Abstract
To develop a structural homolog of mannosylerythritol lipids (MELs), Pseudozyma tsukubaensis JCM16987 (known to be a specific producer of the diastereomer type of mono-acetylated MEL (MEL-B)) was cultivated in medium containing 4 % (w/v) olive oil as the primary carbon source and 4 % l-arabitol as the supplemental sugar alcohol. Based on thin-layer chromatography (TLC), the glycolipid extract showed two major spots corresponding to MEL-B and an unknown glycolipid (GL1). Based on high-performance liquid chromatography after acid hydrolysis, GL1 from the l-arabitol culture showed two primary peaks identical to mannose and arabitol using the sugar analysis column, and one peak identical to l-arabitol was detected using the chiral resolution column. Based on NMR analysis, GL1 was identified as mono-acetylated mannosyl-l-arabitol lipid (MLAL-B) consisting of mannose, with l-arabitol as the sugar moiety. The observed critical micelle concentration (CMC) and surface tension at the CMC (γCMC) of MLAL-B were 1.2 × 10−5 M and 32.8 mN/m, which were significantly higher than MEL-B (CMC = 3.1 × 10−6 M and γcmc = 26.1 mN/m). Furthermore, based on a water-penetration scan, MLAL-B efficiently formed lamellar phase (Lα) and myelins at a broad concentration range. Thus, the present glycolipid showed higher hydrophilicity and/or water solubility and increased our understanding of environmentally advanced biosurfactants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
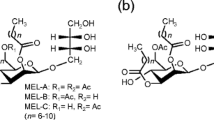
Biosurfactants are surface-active compounds produced by various microorganisms and have attracted considerable interest in recent years due to their unique properties (Lang 2002; Kitamoto et al. 2002, 2009). Mannosylerythritol lipids (MELs) are abundantly produced from vegetable oils by yeast strains of the genus Pseudozyma (Kitamoto et al. 2002; Morita et al. 2007, 2009a). Pseudozyma antarctica (Kitamoto et al. 1990), Pseudozyma aphidis (Rau et al. 2005), and Pseudozyma rugulosa (Morita et al. 2006) are high-level producers that secrete mainly MEL-A, together with MEL-B and MEL-C as minor components (Fig. 1a). The interfacial and self-assemble properties of the MELs depend on structural differences, i.e., the number of acetyl groups cause significant differences in critical micelle concentrations (CMCs) and immediate formation of liquid-crystalline phases (Kitamoto et al. 1993; Imura et al. 2006; Morita et al. 2008a, b). The hydrophilicity of mono-acetylated MELs (MEL-B and MEL-C) is higher than those of di-acetylated MELs (MEL-A).
We previously reported the production of various MEL homologs, such as tri-acylated MEL (Fukuoka et al. 2007a; Morita et al. 2008c) and mono-acylated MEL (Fukuoka et al. 2007b), as well as di-acylated MEL (MEL-A, MEL-B, and MEL-C) with various fatty acid lengths (Morita et al. 2009a). Pseudozyma parantarctica JCM 11752T was further shown to produce three di-acetylated type mannosyl-alditol lipids, e.g., mannosyl-d-mannitol lipid (MDML-A, Fig. 1b), mannosyl-ribitol lipid (MRL-A, Fig. 1c), and mannosyl-d-arabitol lipid (MDAL-A, Fig. 1d), in which the hydrophilic part was converted from erythritol (C4 sugar alcohol) to d-mannitol (C6), ribitol (C5), or d-arabitol (C5) when yeast cells were cultivated in medium containing an excess amount of each sugar alcohol (Morita et al. 2009b, 2012). The critical micelle concentration (CMC) and surface tension at the CMC (γCMC) of MDML-A, MRL-A, and MDAL-A were slightly lower than those of MEL-A. On the other hand, the liquid crystalline structures of MDML-A, MRL-A, and MDAL-A in water were similar to those of MEL-B, which showed more hydrophilicity and contained a mono-acetylated mannose moiety. Therefore, elongation of the sugar alcohol component should increase the hydrophilicity of these MEL homologs, despite maintaining their excellent surface-tension-lowering activity.
On the other hand, Pseudozyma tsukubaensis produces only a diastereomer type of MEL-B (Fig. 1e), 4-O-[6′-O-acetyl-2′,3′-di-O-alka(e)noyl-β-d-mannopyranosyl]-(2R,3S)-erythritol (Fukuoka et al. 2008, 2012). Interestingly, the sugar moiety of the diastereomer type of MEL-B, 4-O-β-d-mannopyranosyl-(2R,3S)-erythritol, is difficult to crystallize and shows good hygroscopic properties, whereas conventional MELs, 4-O-β-d-mannopyranosyl-(2S,3R)-erythritol, crystallizes easily (Fukuoka et al. 2008). In addition, the diastereomer type of MELs showed a higher CMC and hydrophilicity compared to the corresponding MELs (Fukuoka et al. 2012). Moreover, these diastereomers efficiently formed relatively large vesicles and the one-phase lamellar structure at a broader concentration range than the corresponding MELs due to maintaining more water between the polar layers (Fukuoka et al. 2012). These results indicate that differences in MEL carbohydrate configurations significantly affect interfacial properties, self-assembly, and hydration ability.
Based on these results, hydrophilicity of the glycolipids is affected by the characteristics of the hydrophilic domain, such as de-acetylated mannose, elongated sugar alcohol, and/or a different carbohydrate configuration. However, the diastereomer type of mono-acetylated (or non-acetylated) mannosyl-alditol lipids has not been identified, except for MELs.
In this report, we explored the formation of a novel structural homolog of MEL with different carbohydrate configurations from P. tsukubaensis by cultivation in medium containing an excess amount of optical isomers of sugar alcohols. Here, we investigated the supplementation of l-arabitol with vegetable oil using P. tsukubaensis and obtained a novel glycolipid, namely, mono-acetylated mannosyl-l-arabitol lipid (MLAL-B). We also describe its surface-active and self-assembling properties. This is the first report on the microbial formation of mannosyl-arabitol lipid possessing l-arabitol as the sugar component.
Materials and methods
Materials
All reagents and solvents were commercially available and used as received. Mannosyl-d-arabitol lipid (MDAL) was produced by Pseudozyma parantarctica JCM 11752T in our laboratory, as described previously (Morita et al. 2012).
Microorganism
P. tsukubaensis JCM 16987 was obtained from the RIKEN BioResource Center. Stock cultures were cultivated for 3 days at 25 °C on agar medium containing 4 % glucose, 0.3 % NaNO3, 0.03 % MgSO4, 0.03 % KH2PO4, and 0.1 % yeast extract. They were stored at 4 °C and renewed every 2 weeks.
Media preparation and culture conditions
Seed cultures were prepared by inoculating cells grown on slants into test tubes containing growth medium [4 % glucose, 0.3 % NaNO3, 0.03 % MgSO4, 0.03 % KH2PO4, 0.1 % yeast extract (pH 6.0)] at 25 °C on a reciprocal shaker (200 strokes/min) for 2 days. Seed cultures (0.1 ml) were transferred to a flask containing 20 ml of a basal medium [4 % (w/v) olive oil, 0.3 % NaNO3, 0.03 % MgSO4, 0.03 % KH2PO4, 0.1 % yeast extract (pH 6.0)] and 8 % d-arabitol or l-arabitol, and then incubated at 25 °C on a rotary shaker (250 rpm) for 7 days.
Glycolipid isolation
The produced glycolipids were extracted from culture medium with an equal amount of ethyl acetate. The extracts were analyzed using thin-layer chromatography (TLC) on silica plates (silica gel 60F; Wako, Osaka, Japan) with a solvent system consisting of chloroform/methanol/7 N ammonium hydroxide (65:15:2, v/v/v). The compounds on the plates were located by charring at 110 °C for 5 min after applying the anthrone reagent (Kitamoto et al. 1990). The purified MEL fraction including MEL-A, MEL-B, and MEL-C was used as a standard, as reported previously (Morita et al. 2006).
Glycolipid purification
The above ethyl acetate fractions were evaporated. The concentrated glycolipids were dissolved in chloroform and purified using silica gel (Wako-gel C-200) column chromatography with a gradient elution of chloroform/acetone (10:0 to 0:10, v/v) mixtures as solvent systems (Morita et al. 2006). The purified glycolipids were used in the following experiments.
Synthesis of MA by alkaline hydrolysis of MALs
NaOMe (8 mg, ca. 150 μmol) in dry MeOH (2 mL) was added to a mixture of mannosyl-arabitol lipids (MALs) (250 mg, ca. 0.35 mmol) in dry MeOH (2 mL). After stirring at room temperature for 1 h, cation exchange resin (DOWEX H+ form, >400 mg) was added and the mixture was stirred further. After stirring for 15 min, the resin was removed by filtration and the filtrate was evaporated. A small amount of water (1 mL) and ethyl acetate (10 mL) was added to this residue, after which fatty acids were extracted using ethyl acetate. The resulting aqueous layer containing the sugar moiety was concentrated to a colorless syrup (ca. 70 mg).
Structural determination of glycolipids and sugars
Structural determination of the partially purified glycolipids (MALs) and sugars (mannosyl-arabitol (MA)) dissolved in CDCl3 or D2O was performed using 1H, 13C nuclear magnetic resonance (NMR) and two-dimensional NMR analysis, such as 1H-1H correlation spectroscopy (COSY) or heteronuclear multiple quantum correlation (HMQC) using a Btruker AVANCE 400 (400 MHz). Acid degradation was performed by mixing the purified glycolipids (10 mg) with 1 ml of 5 % HCl-methanol reagent (Wako, Osaka, Japan) overnight at room temperature. After the reaction was quenched with water (1 ml), the methyl ester derivatives of the fatty acids were removed with n-hexane. After the water-soluble fraction was neutralized through the Amberlite column pretreated with NaOH, the fraction was applied to HPLC on a SUGAR SH1011 column (Shoko, Tokyo, Japan) with a differential refractive index detector (RI-8020) using 0.01 N H2SO4 as the solvent system. d-Arabitol and l-arabitol were separately detected using HPLC on a CHIRALPAK IF column (Daicel, Tokyo, Japan) with a differential refractive index detector (RI-8020) using acetonitrile as the solvent system.
The fatty acid profile was analyzed using gas chromatography-mass spectrometry (GC-MS) (Hewlett Packard 6890 and 5973 N) with a TC-WAX (GL-science, Tokyo, Japan) and temperature program from 90 °C (held for 3 min) to 240 °C at 5 °C/min.
The molecular weight of glycolipids was measured using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) (autoflex speed TOF/TOF, Bruker Daltonics Inc.) with 2,5-dihydroxybenzoic acid.
Determination of surface tension
The surface tension of the partially purified glycolipids was determined using the Whilhelmy method at 25 °C, which was performed using an apparatus consisting of an automatic Whilhelmy-type automatic tensionmeter (DY-500; Kyowa Interface Science, Saitama, Japan).
Water-penetration scan technique
To examine the lyotropic-liquid-crystalline phase behavior of the partially purified glycolipids, the water-penetration scan technique was used as reported previously (Imura et al. 2006). A polarized optical microscope (ECLIPSE E-600; Nikon, Tokyo, Japan) with crossed-polarizing filters equipped with a charge-coupled-device camera (DS-SM; Nikon) was used for the scan. Birefringent textures from the optical microscopy allowed for the assignment of particular lyotropic phase types to the samples.
Results
Production of glycolipids with l-arabitol by P. tsukubaensis
P. tsukubaensis JCM 16987 was recently isolated as an excellent producer of the diastereomer type of mono-acetylated MEL (MEL-B) (Morita et al. 2010). To obtain novel structural homologs of MEL-B, the yeast strain was cultivated in basal medium containing 8 % d-arabitol or l-arabitol for 7 days at 25 °C. The cultures were extracted with ethyl acetate, and the ethyl acetate fractions were spotted on a TLC plate. A mixture of purified MEL-A, MEL-B, and MEL-C was used as the standard, and the glycolipids were detected with anthrone reagent.
Interestingly, the fraction from the culture with l-arabitol showed two main spots corresponding to MEL-B and an unknown glycolipid (GL1), while the fraction from d-arabitol showed no spot. The control culture without sugar alcohol provided MEL-B as the main product (Fig. 2).
Formation of glycolipids by Pseudozyma tsukubaensis JCM 16987. Samples were extracted from culture medium with ethyl acetate, and the organic solvent fraction was spotted on a TLC plate. Spots were visualized with anthrone reagent. Control was an extract from the culture without supplementation of any sugar alcohols. MEL mannosylerythritol lipid, GL1 unknown glycolipid
Structural determination of glycolipids derived from culture using l-arabitol
To confirm the structure of GL1, glycolipids were partially purified from the ethyl acetate extracts (0.48 g of purified GL1 was obtained from 330 mL of culture medium, corresponding to a production yield of 1.45 g/L) and were analyzed using NMR. Based on 1H and 13C NMR analyses, a glycolipid with a higher R f value on TLC was identified as the diastereomer type of MEL-B, as described previously (Fukuoka et al. 2008). In the same manner, GL1 with the lower R f value on TLC showed a similar 1H NMR spectrum as MEL-B (data not shown). Particularly, the two resonances arising from H-5a and 5b at 3.96 and 3.98 ppm overlapped, which is a primary feature of the carbohydrate configuration similar to the diastereomer type MEL. On the other hand, the spectrum of 13C NMR was clearly different between GL1 and MEL-B (data not shown). There were ten peaks at between 60 and 75 ppm in the 13C NMR chart of GL1, whereas there are nine peaks at the same range in the chart of MEL-B. Five peaks derived from mannose (C2∼5) almost correspond to each other. Therefore, GL1 was presumed to have a C5 sugar alcohol as substitute for erythritol. From these results, GL1 was estimated to be a mannosyl-alditol lipid with C5 sugar alcohol having a carbohydrate configuration similar to the diastereomer of MEL-B (Table 1).
To identify the sugar composition of GL1, the acid-hydrolyzed fraction prepared from GL1 was subjected to HPLC analysis. As expected, the fraction showed the two peaks corresponding to mannose (9.48 min) and arabitol (12.08 min) (Fig. 3a), although d- and l-arabitol were detected at the same retention time with the sugar analysis column. As the control, purified MEL-B yielded three peaks corresponding to mannosyl-erythritol (8.75 min), mannose (9.49 min), and erythritol (10.90 min).
HPLC analysis of the sugar moiety of GL1. Glycolipids produced from olive oil and l-arabitol were degraded with acid solution, and the obtained water-soluble fraction was subjected to HPLC analysis using the sugar analysis column (a). The mixture of mannosylerythritol, d-mannose, meso-erythritol, ribitol, and l-arabitol was used as standard solution. Mannosylerythritol used as the standard was obtained by acid hydrolysis of MEL. The retention time (minutes) of each peak was provided in parentheses. The retention time of d-arabitol was identical to l-arabitol (data not shown). The water-soluble fraction of GL1 and its sugar component derived by alkaline hydrolysis were subjected to HPLC analysis using the chiral resolution column (b). The mixture of d-arabitol and l-arabitol was used as the standard. The retention time (minutes) of each peak was given in parentheses
Subsequently, to detect d- and l-arabitol (9.51 and 8.79 min, respectively) independently, we screened various columns and separation conditions, and found for the first time a chiral resolution column, CHIRALPAC IF, allowing for separation of peaks corresponding to d- and l-arabitol. The acid-hydrolyzed fraction was subjected to the HPLC system equipped with chiral resolution column, and a peak corresponding to l-arabitol (8.76 min) was observed (Fig. 3b). Furthermore, the sugar moiety obtained by alkaline hydrolysis of GL1 was analyzed using the same HPLC system after acid hydrolysis, and l-arabitol was detected (8.78 min). Consequently, GL1 consisted of d-mannose and l-arabitol as the sugar moiety in place of erythritol.
In addition, to clarify the difference in the carbohydrate configuration between GL1 and MDAL (Fig. 1d), we prepared MA using alkaline hydrolysis. Figure 4 shows the partial 1H NMR spectra of two types of MA from GL1 and MDAL, and the chemical shifts of these MAs are summarized in Table 2. Both peak patterns were similar. However, the peaks at H-5a and H-5b were separated and the chemical shifts of H-1′ were slightly different. These results were similar to the difference in the spectra of two diastereomers of MEL and mannosylerythritol (ME), as reported previously (Fukuoka et al. 2008). Although the absolute configuration has not been confirmed using X-ray diffraction, these results were most likely attributed to a difference in the configuration of d- and l-arabitol in these MALs.
As mentioned above, P. tsukubaensis is known to produce the MEL diastereomer containing the 4-O-β-d-mannopyranosyl-(2R,3S)-erythritol moiety, which has a different chirality than conventional MELs. Based on this fact and the above results, GL1 was predicted to be 5-O-(6′-O-acetyl-2′,3′-di-O-alka(e)noyl-β-d-mannopyranosyl)-l-arabitol (MLAL-B, Fig. 5). The fatty acids were mainly composed of C8, C12, and C14, as well as MEL-B (Table 3).
The molecular weights of the main components of GL1 were 664.4 (C8 and C12 acids) and 690.5 (C8 and C14), as determined from the main peaks (687.4 and 713.5 [M+Na]+) on MALDI-TOF/MS analysis. This result is consistent with the above structure.
Surface-active properties of MLAL-B (GL1)
Since MLAL-B consists of the C5 sugar alcohol as the hydrophilic moiety, it was expected to show different surface activities and interfacial properties compared to MEL-B consisting of erythritol as the C4 sugar alcohol. Thus, we evaluated the surface tension of the purified MLAL-B using the Whilhelmy method.
The estimated CMC and γCMC of MLAL-B were 1.2 × 10−5 M and 32.8 mN/m, respectively. On the other hand, those of MEL-B produced by P. tsukubaensis was reported to be 3.1 × 10−6 M (γCMC = 26.1 mN/m) (Fukuoka et al. 2012). These results indicated that MLAL-B is more hydrophilic than MEL-B and maintains excellent interfacial properties, while its surface-tension-lowering activity is lower than that of MEL-B.
Formation of lyotropic liquid crystals from MLAL-B (GL1)
We further explored the self-assembling properties of MLAL-B in aqueous solutions. Here, we examined the formation of lyotropic-liquid-crystalline phases from partially purified MLAL-B using the water-penetration technique. The water penetration scan of the MLAL-B viewed with crossed-polarizing filter clearly showed four different regions that should represent water (W), myelins, the lamellar phase (Lα), and the neat surfactant phase (S). The observed lamellar phase spreads over a wide concentration range. Based on our previous study, MEL-B (which has significantly higher hydrophilicity than MEL-A) efficiently forms the lamellar phase (Lα) and myelins, while MEL-A forms mainly the sponge phase (L3) (Imura et al. 2006). Similar to MEL-B, MLAL-B is also likely to show higher hydrophilicity compared to MEL-A.
Consequently, the present novel glycolipid biosurfactants produced from l-arabitol by P. tsukubaensis have excellent surface-active and self-assembling properties, reflecting the unique structure of the sugar moiety including chirality, chain length, and the degree of acetylation.
Discussion
Here, for the first time, we report the generation of a mono-acetylated mannosyl-l-arabitol lipid (MLAL-B) by P. tsukubaensis JCM 16987 known to be a specific product of the diastereomer type of MEL-B.
Recently, a gene cluster responsible for MEL biosynthesis has been reported for conventional MEL producers, P. antarctica, and Ustilago maydis (Morita et al. 2013, 2014; Hewald et al. 2006). The formation of mannosyl-erythritol, the sugar moiety of MELs, is catalyzed by the reaction of a mannosyl transferase encoded by the gene, emt1. Therefore, the substrate specificity and affinity of this transferase is important to determine the structure of its sugar moiety. Hence, we hypothesized that enzymatic properties of this transferase strongly contribute to structural differences in the sugar moiety.
Previously, three mannosyl-alditol lipids possessing d-mannose, d-arabitol, or ribitol were produced by P. parantarctica using each sugar alcohol as the supplemental source (Fig. 1b–d). However, l-arabitol and xylitol were not suitable as a substrate for converting erythritol into l-arabitol and xylitol, respectively (Morita et al. 2012). In this study, in P. tsukubaensis, l-arabitol was available as the substrate to swap erythritol with l-arabitol, and d-arabitol was not used as a substrate. Xylitol was also not a substrate in P. parantarctica and l-mannitol has not been tested due to its high cost. Based on the configuration of two asymmetric carbons of the sugar alcohol component attached to mannose in P. parantarctica, d-mannitol (4S,5R configuration) and d-arabitol (3S,4R) have the same diol configuration as 4-O-β-d-mannopyranosyl-(2S,3R)-erythritol in conventional MELs, but d-xylose and l-arabitol do not. On the other hand, in P. tsukubaensis, l-arabitol (3R,4S) has the same diol configuration as 4-O-β-d-mannopyranosyl-(2R,3S)-erythritol in the MEL diastereomers, while d-xylose and d-arabitol do not (Fig. 6). These results suggest that sugar alcohols bearing the similar alcohol configuration as erythritol are directly utilized by mannosyl transferase to form each sugar moiety. Further biochemical and genetic studies on P. tsukubaensis would uncover the molecular mechanisms, including the transferase.
On glycolipid surfactants, the structure of the sugar moiety has a critical effect not only on the hydrophilicity but also on the physiochemical properties including self-assembly (Imura et al. 2006). MEL-B consisting of mono-acetylated mannose shows higher hydrophilicity than MEL-A (consisting of di-acetylated mannose) and efficiently forms lamellar structures in water at a broad concentration range. On the other hand, MDML-A shows higher hydrophilicity compared with MEL-A and MEL-B, which consists of mono-acetylated mannose (Morita et al. 2008a, b; Konishi et al. 2008). In this study, MLAL-B showed higher CMC (1.2 × 10−5 M) and γCMC (32.8 mN/m) values than the diastereomer type of MEL-B (3.1 × 10−6 M and 26.1 mN/m, respectively). On the other hand, as reported previously, MDML-A and MDAL-A showed lower CMC and γCMC (2.6 × 10−6 M and 24.2 mN/m, and 1.5 × 10−6 M and 24.2 mN/m, respectively) values than conventional MEL-A (2.7 × 10−6 M and 28.4 mN/m, respectively). Therefore, elongation of the sugar moiety is likely to have a more significant impact on the diastereomer type of mannosyl-alditol lipids compared with conventional mannosyl-alditol lipids. Although detailed effects of the sugar moiety on interfacial and self-assembling properties remain unclear, the number and configuration of the hydroxyl group at the sugar alcohol moiety may play an important role in adsorption at the air-water interface.
In conclusion, we generated a novel MEL derivative, MLAL-B, using l-arabitol, and explored the substrate specificity of the mannosyl-transferase generating the sugar moiety of glycolipids. Furthermore, based on HPLC analysis, we separated peaks between d- and l-arabitol by screening various chiral resolution columns and separation conditions. Consequently, combined with structural analysis, MLAL-B was shown to possess l-arabitol as the hydrophilic component. MLAL-B showed excellent surface activities and unique self-assembling properties, besides increasing the hydrophilicity. Analysis of these glycolipids increases our understanding of the structure-function relationship of glycolipid biosurfactants, and may have a broad range of applications.
References
Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D (2007a) Characterization of new glycolipid biosurfactants, tri-acylated mannosylerythritol lipids, produced by Pseudozyma yeasts. Biotechnol Lett 29:1111–1118
Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D (2007b) Structural characterization and surface-active properties of a new glycolipid biosurfactant, mono-acylated mannosylerythritol lipid, produced from glucose by Pseudozyma antarctica. Appl Microbiol Biotechnol 76:801–810
Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D (2008) A basidiomycetous yeast, Pseudozyma tsukubaensis, efficiently produces a novel glycolipid biosurfactant. The identification of a new diastereomer of mannosylerythritol lipid-B. Carbohydr Res 343:555–560
Fukuoka T, Yanagihara T, Imura T, Morita T, Sakai H, Abe M, Kitamoto D (2012) The diastereomers of mannosylerythritol lipids have different interfacial properties and aqueous phase behavior, reflecting the erythritol configuration. Carbohydr Res 351:81–86
Hewald S, Linne U, Scherer M, Marahiel MA, Kamper J, Bölker M (2006) Identification of a gene cluster for biosynthesis of mannosylerythritol lipids in the basidiomycetous fungus Ustilago maydis. Appl Environ Microbiol 72:5469–5477
Imura T, Ohta N, Inoue K, Yagi H, Negishi H, Yanagishita H, Kitamoto D (2006) Naturally engineered glycolipid biosurfactants leading to distinctive self-assembled structures. Chem Eur J 12:2434–2440
Kitamoto D, Haneishi K, Nakahara T, Tabuchi T (1990) Production of mannosylerythritol lipids by Candida antarctica from vegetable oils. Agric Biol Chem 54:37–40
Kitamoto D, Yanagishita H, Shinbo T, Nakane T, Kamisawa C, Nakahara T (1993) Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J Biotech 29:91–96
Kitamoto D, Isoda H, Nakahara T (2002) Functional and potential application of glycolipid biosurfactants. J Biosci Bioeng 94:187–201
Kitamoto D, Morita T, Fukuoka T, Konishi M, Imura T (2009) Self-assembling properties of glycolipid biosurfactants and their potential applications. Curr Opin Colloid Interface Sci 14:315–328
Konishi M, Morita T, Fukuoka T, Imura T, Kakugawa K, Kitamoto D (2008) Efficient production of mannosylerythritol lipids with high hydrophilicity by Pseudozyma hubeiensis KM-59. Appl Microbiol Biotechnol 78:37–46
Lang S (2002) Biological amphiphiles (microbial biosurfactants). Curr Opin Colloid Int Sci 7:12–20
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2006) Discovery of Pseudozyma rugulosa NBRC 10877 as a novel producer of glycolipid biosurfactants, mannosylerythritol lipids, based on rDNA sequence. Appl Microbiol Biotechnol 73:305–315
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2007) Characterization of the genus Pseudozyma by the formation of glycolipid biosurfactants, mannosylerythritol lipids. FEMS Yeast Res 7:286–292
Morita T, Konishi M, Fukuoka T, Imura T, Yamamoto S, Kitagawa M, Sogabe A, Kitamoto D (2008a) Identification of Pseudozyma graminicola CBS 10092 as a producer of glycolipid biosurfactants, mannosylerythritol lipids. J Oleo Sci 57:123–131
Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto D (2008b) Production of glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma siamensis CBS 9960 and their interfacial properties. J Biosci Bioeng 105:493–502
Morita T, Konishi M, Fukuoka T, Imura T, Sakai H, Kitamoto D (2008c) Efficient production of di- and tri-acylated mannosylerythritol lipids as glycolipid biosurfactants by Pseudozyma parantarctica JCM 11752T. J Oleo Sci 57:557–565
Morita T, Fukuoka T, Imura T, Kitamoto D (2009a) Production of glycolipid biosurfactants by basidiomycetous yeasts. Biotechnol Appl Biochem 53:39–49
Morita T, Fukuoka T, Konishi M, Imura T, Yamamoto S, Kitagawa M, Sogabe A, Kitamoto D (2009b) Production of a novel glycolipid biosurfactant, mannosylmannitol lipid, by Pseudozyma parantarctica and its interfacial properties. Appl Microbiol Biotechnol 83:1017–1025
Morita T, Takashima M, Fukuoka T, Konishi M, Imura T, Kitamoto D (2010) Isolation of basidiomycetous yeast Pseudozyma tsukubaensis and production of glycolipid biosurfactant, a diastereomer type of mannosylerythritol lipid-B. Appl Microbiol Biotechnol 88:678–688
Morita T, Fukuoka T, Imura T, Kitamoto D (2012) Formation of two novel glycolipid biosurfactants, mannosylribitol lipid and mannosylarabitol lipid, by Pseudozyma parantarctica JCM 11752T. Appl Microbiol Biotechnol 96:931–938
Morita T, Koike H, Koyama Y, Hagiwara H, Ito E, Fukuoka T, Imura T, Machida M, Kitamoto D (2013) Genome Sequence of the Basidiomycetous Yeast Pseudozyma antarctica T-34, a Producer of the glycolipid biosurfactants mannosylerythritol lipids. Genome Announc 1:e0006413
Morita T, Koike H, Hagiwara H, Ito E, Machida M, Sato S, Habe H, Kitamoto D (2014) Genome and transcriptome analysis of the basidiomycetous yeast Pseudozyma antarctica producing extracellular glycolipids, mannosylerythritol lipids. PLoS One 9:e86490
Rau U, Nguyen LA, Schulz S, Wary V, Nimtz M, Roeper H, Koch H, Lang S (2005) Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl Microbiol Biotechnol 66:551–559
Acknowledgments
We thank Life Science Development Center, CPI Company, Daicel Co. Ltd. (Japan), for screening various columns and separation conditions of d- and l-arabitol.
Conflict of interest
We declare that we do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, T., Fukuoka, T., Kosaka, A. et al. Selective formation of mannosyl-l-arabitol lipid by Pseudozyma tsukubaensis JCM16987. Appl Microbiol Biotechnol 99, 5833–5841 (2015). https://doi.org/10.1007/s00253-015-6575-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6575-4