the 4-epimerase activity of AglW. Inhibitors were present at 2 mM concentration in β4GalT-coupled 4-epimerase assays (Assay 1) using UDP-Glc as the 4-epimerase substrate. Error bars show the differences between duplicate determinations. All nucleotide sugar derivatives showed inhibition of 4-epimerase but not of the coupled enzyme, β4GalT. Error bars represent the difference between dupicate determinations
Discussion
The novel GT-coupled assays used in this study established that AglW from M. maripaludis is a UDP-Hex/HexNAc 4-epimerase. The enzyme acts on UDP-Glc/UDP-Gal as well as UDP-GalNAc/UDP-GlcNAc as substrates and can thus be classified as a group 2 4-epimerase (Table 1). A number of methods have been used to assay 4-epimerases and determine nucleotide sugar product concentrations. The development of coupled assays in this work to evaluate the activity and substrate specificity of an unknown 4-epimerase has provided a means for the quantitative, sensitive and specific assessment of activity using six different substrates, and allowed the biochemical characterization of AglW. Coupled assays exploit the exquisite donor specificities of GTs and can reduce the reversible reaction of the 4-epimerase. The epimerase assay coupled with bovine β4GalT was the most convenient, feasible and economical method for determining 4-epimerase activity. However, most GTs are highly specific for nucleotide sugar donor substrates; thus, the enzymes chosen in this study could be replaced by other GTs that utilize the nucleotide sugar to be examined as 4-epimerase substrate. These coupled assays can provide an initial screening method for other putative 4-epimerases from any domain or for testing potential inhibitors (Fig. 4). The inhibitors have potential in examining the exact role of 4-epimerase in the biosynthesis of archaeal N-glycans.
The kinetic parameters for the archaeal enzyme are within the ranges found for bacterial and eukaryotic 4-epimerases. It appears that AglW has a higher affinity for UDP-Glc over UDP-Gal, although UDP-GalNAc has a higher catalytic efficiency compared to UDP-GlcNAc (Table 2). This is in contrast to bacterial eGalE that prefers UDP-Gal over UDP-Glc [36]. However, Gne from E. coli O86:B7 showed no preference towards a particular substrate and demonstrated similar affinities for acetylated and non-acetylated UDP-Glc and UDP-Gal [18]. The 4-epimerase from the archaeon P. horikoshii had a preference for UDP-Gal with a KM of 0.23 mM for UDP-Gal compared to 2.24 mM for UDP-Glc [34].
Examination of the reaction conditions for AglW indicated that AglW activity was optimal at slightly alkaline pH over a broad temperature range and not dependent on metal ion or NAD+ addition. Optimal pH ranges for other 4-epimerases are generally reported to be in the pH 7–8.5 range. Many 4-epimerases from the other domains have also been reported to not require metal ions for activity, although the enzyme from P. horikoshii was severely inhibited by Cu2+ [34]. The presence of the highly conserved Rossman fold in AglW indicates that the enzyme is dependent on NAD+ for activity. The lack of effect of added NAD+ is consistent with NAD+ already being tightly bound to the enzyme when it is purified.
Analyses of crystal structures and mutations of human and E. coli 4-epimerases revealed that substrate specificity is defined by the size of the substrate binding site and a limited number of specific amino acids [19, 37]. The mechanism of 4-epimerases has been confirmed in crystal structure analyses [25]. The binding of UDP causes a conformational change in the enzyme. An essential Tyr residue of the highly conserved YxxxK motif (Fig. S2) facilitates the removal of a proton from the 4-OH group of the sugar, while the negative charge is stabilized by the nearby Lys residue. In a Tyr-NAD+ complex, a hydride ion is then transferred to NAD+, forming NADH and a 4-keto sugar intermediate. The sugar then rotates and NADH re-donates the hydride to the opposite face of the sugar, and Tyr donates a proton back to O4 [19, 21, 22, 37,38,39], followed by the departure of UDP-sugar and conformational change of the enzyme. Archaeal AglW may also use this mechanism. The Y151A mutant of AglW was well expressed (Fig. 2b) but showed a complete loss of activity with all substrates suggesting strongly that Tyr151 is an essential catalytic residue. It is not clear why three YxxxK motifs are present in AglW and if any of the other motifs also have a catalytic role. However, a comparison with other 4-epimerases suggests that only the Y151xxxK155 motif in AglW aligns with that motif in other 4-epimerases (Fig. S2). Lys155 may have an additional role in the expression and stability of AglW since good expression of the K155A mutant form was not achieved in E.coli under conditions identical to those used to purify the wildtype and the Y151A mutant form of AglW. These results are consistent with the previous findings that Y151A and K155A mutant versions of AglW were not successful in complementation assays of M. maripaludis aglW mutant cells [12].
AglW is the second gene in a 6 gene operon [MMP_RS05630, MMP_RS05635 (aglW), MMP_RS05640 MMP_RS05645, MMP_RS05650, MMP_RS05655, formerly locus tags MMP1089–1094]. The operon is the boundary of a large collection of genes in this area of the genome devoted to N-linked glycosylation. Of these 6 genes, in frame deletions were previously created in aglW (MMP_RS05635) as well as the next two genes [12]. Only deletion of aglW had an observable effect on the archaellin glycan, resulting in loss of the terminal sugar as well as the threonine on the third sugar. The first gene in the operon (MMP_RS05630) is annotated as a flippase that resembles the protein in Gram negative bacteria that translocates the undecaprenol-linked intermediate in O antigen polysaccharide synthesis across the cytoplasmic membrane [40]. Repeated attempts to delete this gene have been unsuccessful [15] and it is considered an essential gene [41] despite the fact that the N-glycosylation system itself is not essential for M. maripaludis [7]. MMP_RS05640 is annotated as either an ADP or UDP-glucose pyrophosphorylase while MMP_RS05645 is predicted to be an integral membrane protein containing 10 transmembrane domains and annotated as an auxin efflux protein, likely a transporter protein. No role in N-linked glycosylation is evident for these two genes based on the current data. The three other genes in the operon could not be deleted. The last two genes are annotated as phosphopantetheine adenylyltransferase (MMP_RS05650) and phosphoenol pyruvate synthase (MMP_RS05655) and as such are likely involved in intermediary metabolism and not N-glycosylation. Both of these genes have been identified as likely essential in a genome-wide transposon mutagenesis study [41].
Many other Methanococcus species, including M. voltae, have a highly conserved homologue of aglW. M. voltae was the first Methanococcus species to have the structure of the archaellin N-linked glycan determined [42]. While the glycans are related in structure to that of M. maripaludis, in M. voltae the linking sugar was shown to be GlcNAc (not GalNAc) and the glycan was only a trisaccharide with no sugar similar to the unique fourth sugar of the M. maripaludis glycan [43]. Subsequent analysis of another version of the M. voltae PS strain revealed the archaellin N-linked glycan to be a tetrasaccharide [43]. The structure of the fourth sugar was not determined in that study but it’s mass (220 or 260 Da) indicated it is not identical to the terminal sugar (mass 217 Da) in the M. maripaludis glycan, although it may be related and hence require the activity of AglW for its synthesis.
Many of the genes encoding enzymes involved in the pathways for synthesis of the donor substrates and the GTs required to assemble the archaellin tetrasaccharide are now known [11]. However, AglW is the first enzyme in M. maripaludis that has been biochemically characterized. Three enzymes specifically affect the unique terminal sugar of the N-linked tetrasaccharide, i.e. methyltransferase AglV that likely transfers a methyl group to the 5-position of the fourth sugar [8], AglL believed to be the GT responsible for the transfer of the terminal sugar and the 4-epimerase AglW (Fig. 1). The role of AglW appears to be at an early step in the synthesis pathway that ultimately results in the terminal sugar (as shown in Fig. 1) but its exact biological function remains to be determined. In order to study the individual enzyme reactions involving rare or unique substrates, most of the authentic enzyme substrates or substrate analogs would have to be chemically or enzymatically synthesized. It therefore remains an enormous challenge for future research to biochemically characterize the enzymes, mechanisms and pathways in the synthesis of N-glycosylation in archaea.
References
Chaban, B., Ng, S.Y., Kanbe, M., Saltzman, I., Nimmo, G., Aizawa, S., Jarrell, K.F.: Systematic deletion analyses of the fla genes in the flagella operon identify several genes essential for proper assembly and function of flagella in the archaeon, Methanococcus maripaludis. Mol. Microbiol. 66, 596–609 (2007)
Stahl, D.A., de la Torre, J.R.: Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 66, 83–101 (2012)
Offre, P., Spang, A., Schleper, C.: Archaea in biogeochemical cycles. Annu. Rev. Microbiol. 67, 437–457 (2013)
Enzmann, F., Mayer, F., Rother, M., Holtmann, D.: Methanogens: biochemical background and biotechnological applications. AMB Express. 8, 1–22 (2018)
Fortunato, C.S., Larson, B., Butterfield, D.A., Huber, J.A.: Spatially distinct, temporally stable microbial populations mediate biogeochemical cycling at and below the seafloor in hydrothermal vent fluids. Environ. Microbiol. 20, 769–784 (2018)
Jones, W.J., Paynter, M.J.B., Gupta, R.: Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch. Microbiol. 135, 91–97 (1983)
Jarrell, K.F., Ding, Y., Meyer, B.H., Albers, S.-V., Kaminski, L., Eichler, J.: N-linked glycosylation in archaea: a structural, functional, and genetic analysis. Microbiol. Mol. Biol. Rev. 78, 304–341 (2014)
Ding, Y., Jones, G.M., Uchida, K., Aizawa, S.-I., Robotham, A., Logan, S.M., Kelly, J., Jarrell, K.F.: Identification of genes involved in the biosynthesis of the third and fourth sugars of the Methanococcus maripaludis archaellin N-linked tetrasaccharide. J. Bacteriol. 195, 4094–4104 (2013)
Moore, B.C., Leigh, J.A.: Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J. Bacteriol. 187, 972–979 (2005)
Albers, S.V., Jarrell, K.F.: The archaellum: an update on the unique archaeal motility structure. Trends Microbiol. 26, 351–362 (2018)
Siu, S., Robotham, A., Logan, S.M., Kelly, J.F., Uchida, K., Aizawa, S.-I., Jarrell, K.F.: Evidence that biosynthesis of the second and third sugars of the archaellin tetrasaccharide in the archaeon Methanococcus maripaludis occurs by the same pathway used by Pseudomonas aeruginosa to make a di-N-acetylated sugar. J. Bacteriol. 197, 1668–1680 (2015)
Ding, Y., Jones, G.M., Brimacombe, C., Uchida, K., Aizawa, S.-I., Logan, S.M., Kelly, J.F., Jarrell, K.F.: Identification of a gene involved in the biosynthesis pathway of the terminal sugar of the archaellin N-linked tetrasaccharide in Methanococcus maripaludis. Antonie van Leeuwenhoek. 109, 131–148 (2016)
Kelly, J., Logan, S.M., Jarrell, K.F., VanDyke, D.J., Vinogradov, E.: A novel N-linked flagellar glycan from Methanococcus maripaludis. Carbohydr. Res. 344, 648–653 (2009)
Ding, Y., Uchida, K., Aizawa, S.-I., Murphy, K., Berezuk, A., Khursigara, C.M., Chong, J.P.J., Jarrell, K.F.: Effects of N-glycosylation site removal in Archaellins on the assembly and function of Archaella in Methanococcus maripaludis. PLoS One. 10(2) e0116402), 1–23 (2015)
VanDyke, D.J., Wu, J., Logan, S.M., Kelly, J.F., Mizuno, S., Aizawa, S.-I., Jarrell, K.F.: Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. 72, 633–644 (2009)
Westman, E.L., McNally, D.J., Charchoglyan, A., Brewer, D., Field, R.A., Lam, J.S.: Characterization of WbpB, WbpE, and WbpD and reconstitution of a pathway for the biosynthesis of UDP-2,3-diacetamido-2,3-dideoxy-D-mannuronic acid in Pseudomonas aeruginosa. J. Biol. Chem. 284, 11854–11862 (2009)
Jones, G.M., Wu, J., Ding, Y., Uchida, K., Aizawa, S.-I., Robotham, A., Logan, S.M., Kelly, J., Jarrell, K.F.: Identification of genes involved in the acetamidino group modification of the flagellin N-linked glycan of Methanococcus maripaludis. J. Bacteriol. 194, 2693–2702 (2012)
Guo, H., Li, L., Wang, P.G.: Biochemical characterization of UDP-GlcNAc/Glc 4-epimerase from Escherichia coli O86:B7. Biochemistry. 45, 13760–13768 (2006)
Schulz, J.M., Watson, A.L., Sanders, R., Ross, K.L., Thoden, J.B., Holden, H.M., Fridovich-Keil, J.L.: Determinants of function and substrate specificity in human UDP-galactose 4′-epimerase. J. Biol. Chem. 279, 32796–32803 (2004)
Bengoechea, J.A., Pinta, E., Salminen, T., Oertelt, C., Holst, O., Radziejewska-Lebrecht, J., Piotrowska-Seget, Z., Venho, R., Skurnik, M.: Functional characterization of Gne (UDP-N-Acetylglucosamine-4-epimerase), Wzz (chain length determinant), and Wzy (O-antigen polymerase) of Yersinia enterocolitica serotype O:8. J. Bacteriol. 184, 4277–4287 (2002)
Ishiyama, N., Creuzenet, C., Lam, J.S., Berghuis, A.M.: Crystal structure of WbpP, a genuine UDP-N-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa substrate specificity in UDP-hexose 4-epimerases. J. Biol. Chem. 279, 22635–22642 (2004)
Bhatt, V.S., Guan, W., Xue, M., Yuan, H., Wang, P.G.: Insights into role of the hydrogen bond networks in substrate recognition by UDP-GalNAc 4-epimerases. Biochem. Biophys. Res. Commun. 412, 232–237 (2011)
Rush, J.S., Alaimo, C., Robbiani, R., Wacker, M., Waechter, C.J.: A novel epimerase that converts GlcNAc-P-P-undecaprenol to GalNAc-P-P-undecaprenol in Escherichia coli O157. J. Biol. Chem. 285, 1671–1680 (2010)
Cunneen, M.M., Liu, B., Wang, L., Reeves, P.R.: Biosynthesis of UDP-GlcNAc, UndPP-GlcNAc and UDP-GlcNAcA involves three easily distinguished 4-epimerase enzymes, Gne, gnu and GnaB. PLoS One. 8(6) e67646), 1–9 (2013)
Beerens, K., Soetaert, W., Desmet, T.: UDP-hexose 4-epimerases: a view on structure, mechanism and substrate specificity. Carbohydr. Res. 414, 8–14 (2015)
Wang, S., Czuchry, D., Liu, B., Vinnikova, A., Gao, Y., Vlahakis, J.Z., Szarek, W.A., Feng, L., Wang, L., Brockhausen, I.: Characterization of two UDP-gal: GalNAc-diphosphate-lipid β1,3-galactosyltransferases WbwC from Escherichia coli serotypes O104 and O5. J. Bacteriol. 196, 3122–3133 (2014)
Gao, Y., Liu, B., Strum, S., Schutzbach, J., Druzhinina, T.N., Utkina, N.S., Torgov, V.I., Danilov, L.L., Veselovsky, V.V., Vlahakis, J.Z., Szarek, W.A., Wang, L., Brockhausen, I.: Biochemical characterization of WbdN, a beta1,3-glucosyltransferase involved in O-antigen synthesis in enterohemorrhagic Escherichia coli O157. Glycobiology. 22, 1092–1102 (2012)
Xu, C., Liu, B., Hu, B., Han, Y., Feng, L., Allingham, J., Szarek, W.A., Wang, L., Brockhausen, I.: Biochemical characterization of UDP-gal:GlcNAc-pyrophosphate-lipid beta1,4-galactosyltransferase WfeD, a new enzyme from Shigella boydii type 14 that catalyzes the second step in O-antigen repeating unit synthesis. J. Bacteriol. 193, 449–459 (2011)
Piller, F., Hanlon, M.H., Hill, R.L.: Co-purification and characterization of UDP-glucose 4-epimerase and UDP-N-acetylglucosamine 4-epimerase from porcine submaxillary glands. J. Biol. Chem. 258, 10774–10778 (1983)
Brockhausen, I., Benn, M., Bhat, S., Marone, S., Riley, J.G., Montoya-Peleaz, P., Vlahakis, J.Z., Paulsen, H., Schutzbach, J.S., Szarek, W.A.: UDP-gal: GlcNAc-R beta1,4-galactosyltransferase a target enzyme for drug design. Acceptor specificity and inhibition of the enzyme. Glycoconj. J. 23, 525–541 (2006)
Reck, F., Springer, M., Meinjohanns, E., Paulsen, H., Brockhausen, I., Schachter, H.: Synthetic substrate analogues for UDP-GlcNAc: Man alpha1-3R beta1-2-N-acetylglucosaminyltransferase I. substrate specificity and inhibitors for the enzyme. Glycoconj. J. 12, 747–754 (1995)
Elhammer, A., Poorman, R., Brown, E., Maggiora, L., Hoogerheide, J., Kézdy, F.: The specificity of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase as inferred from a database of in vivo substrates and from the in vitro glycosylation of proteins and peptides. J. Biol. Chem. 268, 10029–10038 (1993)
Brockhausen, I., Dowler, T., Paulsen, H.: Site directed processing: role of amino acid sequences and glycosylation of acceptor glycopeptides in the assembly of extended mucin type O-glycan core 2. Biochim. Biophys. Acta. 1790, 1244–1257 (2009)
Chung, S.K., Ryu, S.I., Lee, S.B.: Characterization of UDP-glucose 4-epimerase from Pyrococcus horikoshii: regeneration of UDP to produce UDP-galactose using two-enzyme system with trehalose. Bioresour. Technol. 110, 423–429 (2012)
Jensen, J.W., Schutzbach, J.S.: Characterization of mannosyl-transfer reactions catalyzed by dolichyl-mannosyl-phosphate-synthase. Carbohydr. Res. 149, 199–208 (1986)
Chen, X., Kowal, P., Hamad, S., Fan, H., Wang, P.G.: Cloning, expression and characterization of a UDP-galactose 4-epimerase from Escherichia coli. Biotechnol. Lett. 21, 1131–1135 (1999)
Thoden, J.B., Henderson, J.M., Fridovich-Keil, J.L., Holden, H.M.: Structural analysis of the Y299C mutant of Escherichia coli UDP-galactose 4-epimerase. Teaching an old dog new tricks. J. Biol. Chem. 277, 27528–27534 (2002)
Shaw, M.P., Bond, C.S., Roper, J.R., Gourley, D.G., Ferguson, M.A.J., Hunter, W.N.: High-resolution crystal structure of Trypanosoma brucei UDP-galactose 4′-epimerase: a potential target for structure-based development of novel trypanocides. Mol. Biochem. Parasitol. 126, 173–180 (2003)
Yoneda, K., Sakuraba, H., Muraoka, I., Oikawa, T., Ohshima, T.: Crystal structure of UDP-galactose 4-epimerase-like l-threonine dehydrogenase belonging to the intermediate short-chain dehydrogenase-reductase superfamily. FEBS J. 277, 5124–5132 (2010)
Islam, S.T., Lam, J.S.: Wzx flippase-mediated membrane translocation of sugar polymer precursors in bacteria. Environ. Microbiol. 15, 1001–1015 (2013)
Sarmiento, F., Mrázek, J., Whitman, W.B.: Genome-scale analysis of gene function in the hydrogenotrophic methanogenic archaeon Methanococcus maripaludis. Proceed. Nat. Acad. Sci. 110, 4726–4731 (2013)
Voisin, S., Houliston, R.S., Kelly, J., Brisson, J.-R., Watson, D., Bardy, S.L., Jarrell, K.F., Logan, S.M.: Identification and characterization of the unique N-linked glycan common to the flagellins and S-layer glycoprotein of Methanococcus voltae. J. Biol. Chem. 280, 16586–16593 (2005)
Chaban, B., Logan, S.M., Kelly, J.F., Jarrell, K.F.: AglC and AglK are involved in biosynthesis and attachment of Diacetylated glucuronic acid to the N-glycan in Methanococcus voltae. J. Bacteriol. 191, 187–195 (2009)
Acknowledgements
This research was supported by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to I.B and to K.F.J.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 636 kb)
Rights and permissions
About this article
Cite this article
Sharma, S., Ding, Y., Jarrell, K.F. et al. Identification and characterization of the 4-epimerase AglW from the archaeon Methanococcus maripaludis. Glycoconj J 35, 525–535 (2018). https://doi.org/10.1007/s10719-018-9845-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9845-4