Abstract
In order to develop novel glycolipid biosurfactants, Pseudozyma parantarctica JCM 11752T, which is known as a producer of mannosylerythritol lipids (MEL), was cultivated using different sugar alcohols with the presence of vegetable oil. When cultivated in a medium containing 4 % (w/v) olive oil and 4 % d-ribitol or d-arabitol, the yeast strain provided different glycolipids, compared to the case of no sugar alcohol. On TLC, both of the extracted glycolipid fractions gave two major spots corresponding to MEL-A (di-acetylated MEL) and MEL-B (mono-acetylated MEL). Based on 1H NMR analysis, one glycolipid was identified as MEL-A, but the other was not MEL-B. On high-performance liquid chromatography after acid hydrolysis, the unknown glycolipid from the d-ribitol culture provided mainly two peaks identical to d-mannose and d-ribitol, and the other unknown glycolipid from the d-arabitol culture did two peaks identical to d-mannose and d-arabitol. Accordingly, the two unknown glycolipids were identified as mannosylribitol lipid (MRL) and mannosylarabitol lipid (MAL), respectively. The observed critical micelle concentration (CMC) and surface tension at CMC of MRL were 1.6 × 10−6 M and 23.7 mN/m, and those of MAL were 1.5 × 10−6 M and 24.2 mN/m, respectively. These surface-tension-lowering activities were significantly higher compared to conventional MEL. Furthermore, on a water-penetration scan, MRL and MAL efficiently formed not only the lamella phase (Lα) but also the myelins at a wide range of concentrations, indicating their excellent self-assembling properties and high hydrophilicity. The present two glycolipids should thus facilitate the application of biosurfactants as new functional materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
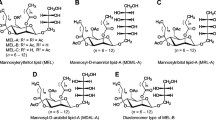
Biosurfactants are surface-active compounds produced by various microorganisms and have attracted considerable interest in recent years due to their unique properties (Lang 2002; Kitamoto et al. 2002). Mannosylerythritol lipids (MEL) are abundantly produced more than 100 g/l from vegetable oils by yeast strains belonging to the genus Pseudozyma and are thus one of the most promising biosurfactants known (Kitamoto et al. 2002; Morita et al. 2007, 2009a). Pseudozyma antarctica (Kitamoto et al. 1990), Pseudozyma aphidis (Rau et al. 2005), and Pseudozyma rugulosa (Morita et al. 2006) are high-level producers, and secrete mainly MEL-A, together with MEL-B and MEL-C as minor components (Fig. 1a).
MEL exhibits not only excellent surface- and interfacial-tension-lowering activities (Kitamoto et al. 1993) but also distinctive self-assembling properties generating different lyotropic liquid crystalline structures, such as sponge (L3), bicontinuous (V2), and lamella (Lα) phases, in the broad range of the glycolipid concentrations (Imura et al. 2004, 2006, 2007a). Moreover, MEL-A shows versatile biochemical actions including differentiation induction with respect to human leukemia (Isoda et al. 1997), rat pheochromocytoma (Wakamatsu et al. 2001), and mouse melanoma cells (Zhao et al. 2001) as well as high affinity binding towards different immunoglobulins (Imura et al. 2007b; Ito et al. 2007) and lectins (Konishi et al. 2007).
We previously reported the production of different MEL derivatives, such as tri-acylated MEL (Fukuoka et al. 2007a; Morita et al. 2008a) and mono-acylated MEL (Fukuoka et al. 2007b), in addition to di-acylated MEL (MEL-A, MEL-B, and MEL-C) with various lengths of fatty acids (Morita et al. 2009a). Moreover, Pseudozyma parantarctica JCM 11752T was recently reported to produce mannosylmannitol lipid (MML) possessing mannitol (C6) as the hydrophilic part instead of erythritol (C4) when cultivated in a medium containing an excess amount of d-mannitol (Morita et al. 2009b) (Fig. 1b). MML provided the similar critical micelle concentration (CMC) and surface tension at CMC (γcmc) to those of MEL-A, which has a “di-acetylated mannose” moiety. On the other hand, the liquid crystalline structures of MML in water were similar to those of MEL-B, which has a “mono-acetylated mannose” moiety. Therefore, the presence of a mannitol moiety instead of erythritol was assumed to provide the high hydrophilicity and/or water solubility compared to MEL-A.
These MEL derivatives have the difference in the surface activities and the self-assembling properties generating different lyotropic liquid crystalline structures. Increase of the structural variety of the glycolipid biosurfactants not only expands the industrial application but also facilitates a better understanding of the structure–function relationship. We thus focused our attention on the control of the hydrophilic part of MEL by the introduction of different sugar alcohols other than erythritol and mannitol.
Here, we investigated the supplementation of C5 sugar alcohols with the presence of vegetable oil using P. parantarctica and succeeded in obtaining two novel glycolipid biosurfactants; mannosylribitol lipid (MRL) bearing ribitol as the sugar moiety, and mannosylarabitol lipid (MAL) bearing arabitol as the sugar moiety. We also describe their surface-active and self-assembling properties. This is the first report on the microbial formation of MRL and MAL.
Materials and methods
Microorganism
P. parantarctica JCM 11752T was obtained from the RIKEN BioResource Center. Stock cultures were cultivated for 3 days at 25 °C on an agar medium containing 4 % glucose, 0.3 % NaNO3, 0.03 % MgSO4, 0.03 % KH2PO4, and 0.1 % yeast extract. They were stored at 4 °C and renewed every 2 weeks.
Media preparation and culture condition
Seed cultures were prepared by inoculating cells grown on slants into test tubes containing a growth medium [4 % glucose, 0.3 % NaNO3, 0.03 % MgSO4, 0.03 % KH2PO4, 0.1 % yeast extract (pH 6.0)] at 30 °C on a reciprocal shaker (200 strokes/min) for 2 days. Seed cultures (0.1 ml) were transferred to a flask containing 20 ml of a basal medium [4 % (w/v) olive oil, 0.3 % NaNO3, 0.03 % MgSO4, 0.03 % KH2PO4, 0.1 % yeast extract (pH 6.0)], and then incubated at 35 °C on a rotary shaker (300 rpm) for 7 days.
Isolation of glycolipids
The produced glycolipids were extracted from the culture medium with an equal amount of ethyl acetate. The extracts were analyzed by thin-layer chromatography (TLC) on silica plates (Silica gel 60F; Wako, Osaka, Japan) with a solvent system consisting of chloroform/methanol/7 N ammonium hydroxide (65:15:2, v/v/v). The compounds on the plates were located by charring at 110 °C for 5 min after spraying the anthrone reagent (Kitamoto et al. 1990). The purified MEL fraction including MEL-A, MEL-B, and MEL-C was used as a standard as reported previously (Morita et al. 2006).
Purification of glycolipids
The above ethyl acetate fractions were evaporated. The concentrated glycolipids were dissolved in chloroform and then purified by silica-gel (Wako-gel C-200) column chromatography using a gradient elution of chloroform/acetone (10:0 to 0:10, v/v) mixtures as solvent systems (Morita et al. 2006). The purified glycolipids were used in the following experiments.
Structural analysis
The structure of the partially purified glycolipids was characterized by 1H and 13C nuclear magnetic resonance (NMR) with a Varian INOVA 400 (400 MHz) at 30 °C using the CD3OD solution (Table 1). In NMR study, the purified MEL-A was used as a reference. The chemical shifts of the hydrophilic moiety of MEL-A produced by P. parantarctica is as follows (Morita et al. 2006): 1H NMR (CDCl3, 400 MHz): δ 5.51 (d, H-2′), 5.24 (t, H-4′), 5.06 (dd, H-3′), 4.71 (d, H-1′), 4.23 (m, H-6′), 3.99 (dd, H-4b), 3.86 (dd, H-4a), 3.75 (m, H-1), 3.74 (m, H-3), 3.71 (m, H-5′), 3.68 (m, H-2), 2.43 (m, –CH2C=O at the C-2′ position), 2.22 (m, –CH2C=O at the C-3′ position), 2.10 (s, CH3C=O at the C-6′ position), 2.03 (s, CH3C=O at the C-4′ position), 1.26–1.40 (br, –CH2–), 0.87 (t, –CH3). The chemical shifts of the hydrophilic moiety of MEL-A produced by Pseudozyma tsukubaensis is as follows (Fukuoka et al. 2008): 1H NMR (CDCl3, 400 MHz): δ 5.48 (dd, H-2′), 4.94 (dd, H-3′), 4.76 (d, H-1′), 4.36–4.49 (m, H-6′), 3.99 (dd, H-4b), 3.87 (dd, H-4a), 3.70–3.82 (m, H-3 and H-4′), 3.57–3.82 (m, H-1), 3.57–3.64 (m, H-2 and H-5′), 2.40 (m, –CH2C=O at the C-2′ position), 2.30 (m, –CH2C=O at the C-3′ position), 2.13 (s, CH3C=O at the C-6′ position), 1.20–1.40 (br, –CH2–), 0.85–0.93 (br, –CH3).
Acid degradation was performed by mixing the purified glycolipids (10 mg) with 1 ml of 5 % HCl–methanol reagent (Wako) overnight at room temperature. After the reaction was quenched with water (1 ml), the methyl ester derivatives of the fatty acids were removed with n-hexane. After the water-soluble fraction was neutralized through the Amberlite column pretreated with NaOH, the fraction was supplied to HPLC on a SUGAR SH1011 column (Shoko, Tokyo, Japan) with a differential refractive index detector (RI-8020) using 0.01 N H2SO4 as the solvent system.
The fatty acid profile were analyzed by gas chromatography–mass spectrometry (GC–MS) (Hewlett Packard 6890 and 5973N) with a TC-WAX (GL-science, Tokyo, Japan) with the temperature programmed from 90 °C (held for 3 min) to 240 °C at 5 °C/min.
The molecular weight of glycolipids was measured by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) (Voyager-DE PRO) with an α-cyano-4-hydroxycinnamic acid matrix.
Determination of surface tension
The surface tension of the partially purified glycolipids was determined by the Whilhelmy method at 25 °C, which was performed using the apparatus consisting of an automatic Whilhelmy-type automatic tensiometer (CBVP-A3; Kyowa Interface Science, Saitama, Japan).
Water-penetration scan technique
To examine the lyotropic-liquid-crystalline phase behavior of the partially purified glycolipids, the water-penetration scan technique was used as reported previously (Imura et al. 2006). A polarized optical microscope (ECLIPSE E-600; Nikon, Tokyo, Japan) with crossed polarizing filters equipped with a charge-coupled-device camera (DS-SM; Nikon) was used for the scan. Birefringent textures from the optical microscopy allowed the assignment of the particular lyotropic phase types to the samples.
Results
Production of glycolipids with C5 sugar alcohol
P. parantarctica JCM 11752T, an excellent producer of MELs, was recently found to produce mannosylmannitol lipid (MML) from vegetable oil and d-mannitol (Morita et al. 2009b). Aiming to obtain novel glycolipid biosurfactants, the yeast strain was thus cultivated in the basal medium containing 4 % (w/v) of d-xylitol, d-ribitol, d-arabitol, or l-arabitol for 7 days at 35 °C. The cultures were extracted with ethyl acetate, and the ethyl acetate fractions were spotted on a TLC plate. A mixture of purified MEL-A, MEL-B, and MEL-C was used as the standard, and the glycolipids were detected with the anthrone reagent.
The glycolipid pattern of the control without sugar alcohol was mainly MEL-A together with MEL-C as the minor product (Fig. 2). In case of d-xylitol, the fraction gave the similar glycolipid pattern as the control. l-Arabitol gave only a small amount of glycolipids. Interestingly, the fraction from the d-ribitol culture (272.0 mg from 20 ml of culture medium) showed two main spots corresponding to MEL-A and MEL-B, while the fraction from d-arabitol (350.4 mg from 20 ml of culture medium) did the major spot corresponding to MEL-B, together with the minor spot corresponding to MEL-A.
Production of glycolipids by Pseudozyma parantarctica JCM 11752T. Samples were extracted from the culture medium with ethyl acetate, and the organic solvent fraction was spotted on to a TLC plate. The spots were visualized with the anthrone reagent. White arrows show the spots of the unknown glycolipids. Control is an extract from the culture without supplementation of any sugar alcohols
Structural determination of the glycolipids from d-ribitol and d-arabitol
To confirm the structure of these four glycolipids, the ethyl acetate fractions were partially purified and then studied by 1H NMR spectroscopy. Based on 1H NMR study, two glycolipids with the higher R f value on TLC were identified as previously reported MEL-A (Kitamoto et al. 2000) (data not shown). Two other glycolipids with the lower R f value on TLC (indicated by a white arrow in Fig. 2) showed a very similar spectrum to that of MEL-A, but the spectrum was clearly different from that of MEL-B (Table 1). Unfortunately, the carbon number of the two glycolipids was obscured on 13C NMR analysis because of the contamination of significant amount of MEL-B. We tried to completely separate the present glycolipid and MEL-B by the column chromatography, but failed to do it. According to the 1H NMR study, these two glycolipids were estimated to contain at least 20 % of MEL-B.
To identify the sugar composition of the two glycolipids, they were subjected to HPLC analysis after acid hydrolysis. As we expected, the acid-hydrolyzed fraction prepared from the d-ribitol culture showed the two peaks corresponding to d-mannose (6.07 min) and d-ribitol (7.56 min) (Fig. 3a). Likewise, the fraction from the d-arabitol culture gave the two peaks corresponding to d-mannose (6.07 min) and d-arabitol (7.71 min) (Fig. 3b). The small erythritol peak in each fraction is probably due to the contaminated MEL-B. The two glycolipids should thus be composed of mannose and d-ribitol or d-arabitol as the hydrophilic part.
HPLC analysis of sugar moiety of the two glycolipids. The glycolipids produced from olive oil and d-ribitol (a) and d-arabitol (b) were treated with acid solution, and the obtained water-soluble fraction was subjected to HPLC analysis (lower chart). The mixture of mannosylerythritol, d-mannose, meso-erythritol, and d-ribitol or d-arabitol was used as the standard solution (upper chart). Mannosylerythritol used as the standard was obtained by the deacylated treatment of MEL. The retention time (minutes) of each peak is given in parentheses
The molecular weight of the glycolipid from the d-ribitol culture was 678.8 (C8 and C10 acids) as determined from the main peak (701.8 [M + Na]+) on MALDI-TOF/MS analysis. Likewise, that of the glycolipid from the d-arabitol culture was 678.9 (C8 and C10 acids) as determined from the main peak (701.9 [M + Na]+). These results are well consistent with the above structures. Consequently, the new glycolipid produced from olive oil and d-ribitol was identified as 1-O-alka(e)noyl-4-O-[(4′,6′-di-O-acetyl-2′,3′-di-O-alka(e)noyl)-β-d-mannopyranosyl]-d-ribitol, mannosylribitol lipid (MRL, Fig. 4a). The other new glycolipid produced from olive oil and d-arabitol was identified as 1-O-alka(e)noyl-4-O-[(4′,6′-di-O-acetyl-2′,3′-di-O-alka(e)noyl)-β-d-mannopyranosyl]-d-arabitol, mannosylarabitol lipid (MAL, Fig. 4b).
In both of MRL and MAL, the fatty acids were mainly composed of C8 and C10 as well as that of MML (Table 2).
Surface-active properties of MRL and MAL
As MRL and MAL consist of C5 sugar alcohol as the hydrophilic moiety, they were expected to show different surface activities and interfacial properties compared to conventional MEL consisting of erythritol as C4 sugar alcohol. We thus evaluated the surface tension of the partially purified MRL and MAL by the Whilhelmy method.
Figure 5a shows the surface (air–water) tension vs. concentration plot of the partially purified MRL in distilled water. The estimated critical micelle concentration (CMC) and surface tension at CMC (γcmc) of the MRL were 1.6 × 10−6 M and 23.7 mN/m, respectively. Figure 5b shows the surface tension vs. concentration plot of the partially purified MAL in distilled water. The estimated CMC and γcmc of the MRL were 1.5 × 10−6 M and 24.2 mN/m, respectively. On the other hand, those of MEL-A and MEL-B produced by P. antarctica T-34 were 2.7 × 10−6 M (γcmc = 28.4 mN/m) and 4.5 × 10−6 M (γcmc = 28.2 mN/m), respectively (Kitamoto et al. 1993). Therefore, both of the new glycolipids generate an excellent surface-tension-lowering activity besides the presence of more hydrophilic sugar alcohol than erythritol.
Formation of lyotropic liquid crystals from MRL and MAL
We further tentatively investigated the self-assembly properties of MRL and MAL in aqueous solutions. Here, we examine the formation of lyotropic-liquid-crystalline phases from the partially purified MRL and MAL by the water-penetration technique.
Figure 6 shows water-penetration scans of the partially purified MRL and MAL viewed with (bottoms, POL) and without (upper, DIC) crossed polarizing filters. In both cases, the photographs clearly indicate four different regions that should represent water (W), myelins, the lamellar phase (Lα), and the neat surfactant phase (S). Interestingly, the observed lamellar phase spreads over a wide concentration range. Based on our previous study, MEL-B, which has significantly higher hydrophilicity than MEL-A, efficiently forms the lamellar phase (Lα) and myelins, while MEL-A forms mainly the sponge phase (L3) (Imura et al. 2006). Similar to MEL-B, MRL and MAL are also very likely to show higher hydrophilicity compared to MEL-A.
Consequently, the present new glycolipid biosurfactants produced by P. parantarctica have excellent surface-active and self-assembling properties, reflecting the unique hydrophilic part consisting of C5 sugar alcohol.
Discussion
Here, we reported the formation of two new glycolipid biosurfactants, namely MRL and MAL by P. parantarctica JCM 11752T known as a MEL producer. As mentioned, these glycolipids were obtained when the strain JCM 11752T was grown in the medium containing olive oil and d-ribitol and d-arabitol, respectively.
Conventional MEL hitherto reported (MEL-A, MEL-B, and MEL-C) show significant differences in the structure as to the number and position of acetyl group at the mannose moiety and/or to the fatty acid composition (Fig. 1). However, mannosylerythritol as the hydrophilic part is the same among them all. MML bearing C6 sugar alcohol as the hydrophilic part was the first MEL derivative with a different sugar backbone. The present MRL and MAL bearing C5 sugar alcohol showed different surface-active and self-assembling properties, and would thus allow us to obtain a better understanding for the structure–function relationship of glycolipid biosurfactants.
Recently, the biosynthetic pathway of MEL was deduced in Ustilago maydis, which is also known as a MEL producer (Hewald et al. 2006). Although the substrate specificity of a mannosyl transferase, which catalyzes the synthesis of mannosylerythritol by transfer of GDP-mannose to erythritol, still remains unknown, we succeeded in obtaining MML from olive oil with P. parantarctica by supplementation of mannitol in the culture medium (Morita et al. 2009b). In this study, we found that C5 sugar alcohols such as d-ribitol and d-arabitol also serve as the donor to create new glycolipids, but d-xylose and l-arabitol do not. Interestingly, d-mannitol (at C4 and C5), d-ribitol (at C3 and C4), and d-arabitol (at C3 and C4) have the same diol configuration as meso-erythritol (at C2 and C3), but d-xylose and l-arabitol do not. These results may suggest that sugar alcohols bearing the similar alcohol configuration as meso-erythritol are directly utilized by the mannosyl transferase to form new mannose derivatives other than mannosylerythritol. Nevertheless, detailed biochemical studies should be done on the transferase to shed light on the mechanism generating a “new hydrophilic part”.
Generally, on glycolipid surfactants, the structure of a sugar backbone gives a critical effect not only on the hydrophilicity but also on the physiochemical properties including its manner of self-assembly (Imura et al. 2006). Indeed, MEL-B consisting of “mono-acetylated mannose” shows higher hydrophilicity than MEL-A consisting of “di-acetylated mannose” and efficiently forms the lamellar structures in water at a wide concentration range. Furthermore, MEL-C consisting of “mono-acetylated mannose” shows higher hydrophilicity compared MEL-A and MEL-B, and provides various self-assembling properties depending on the chain length of fatty acids (Morita et al. 2008b, c; Konishi et al. 2008).
In our previous study, we investigated the modification of the sugar backbone of MEL by supplementation of sugar alcohols other than meso-erythritol, and obtained MML by P. parantarctica using d-mannitol and olive oil. MML bearing mannosylmannitol showed the similar CMC (2.6 × 10−6 M) but lower γcmc (24.2 mN/m) to that of MEL-A (2.7 × 10−6 M and 28.4 mN/m, respectively). As we expected, the present MRL and MAL showed higher surface activities compared to conventional MEL. Although the reason why the present glycolipids shows very high surface activities is not clear, the number and configuration of the hydroxyl group at the sugar alcohol moiety should play an important role on the adsorption at air–water interface.
On a water-penetration scan, MAL and MRL immediately formed myelin and lamellar phase as well as MML, in contrast to the case of MEL-A. These results strongly support that the presence of longer-chain sugar alcohol than erythritol (C4) should contribute to provide higher hydrophilicity and/or water solubility compared to conventional MEL.
In conclusion, we investigated the production of new MEL derivatives using different sugar alcohols and vegetable oil, and obtained two new glycolipids, namely MRL and MAL from d-ribitol and d-arabitol, respectively. Both of the glycolipids were identified to possess C5 sugar alcohol as the hydrophilic part, and exhibited excellent surface activities and unique self-assembling properties. Thus, the present glycolipids would enable us to obtain a better understanding of the structure–function relationship of glycolipid biosurfactants and facilitate a broad range of their applications.
References
Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D (2007a) Characterization of new glycolipid biosurfactants, tri-acylated mannosylerythritol lipids, produced by Pseudozyma yeasts. Biotechnol Lett 29:1111–1118
Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D (2007b) Structural characterization and surface-active properties of a new glycolipid biosurfactant, mono-acylated mannosylerythritol lipid, produced from glucose by Pseudozyma antarctica. Appl Microbiol Biotechnol 76:801–810
Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D (2008) A basidiomycetous yeast, Pseudozyma tsukubaensis, efficiently produces a novel glycolipid biosurfactant. The identification of a new diastereomer of mannosylerythritol lipid-B. Carbohydr Res 343:555–560
Hewald S, Linne U, Scherer M, Marahiel MA, Kamper J, Bölker M (2006) Identification of a gene cluster for biosynthesis of mannosylerythritol lipids in the basidiomycetous fungus Ustilago maydis. Appl Environ Microbiol 72:5469–5477
Imura T, Yanagishita H, Kitamoto D (2004) Coacervate formation from natural glycolipid: one acetyl group on the headgroup triggers coacervate-to-vesicle transition. J Am Chem Soc 126:10804–10805
Imura T, Ohta N, Inoue K, Yagi H, Negishi H, Yanagishita H, Kitamoto D (2006) Naturally engineered glycolipid biosurfactants leading to distinctive self-assembled structures. Chem Eur J 12:2434–2440
Imura T, Hikosaka Y, Worakitkanchanakul W, Sakai H, Abe M, Konishi M, Minamikawa H, Kitamto D (2007a) Aqueous-phase behavior of natural glycolipid biosurfactants mannosylerythritol lipid A: sponge, cubic, and lamella phases. Langmuir 23:1659–1663
Imura T, Ito S, Azumi R, Yanagishita H, Sakai H, Abe M, Kitamoto D (2007b) Monolayers assembled from a glycolipid biosurfactant from Pseudozyma (Candida) antarctica serve as a high-affinity ligand system for immunoglobulin G and M. Biotechnol Lett 29:865–870
Isoda H, Kitamoto D, Shinomoto H, Matsumura M, Nakahara T (1997) Microbial extracellular glycolipid induction of differentiation and inhibition of the protein kinase C activity of human promyelocytic leukemia cell line activity of human promyelocytic leukemia cell line HL60. Biosci Biotechnol Biochem 61:609–614
Ito S, Imura T, Fukuoka T, Morita T, Sakai H, Abe M, Kitamoto D (2007) Kinetic studies on the interactions between glycolipid biosurfactants assembled monolayers and various classes of immunoglobulins using surface plasmon resonance. Colloid Surf B 58:165–171
Kitamoto D, Haneishi K, Nakahara T, Tabuchi T (1990) Production of mannosylerythritol lipids by Candida antarctica from vegetable oils. Agric Biol Chem 54:37–40
Kitamoto D, Yanagishita H, Shinbo T, Nakane T, Kamisawa C, Nakahara T (1993) Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J Biotech 29:91–96
Kitamoto D, Ghosh S, Ourisson G, Nakatani Y (2000) Formation of giant vesicles from diacylmannosylerythritols, and their binding to concanavalin A. Chem Commun 10:861–862
Kitamoto D, Isoda H, Nakahara T (2002) Functional and potential application of glycolipid biosurfactants. J Biosci Bioeng 94:187–201
Konishi M, Imura T, Morita T, Fukuoka T, Kitamoto D (2007) A yeast glycolipid biosurfactant, mannosyl-erythritol lipid, shows high binding affinity towards lectins on a self-assembled monolayer system. Biotechnol Lett 29:473–480
Konishi M, Morita T, Fukuoka T, Imura T, Kakugawa K, Kitamoto D (2008) Efficient production of mannosylerythritol lipids with high hydrophilicity by Pseudozyma hubeiensis KM-59. Appl Microbiol Biotechnol 78:37–46
Lang S (2002) Biological amphiphiles (microbial biosurfactants). Curr Opin Coll Int Sci 7:12–20
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2006) Discovery of Pseudozyma rugulosa NBRC 10877 as a novel producer of glycolipid biosurfactants, mannosylerythritol lipids, based on rDNA sequence. Appl Microbiol Biotechnol 73:305–315
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2007) Characterization of the genus Pseudozyma by the formation of glycolipid biosurfactants, mannosylerythritol lipids. FEMS Yeast Res 7:286–292
Morita T, Konishi M, Fukuoka T, Imura T, Sakai H, Kitamoto D (2008a) Efficient production of di- and tri-acylated mannosylerythritol lipids as glycolipid biosurfactants by Pseudozyma parantarctica JCM 11752T. J Oleo Sci 57:557–565
Morita T, Konishi M, Fukuoka T, Imura T, Yamamoto S, Kitagawa M, Sogabe A, Kitamoto D (2008b) Identification of Pseudozyma graminicola CBS 10092 as a producer of glycolipid biosurfactants, mannosylerythritol lipids. J Oleo Sci 57:123–131
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2008c) Production of glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma siamensis CBS 9960 and their interfacial properties. J Biosci Bioeng 105:493–502
Morita T, Fukuoka T, Imura T, Kitamoto D (2009a) Production of glycolipid biosurfactants by basidiomycetous yeasts. Biotechnol Appl Biochem 53:39–49
Morita T, Fukuoka T, Konishi M, Imura T, Yamamoto S, Kitagawa M, Sogabe A, Kitamoto D (2009b) Production of a novel glycolipid biosurfactant, mannosylmannitol lipid, by Pseudozyma parantarctica and its interfacial properties. Appl Microbiol Biotechnol 83:1017–1025
Rau U, Nguyen LA, Schulz S, Wary V, Nimtz M, Roeper H, Koch H, Lang S (2005) Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl Microbiol Biotechnol 66:551–559
Wakamatsu Y, Zhao X, Jin C, Day N, Shibahara M, Nomura N, Nakahara T, Murata T, Yokoyama KK (2001) Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK-related signal cascade. Eur J Biochem 268:0374–0383
Zhao XX, Murata T, Ohno S, Day N, Song J, Nomura N, Nakahara T, Yokoyama KK (2001) Protein kinase C alpha plays a critical role in mannosylerythritol lipid-induced differentiation of melanoma B16 cells. J Biological Chemist 276:39903–39910
Acknowledgment
This work was supported by the Industrial Technology Research Grant Program in 06A17501c from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, T., Fukuoka, T., Imura, T. et al. Formation of the two novel glycolipid biosurfactants, mannosylribitol lipid and mannosylarabitol lipid, by Pseudozyma parantarctica JCM 11752T . Appl Microbiol Biotechnol 96, 931–938 (2012). https://doi.org/10.1007/s00253-012-4230-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4230-x