Abstract
Mannosylerythritol lipids (MELs) are one of the most promising biosurfactants known because of their multifunctionality and biocompatibility. A previously isolated yeast strain, Pseudozyma sp. KM-59, mainly produced a hydrophilic MEL, namely MEL-C (4-O-[4′-O-acetyl-2′,3′-di-O-alka(e)noyl-β-d-mannopyranosyl]-d-erythritol). In this study, we taxonomically characterize the strain in detail and investigate the culture conditions. The genetic, morphological, and physiological characteristics of the strain coincided well with those of Pseudozyma hubeiensis. On batch culture for 4 days under optimal conditions, the yield of all MELs was 21.8 g/l; MEL-C comprised approximately 65% of the all MELs. Consequently, on fed-batch culture for 16 days, the yield reached 76.3 g/l; the volumetric productivity was approximately 4.8 g l−1 day−1. We further examined the surface-active and self-assembling properties of the hydrophilic MELs produced by the yeast strain. They showed higher emulsifying activities against soybean oil and a mixture of hydrocarbons (2-methylnaphtarene and hexadecane, 1:1) than the synthetic surfactants tested. On water penetration scans, they efficiently formed lyotropic liquid crystalline phases such as myelines and lamella (\( {\text{L}}_{\alpha } \)) in a broad range of their concentrations, indicating higher hydrophilicity than conventional MELs. More interestingly, there was little difference in the liquid crystal formation between the crude product and purified MEL-C. The present glycolipids with high hydrophilicity are thus very likely to have practical potential without further purification and to expand the application of MELs especially their use in washing detergents and oil-in-water-type emulsifiers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mannosylerythritol lipids (MELs) are abundantly produced at more than 100 g/l from vegetable oils by yeast strains belonging to the genus Pseudozyma and are one of the most promising biosurfactants known (Lang 2001; Kitamoto et al. 2002; Morita et al. 2006, 2007). There are three types of MELs, namely MEL-A, MEL-B, and MEL-C (Fig. 1). Pseudozyma antarctica (Kitamoto et al. 1990), Pseudozyma aphidis (Rau et al. 2005a), and Pseudozyma rugulosa (Morita et al. 2006) are high-level producers and secrete mainly MEL-A, the most hydrophobic one, together with MEL-B and -C as minor components.
MEL-A exhibits not only excellent surface- and interfacial-tension-lowering activities (Kitamoto et al. 1993) but also distinctive self-assembling properties generating different lyotropic liquid crystalline structures such as sponge (L3), bicontinuous (V2), and lamella (\( {\text{L}}_{\alpha } \)) phases in a broad range of glycolipid concentrations (Imura et al. 2004, 2006, 2007b). Moreover, MEL-A shows versatile biochemical actions including differentiation induction with respect to human leukemia (Isoda et al. 1997), rat pheochromocytoma (Wakamatsu et al. 2001), and mouse melanoma cells (Zhao et al. 2001), as well as high affinity binding towards different immunoglobulins (Imura et al. 2007a, Ito et al. 2007) and lectins (Konishi et al. 2007a). However, MEL-A, which has two moles of fatty acids and two acetyl groups on the mannose moiety, possesses a significant drawback in handling as an aqueous solution due to its low water solubility and hydrophilicity (Imura et al. 2006): This has partially limited practical application of these promising biosurfactants.
We recently reported that the newly isolated stain of Pseudozyma sp. KM-59 produced MELs including MEL-C as the major component at a yield of 23 g/l for 10 days cultivation. Interestingly, of the yeast product, MEL-C comprised more than 60% of all the MELs (Konishi et al. 2007b). To the best of our knowledge, there is no report on dominant production of MEL-C, only the exception of our previous study. Based on our series of studies on MELs, MEL-C is very likely to show a higher water solubility and hydrophilicity than MEL-A and MEL-B and to be suitable for use in oil-in-water-type emulsifiers, dispersants, and washing detergents. We thus focused our attention on the efficient production of MEL-C using the strain KM-59.
In this study, we taxonomically classified the strain KM-59 in detail and then developed the culture conditions leading to the maximal production of MEL-C using fed-batch cultivation. Furthermore, we investigated the surface-active and self-assembling properties of the yeast products so as to facilitate its use in a broad range of applications.
Materials and methods
Microorganism
Pseudozyma sp. KM-59 used in this study was isolated as a MEL producer using soybean oil as the sole carbon source (Konishi et al. 2007b). Pseudozyma hubeiensis CBS10077T, the type strain of the species, was kindly supplied by the Centraalbureau voor Schimmelcultures, the Netherlands. Stock cultures were cultivated for 3 days by YM medium containing 10 g/l glucose, 5 g/l peptone, 3 g/l malt extract, and 3 g/l yeast extract. They were stocked at −80°C before use.
Molecular phylogenetic analysis
Culture broth of the strain KM-59 was prepared by cultivating with YM medium at 25°C. DNA extraction was performed using DNeasy plant mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. puReTaq Ready-To-Go polymerase chain reaction (PCR) beads (Amersham Biosciences, NJ, USA) were used for PCR amplification using initial at 95°C for 7 min, 35 cycles with denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min 30 s, and final at 72°C for 7 min. The sequences of D1/D2 region of 26S ribosomal RNA (rRNA) gene were defined using primers NL1, NL2, NL3, and NL4 (O’Donnell 1993). Sequence reaction were performed employing the ABI PRISM™ BigDye™ Terminator, v3.1 Kit (Applied Biosystems, CA, USA) as directed by the manufacturer. The PCR products were sequenced using the ABI PRISM™ 3100 genetic analyzer system (Applied Biosystems).
The KM-59 sequence was compared with those of closely related species obtained from the DNA Data Bank of Japan (http://ddbj.nig.ac.jp) via the BLAST search program and aligned using Clustal W software (Thompson et al. 1994). The phylogenetic tree was visualized by TreeView software (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
Morphological, physiological, and biochemical characteristics
The morphological and physiological characteristics were examined using the methods described by Barnett et al. (2000) and Boekhout and Nakase (1998).
Culture conditions for MEL production
To examine MEL production, seed cultures were prepared by incubating a freeze stock (0.5 ml) in a growth medium [40 g/l glucose, 3.0 g/l NaNO3, 0.3 g/l MgSO4, 0.3 g/l KH2PO4, and 2.0 g/l yeast extract (pH 6.0)] at 28°C for 2 days.
Seed cultures (1.5 ml) were transferred to Erlenmeyer flasks containing 30 ml of an experimental medium [40 g/l soybean oil, 3 g/l NaNO3, 0.3 g/l MgSO4, 0.3 g/l KH2PO4, and 2.0 g/l yeast extract (pH 6.0)] and then incubated at 28°C at 250 rpm using a shaker G·BR-300 (Taitec, Saitama, Japan) unless otherwise indicated.
In fed-batch culture, the following different feeding media were employed: (a) medium [40 g/l soybean oil], (b) [40 g/l soybean oil and 2.0 g/l yeast extract], and (c) [40 g/l soybean oil, 20 g/l glucose and 2.0 g/l yeast extract]. Each feeding medium was directly added to the above flasks every 4 days; this procedure was repeated two or three times.
Isolation and purification of MELs
After cultivation, the culture broth including MELs was extracted with an equal volume of ethyl acetate. The organic layer was separated and evaporated. The concentrate was dissolved with chloroform and then purified by silica-gel chromatography using chloroform–acetone as reported previously (Kitamoto et al. 2000). Purified MEL-A, -B, and -C, which were prepared from soybean oil by P. antarctica T-34 (Kitamoto et al. 1990), were used in the following experiments as a standard.
Thin-layer chromatography
The above ethyl acetate extracts were analyzed by thin-layer chromatography (TLC) on silica plates (Silica gel 60F; Merck) with a solvent system consisting of chloroform-methanol-7 N ammonium hydroxide (65:15:2, v/v). The compounds on the plates were located by charring at 110°C for 5 min after spraying the anthrone reagent as previously reported (Konishi et al. 2007b).
High-performance liquid chromatography
To quantify the produced MELs, high-performance liquid chromatography (HPLC) analysis was carried out with a HPLC system (SSPC; Tosoh, Tokyo, Japan) equipped a silica gel column (Inertsil SIL-100A 5 μm, 4.6 × 250 mm; GL science, Japan) with a low temperature evaporative light scattering detector (Shimadzu, Kyoto, Japan) using a gradient solvent program consisting of various proportions of chloroform and methanol (from 100:0 to 0:100) at a flow rate of 1 ml/min. The quantification of each MEL was performed based on the standard curves of purified MEL derivatives. All measurements reported here are the means calculated from at least three time independent experiments.
Determination of surface tension
The surface tension of the purified MEL-C was determined by the pendant drop method at 25°C, which was performed using the apparatus consisting of an automatic Whilhelmy-type automatic tensiometer (CBVP-A3, Kyowa Interface Science, Japan).
Estimation of emulsifying activity
The emulsifying activity of the purified MEL-C was determined using a modified colorimetric method as previously reported (Kitamoto et al. 2001): 100 μl of hydrophobic liquid phase was added to 5 ml of distilled water containing the purified surfactants (250 μg) in a test tube. The tubes were then vortexed thoroughly for exactly 3 min and allowed to stand at room temperature. After 3 h, the lower 1 ml was transferred to a cuvette, and the turbidity was measured at 620 nm. The solution containing no surfactant was used as a standard. Soybean oil and a mixture of n-hexadecane/2-methylnaphthalene (1:1, v/v) were used as the hydrophobic liquid phase. Polyoxyethylene (20) sorbitan monooleate (Tween80), lauryl benzene sulfonate (LAS), and sodium dodecyl sulfonate (SDS) were also used as reference. All measurements reported here are the means calculated from at least three time independent experiments.
Water penetration scan technique
To examine the lyotropic liquid crystalline phase behavior of MELs, the water penetration scan technique was used as reported previously (Imura et al. 2005). A polarized optical microscope (ECLIPSE E-600, Nikon, Japan) with crossed-polarizing filters equipped with a charge-coupled-device camera (DS-SM, Nikon) was used for the scan. Birefringent textures from the optical microscopy allowed the assignment of the particular lyotropic phase types to the samples. The purified MEL-C and crude MEL products were used as samples. The crude MELs were obtained by extraction with ethyl acetate from the culture broth, which was prepared using the experimental medium at 28°C for 4 days.
Results
Identification of the strain KM-59 producing MEL-C as the major component
Molecular phylogenetic analysis
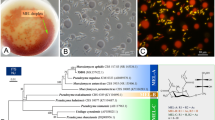
Figure 2 shows the molecular phylogenetic tree constructed using the D1/D2 region of 28S rRNA gene of the strain KM-59 and related species. The analysis showed that the sequence of KM-59 was the same as that of P. hubeiensis (Wang et al. 2006), with the nucleic acid identity of 100% (605/605 bp). The sequence, however, showed a little lower similarity of 96.7 and 96.5% (585/605 bp) to that of P. antarctica as a MEL-A producer (Kitamoto et al. 2001) and P. tsukubaensis as a MEL-B producer (Morita et al. 2007), respectively. The obtained result corresponded well with our previous results on the phylogenetic analysis using the internal transcribed spacer (ITS) region of the gene (Konishi et al. 2007b).
Morphological and physiological characteristics
Figure 3 shows the optical micrograph of the strain KM-59 grown on YM agar at 25°C for 7 days. The cells were elongate or columnar, (5–10) × (1–2.5) μm, and grew normally with polar budding. Pseudomycelium was developed. Within 30 days, no sexual reproduction was observed. The morphological and physiological characteristics (e.g., assimilation of carbon and nitrogen compounds and carbohydrate fermentation) of the strain KM-59 corresponded to those of P. hubeiensis CBS10077T, the type strain of the species (Wang et al. 2006; Table 1).
The strain KM-59 assimilated galactose, melibiose, l-rhammnose, erythritol, d-mannitol, d-glucitol, inositol, ethylamine, l-lysine, and cadaverine and showed a growth on vitamin-free medium and no starch formation. Except for the assimilation of lactose and ethanol, other physiological properties of the strains KM-59 coincided well with those observed for P. hubeiensis. From these results, the present strain KM-59 was identified as P. hubeiensis.
MEL production by the strains of P. hubeiensis
We then compared the strain KM-59 with the type strain on MEL productivity, considering the above results on the taxonomy. MEL production was undertaken using the experimental medium at 28°C for 4 days. Figure 4a shows the TLC patterns for the ethyl acetate extracts obtained with the strain KM-59 and the strain CBS 10077. The type strain was found to produce a mixture of glycolipid corresponding to MEL-A and MEL-C. The major product (lower spot, approximately 70%) was identified as MEL-C after the purification and structural characterization (data not shown). Although there was little difference in the cell growth between the two strains, strain KM-59 produced more than 20 g/l of MELs, while the type strain produced only 0.2 g/l (Fig. 4b). In the case of strain KM-59, no residual soybean oils were detected on both TLC and HPLC.
These results suggested that P. hubeiensis possesses a unique biosynthetic manner intensively generating MEL-C, different from conventional MEL producers such as P. antarctica, P. rugulosa, and P. aphidis. Therefore, P. hubeiensis KM-59 was exclusively used in the following experiments, aiming at developing the efficient production of MELs containing MEL-C as the major component.
Factors affecting MEL production by P. hubeiensis KM-59
-
(1)
The effect of yeast extract
Yeast extract is widely used for supplying minor vitamin and mineral components in fermentation processes. The effect of yeast extract on MEL-C production by the strain KM-59 was thus examined using the experimental medium containing different concentrations of yeast extract at 28°C for 4 days (Fig. 5). Although the concentrations of MELs increased with an increase in the concentration of yeast extract up to 2.0 g/l, further increases in the concentration caused a significant decrease of MEL production. Consequently, the highest yield over 20 g/l was obtained with 2.0 g/l of yeast extract. The experimental medium containing 2 g/l of yeast extract was, thus, exclusively used in the following experiments.
-
(2)
The effect of nitrogen sources
The type of nitrogen source and its concentration is also one of the most important factors in yeast fermentation processes. The effect of nitrogen sources on MEL production by the strain KM-59 was, thus, examined using the above experimental medium containing 3 g/l of different nitrogen sources at 28°C for 4 days. The strain showed a good growth with all the nitrogen sources tested. Interestingly, both NaNO3 and KNO3 gave high yields more than 20 g/l, whereas other nitrogen sources gave no production. The observed effects of nitrogen sources were very similar to those obtained with MEL-A producers such as P. antarctica (Kitamoto et al. 1990), P. aphidis (Rau et al. 2005a), and P. rugulosa (Morita et al. 2006).
-
(3)
The effect of carbon sources
Due to the difference in the chain length of the fatty acids, the types of vegetable oils often drastically affect MEL production by Pseudozyma yeasts (Kitamoto et al. 2001; Morita et al. 2006). Effects of carbon sources on MEL production were also examined with the experimental medium at 28°C for 4 days (Fig. 6). Except for coconut oil, all the vegetable oils tested gave MEL production yields higher than 15 g/l. There was no significant difference in the MEL productivity between olive, corn, safflower, and soybean oils. Soybean oil was, thus, employed as the main carbon source in the following experiments, considering that the oil is most intensively used for MEL production (Kitamoto et al. 1990; Morita et al. 2006, 2007; Rau et al. 2005a, b) and costs less than other vegetable oils.
-
(4)
Effect of hydrophilic precursors
Mannose and erythritol are the invariant structural elements of MEL and have to be synthesized via gluconeogenesis from vegetable oils by the yeast. The effect of supplementation of different precursors on MEL production was further examined using the experimental medium at 28°C for 4 days (Fig. 7). The amount of MELs increased with an increase in the concentration of glucose up to 60 g/l. Further increases in the concentration caused little effect on MEL production. Consequently, MELs reached more than 27 g/l. On the other hand, the addition of mannose gave no significant effect on MEL production, while erythritol provided a slight increase of MEL only when added at 20 g/l.
MEL production by P. hubeiensis KM-59 using fed-batch culture
Figure 8 illustrates the results on MEL production in the batch and fed-batch cultures. In the batch culture with the experimental medium (Fig. 8, Batch), the MEL yield reached 21.8 g/l (0.55 g/g on a weight basis to soybean oil supplied) after 4 days where the content of MEL-C remained approximately 65% of all the MELs. However, the amount of MELs significantly decreased below 10 g/l after 4 days, indicating that most of the carbon source supplied will be consumed within 4 days.
Fed-batch culture is one of the most effective methods leading to efficient production of biosurfactants (Kitamoto et al. 2001; Morita et al. 2006; Rau et al. 2005b), so MEL production using different feeding media was carried out in fed-batch culture. As expected, feeding of soybean oil into the batch culture clearly increased the MEL production [Fig. 8, fed-(a)]. However, a slight decrease of the production was observed after 8 days. Feeding of soybean oil and yeast extract enhanced the cell growth and production to give a yield of 37.4 g/l [Fig. 8, fed-(b)], but provided no significant increase of MELs after 12 days.
These results imply that supplements other than soybean oil and yeast extract are essential to facilitate MEL biosynthesis from the carbon source. The effect of feeding of glucose in addition to soybean oil and yeast extract was thus investigated, taking the distinct effect of glucose into consideration [Fig. 8, fed-(c)]. Interestingly, feeding of these three components to the batch culture maintained efficient production of MELs for 16 days. The total amount of MELs reached 74.3 g/l; the volumetric productivity was 4.6 g l−1 day−1.
Surface-active properties of MEL-C produced by P. hubeiensis KM-59
MEL-C has only one acetyl group in the mannose moiety (Fig. 1) and was, thus, expected to show different surface activities compared to MEL-A, the most intensively studied MEL. We therefore determined the surface tension of the purified MEL-C by the pendant drop method. The used MEL-C showed a single peak on the above HPLC analysis, and the purity was judged to be more than 99%. Figure 9 shows the surface (air–water) tension vs concentration plot of the purified MEL-C in distilled water. The estimated critical micelle concentration (CMC) and surface tension at CMC (γCMC) were 6.0 × 10−6 M and 25.1 mN/m, respectively. On the other hand, those of MEL-A produced by P. antarctica T-34 were 2.7 × 10−6 M and 28.4 mN/m, while those of MEL-B were 4.5 × 10−6 M and 28.2 mN/m, respectively (Kitamoto et al. 1993). As expected, the present MEL-C showed a higher CMC and hydrophilicity compared to conventional MELs, retaining an excellent surface-tension-lowering activity.
We also tentatively examined the emulsifying activity of the purified MEL-C. Here, the activity of the MEL-C was comparatively evaluated using MEL-A produced by P. antarctica T-34 and synthetic surfactants. With respect to soybean oil and a mixture of hydrocarbon (2-methylnaphtarene/n-hexadecane), the present MEL-C showed nearly the same activities as those of MEL-A as well as higher activities than those of LAS, Tween80, and SDS (Fig. 10). Consequently, the present MEL-C showed not only comparable surface activity to MEL-A, but also higher hydrophilicity than the di-acetylated MEL.
Formation of lyotropic liquid crystals from MELs produced by P. hubeiensis KM-59
We further tentatively investigated the self-assemble properties of the present MELs in aqueous solutions. Here, we examined the formation of lyotropic liquid crystalline phases from the purified MEL-C and crude MELs by the water penetration scan as reported previously (Imura et al. 2006). The crude MELs were prepared as above and consisted of MEL-A (22%), MEL-B (13%), and MEL-C (65%); they contained no detectable amount of residual soybean oils or free fatty acids.
Figure 10a and b shows the water penetration scans of the purified MEL-C and crude MELs, respectively, viewed with (right, POL) and without (left, DIC) crossed-polarizing filters. In both samples, the photographs clearly indicated four different regions that should represent water (W), myelines, the lamellar phase (\( {\text{L}}_{\alpha } \)), and the neat surfactant phase (S). Based on our previous study, MEL-B that has much higher hydrophilicity than MEL-A forms the lamellar phase (\( {\text{L}}_{\alpha } \)) and myelines, whereas MEL-A forms the sponge phase (L3; Imura et al. 2006).
As expected, both the purified MEL-C and crude MELs showed an immediate formation of the lamellar and myeline structures, and the two crystalline phases spread over a broad range of the concentrations. Interestingly, there was little difference in the liquid crystal formation between the two samples. Moreover, on both samples, the myeline formation took place much faster than the case of MEL-B (data not shown). From these results, the crude MELs produced by P. hubeiensis KM-59 has much higher hydrophilicity than conventional MELs due to the high content of MEL-C and would show practical properties without further purification (Fig. 11).
Discussion
The strain KM-59 has been previously isolated as a MEL-C producer and was characterized as the genus Pseudozyma based on the sequence of the ITS1 and ITS2 regions of 5.8S rRNA gene (Konishi et al. 2007b). In this study, we undertook the identification of the yeast by morphological and physiological characterization as well as phylogenetic analysis using the D1/D2 region of the 28S rRNA gene.
According to the present taxonomical studies, the strain KM-59 was clearly identified as P. hubeiensis, which has been recently isolated from plant leaves and identified as a new species of the genus (Wang et al. 2006). The strain KM-59 showed complete similarity to P. hubeiensis on the phylogenetic tree, but a lower similarity to P. antarctica as a MEL-A producer, or to P. tsukubaensis as a MEL-B producer. These results revealed that the biosynthetic pathway of MEL significantly varies among the genus Pseudozyma, especially on the level of acetyltransferease, which generates the structural difference between the three types of MELs.
Interestingly, the strain KM-59 showed remarkably higher productivity of MEL compared to P. hubeiensis CBS10077, the type of the species, and was slightly different on the carbon source assimilation from the type stain. The strain KM-59 is, thus, very likely to be a novel strain of P. hubeiensis. Nevertheless, to the best of our knowledge, this is the first report on the efficient production of MELs by P. hubeiensis.
In the present study, with the newly identified strain KM-59, we also designed the experiments leading to the maximum production of MELs including MEL-C as the major component, aiming at facilitating a broad range of applications of the potential biosurfactants. According to the experiments, yeast extract was a crucial factor for MEL production. Indeed, the feeding of yeast extract in fed-batch culture significantly enhanced the production of MELs. The requirement of yeast extract by the strain KM-59, however, seemed a little higher compared to other MEL producers such as P. antarctica T-34 (Kitamoto et al. 1990), P. rugulosa NBRC10877 (Morita et al. 2006), and P. aphidis DSM14930 (Rau et al. 2005a). From the practical point of view, the replacement of yeast extract with other inexpensive organic supplements remains to be attained, considering that the strain is able to growth on a vitamin-free medium (Table 1).
Nitrates such as NaNO3 and KNO3 gave a good production of MELs by the strain KM-59. The observed effects of nitrogen sources on MEL production were nearly the same to those obtained on MEL-A producers (Rau et al. 2005a; Morita et al. 2006). The strain KM-59 efficiently produces MELs from different vegetable oils including palm, olive, corn, safflower, and rapeseed oil as well as soybean oil (Fig. 7). Only coconut oil gave a low yield of MELs, probably due to the high contents of shorter chain acids ranging from C8 to C12. Kitamoto et al (2001) reported that the chain length of the carbon substrate gives a significant effect on MEL production by P. antarctica, and that fatty acids shorter than C12 gave the low productivity.
Supplementation of glucose also significantly affected MEL production by the strain KM-59. Glucose enhanced MEL production, but neither mannose nor erythritol gave a positive effect. On MEL-A producers such as P. rugulosa NBRC10877 (Morita et al. 2006) and P. aphidis DSM70725 (Rau et al. 2005b), both mannose and erythritol considerably enhance MEL production from soybean oil, indicating that the biosynthesis of mannosylerythritol (ME) from glucose limits the rate of the production. The strain KM-59 is, thus, likely to have higher enzymatic activities on the formation of ME from glucose compared to known MEL producers, and may be a potential ME producer.
Accordingly, fed-batch culture with the feeding of soybean oil, glucose, and yeast extract provided the strain KM-59 with notably high yield of MELs (74.3 g/l) for 16 days. More significantly, the obtained volumetric productivity of MELs was 4.6 g l−1 day−1; this is almost comparable to that of high-level MEL producers such as P. antarctica T-34 (Kitamoto et al. 2001) and P. rugulosa (Morita et al. 2006). The present strain would have a practical potential for an efficient production of the most hydrophilic MEL among the known MEL derivatives.
Characterizing the interfacial properties of biosurfactants is crucial for a broad range of applications. We, thus, further investigated the surface-active and self-assembling properties of MEL-C produced by the strain KM-59. The purified MEL-C exhibited a higher CMC and lower γCMC compared to MEL-A and MEL-B produced by P. antarctica T-34 (Kitamoto et al 1993, Imura et al. 2006; Fig. 9). It also showed much higher emulsifying activities toward soybean oil and hydrocarbons than the representative synthesis surfactants.
Water penetration scan technique tentatively demonstrated that MEL-C immediately self-assembles to form lyotropic crystalline phases of the lamella (\( {\text{L}}_{\alpha } \)) and myelines. Interestingly, the crystalline phase pattern of the purified MEL-C was quite different from that of MEL-A, which predominantly forms sponge phase (L3). In addition, the purified MEL-C provided the lamella and myeline structures much faster than the case of MEL-B. These results clearly indicated that the present MEL-C has much superior “hydrophilicity” compared to conventional MEL-A and MEL-B. Indeed, the MEL-C showed higher hydrophilicity than MEL-B on TLC. This is probably to due the presence of a primary hydroxyl group at C′-6 on the mannose moiety, different from two other MELs. The formation of \( {\text{L}}_{\alpha } \) phase and/or myelines in aqueous solutions provides surfactants with great advantages for the use in oil-in-water-type emulsifiers and washing detergents (Nakashima et al. 2000).
More interestingly, the crude MELs showed nearly the same crystalline phase pattern as that of the purified MEL-C. It seems reasonable that there is little difference in the self-assembly between them, considering the high content of MEL-C (more than 65%) in the crude sample.
In general, the downstream processes such as recovery and purification comprise at least 60 to 70% of the total cost of microbial production systems (Zeikus et al. 1999). Especially, purification processes using chromatography often cause a significant loss of products. In the present study, the final recovery yield of MEL-C resulted in low levels at around 50% after the purification with column chromatography (data not shown). Therefore, the direct use of the crude MELs including MEL-C without purification should enable us to simplify the downstream and to reduce the production cost.
From these results, the newly identified strain P. hubeiensis KM-59 was found to efficiently produce MELs with different interfacial and self-assembling properties from those of known MELs and would, thus, open a new avenue for a wide range of applications of the yeast biosurfactants.
References
Barnett JA, Payne RW, Yarrow D (2000) Yeasts: characteristics and identification, 3rd edn. Cambridge University Press, Cambridge
Boekhout T, Nakase T (1998) Pseudozyma Bandoni emend. Boekout and a comparison with the yeast state of Ustilago maydis (de candolle) corda. In: Kurtzman CP, Fell JW (eds) The yeasts a taxonomic study. Elsevier, Amsterdam, Netherlands, pp 790–797
Imura T, Yanagishita H, Kitamoto D (2004) Coacervate formation from natural glycolipid: one acetyl group on the headgroup triggers coacervate-to-vesicle transition. J Am Chem Soc 126:10804–10805
Imura T, Yanagishita H, Ohira J, Sakai H, Abe M, Kitamoto D (2005) Thermodynamically stable vesicle formation from glycolipid biosurfactant sponge phase. Colloids Surf B Biosurfaces 43:114–121
Imura T, Ohta N, Inoue K, Yagi H, Negishi H, Yanagishita H, Kitamoto D (2006) Naturally engineered glycolipid biosurfactants leading to distinctive self-assembled structures. Chem Eur J 12:2434–2440
Imura T, Ito S, Azumi R, Yanagishita H, Sakai H, Abe M, Kitamoto D (2007a) Monolayers assembled from a glycolipid biosurfactant from Pseudozyma (Candida) antarctica serve as a high-affinity ligand system for immunoglobulin G and M. Biotechnol Lett 29:865–870
Imura T, Hikosaka Y, Worakitkanchanakul W, Sakai H, Abe M, Konishi M, Minamikawa H, Kitamoto D (2007b) Aqueous-phase behavior of natural glycolipid biosurfactants mannosylerythritol lipid A: sponge, cubic, and lamella phases. Langmuir 23:1659–1663
Isoda H, Shinmoto H, Kitamoto D, Matsumura M, Nakahara T (1997) Differentiation of human promyelocytic leukemia cell line HL60 by microbial extracellular glycolipids. Lipids 32:263–271
Ito S, Imura T, Fukuoka T, Morita T, Sakai H, Abe M, Kitamoto D (2007) Kinetic studies on the interactions between glycolipid biosurfactants assembled monolayers and various classes of immunoglobulins using surface plasmon resonance. Colloid Surf B 58:165–171
Kitamoto D, Haneishi K, Nakahara T, Tabuchi T (1990) Production of mannosylerythritol lipids by Candida antarctica from vegetable oil. Agric Biol Chem 54:37–40
Kitamoto D, Yanagishita H, Shinbo T, Nakane T, Kamisawa C, Nakahara T (1993) Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J Biotechnol 29:91–96
Kitamoto D, Ghosh S, Ourisson G, Nakatani Y (2000) Formation of giant vesicles from diacylmannosylerythritols, and their binding to concanavalin A. Chem Commun 10:861–862
Kitamoto D, Ikegami T, Suzuki GT, Sasaki A, Takeyama Y, Idemoto Y, Koura N, Yanagishita H (2001) Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma (Candida antarctica). Biotechnol Lett 23:1709–1714
Kitamoto D, Isoda H, Nakahara T (2002) Functions and potential applications of glycolipid biosurfactants: from energy-saving materials to gene delivery carriers. J Biosci Bioeng 94:187–201
Konishi M, Imura T, Morita T, Fukuoka T, Kitamoto D (2007a) A yeast glycolipid biosurfactant, mannosyl-erythritol lipid, shows high binding affinity towards lectins on a self-assembled monolayer system. Biotechnol Lett 29:473–480
Konishi M, Morita T, Fukuoka T, Imura T, Kakugawa, K, Kitamoto D (2007b) Production of different types of mannosylerythritol lipids as biosurfactants by the newly isolated yeast strains belonging to the genus Pseudozyma. Appl Microbiol Biotechnol 75:521–531
Lang S (2001) Biological amphiphiles (microbial biosurfactants). Curr Opin Colloids Interface Sci 7:11–20
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2006) Discovery of Pseudozyma rugulosa NBRC 10877 as a novel producer of glycolipid biosurfactants, mannosylerythritol lipids, based on rDNA sequence. Appl Microbiol Biotechnol 73:305–313
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto H, Kitamoto D (2007) Characterization of the genus Pseudozyma by the formation of glycolipid biosurfactants, mannosylerythritol lipids. FEMS Yeast Res 7:286–292
Nakashima T, Shimizu M, Kukizaki M (2000) Particle control of emulsion by membrane emulsification and its application. Adv Drug Deliv Rev 45:47–56
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK, pp 225–233
Rau U, Nguyen LA, Schulz S, Wray V, Nimtz M, Roper H, Koch H, Lang S (2005a) Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl Microbiol Biotechnol 66:551–559
Rau U, Nguyen LA, Roeper H, Koch H, Lang S (2005b) Fed-batch bioreactor production of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl Microbiol Biotechnol 68:607–613
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting. Position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wakamatsu Y, Zhao X, Jin C, Day N, Shibahara M, Nomura N, Nakahara T, Murata T, Yokoyama KK (2001) Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK-related signal cascade. Eur J Biochem 268:374–383
Wang Q-M, Jia J-H, Bai F-Y (2006) Pseudozyma hubeiensis sp. and Pseudozyma shanxiensis sp. nov. novel ustilaginomycetous anamorphic yeast species from plant leaves. Int J Syst Evol Microbiol 56:289–293
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552
Zhao XX, Murata T, Ohno S, Day N, Song J, Nomura N, Nakahara T, Yokoyama KK (2001) Protein kinase C alpha plays a critical role in mannosylerythritol lipid-induced differentiation of melanoma B16 cells. J Biol Chem 276:39903–39910
Acknowledgment
The authors thank Ms. Sugimura, a fellow of the Japan Industrial Technology Association, for her technical assistance. This work was supported by the Industrial Technology Research Grant Program in 06A17501c from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konishi, M., Morita, T., Fukuoka, T. et al. Efficient production of mannosylerythritol lipids with high hydrophilicity by Pseudozyma hubeiensis KM-59. Appl Microbiol Biotechnol 78, 37–46 (2008). https://doi.org/10.1007/s00253-007-1292-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1292-2