Abstract
Azo dye decolorization was studied with Shewanella strains under saline conditions. Growing cells of Shewanella algae and Shewanella marisflavi isolated from marine environments demonstrated better azo dye decolorization capacities than the other three strains from non-saline sources. Cell suspensions of S. algae and S. marisflavi could decolorize single or mixed azo dyes with different structures. Decolorization kinetics were described with Michaelis–Menton equation, which indicated better decolorization performance of S. algae over S. marisflavi. Lactate and formate were identified as efficient electron donors for amaranth decolorization by the two strains. S. algae and S. marisflavi could decolorize amaranth at up to 100 g L−1 NaCl or Na2SO4. However, extremely low concentration of NaNO3 exerted strong inhibition on decolorization. Both strains could remove the color and COD of textile effluent during sequential anaerobic–aerobic incubation. Lower concentrations of NaCl (20–30 g L−1) stimulated the activities of azoreductase, laccase, and NADH-DCIP reductase. The decolorization intermediates were identified by high-performance liquid chromatography and Fourier transform infrared spectroscopy. Decolorization metabolites of amaranth were less toxic than original dye. These findings improved our knowledge of azo-dye-decolorizing Shewanella species and provided efficient candidates for the treatment of dye-polluted saline wastewaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large amounts of azo dyes are produced annually around the world and used extensively in textile, printing, cosmetic, plastic, food, pharmaceutical, and many other industries due to their ease of synthesis and chemical stability (Stolz 2001). The loss of azo dyes during their application may vary from 2 % for basic dyes to as high as 50 % for reactive dyes, resulting in the generation of azo dye wastewater (Boer et al. 2004; Khalid et al. 2008). Effluents containing azo dyes must be treated before discharge into environment not only because they may affect the transparency and aesthetics of water bodies but also because many azo dyes and their breakdown derivatives have been suggested to be toxic/mutagenic to life (Brown and DeVito 1993; Xu et al. 2007a). Additionally, high concentrations (40–100 g L−1) of salts (especially NaCl) were generally used in dye baths to achieve maximal fixation of dyes to fibers in textile industry (Ogugbue et al. 2010). Thus, salt concentrations in dye-stuff industry wastewaters have been found to be as high as 150–200 g L−1 (EPA 1997).
Biological treatment of azo dye wastewater has attracted more interests due to its cost effectiveness, lower sludge production, and environmental friendliness (Dubrow et al. 1996). Azo dyes are initially reduced/decolorized by various microbes under anaerobic or anoxic conditions to corresponding aromatic amines, some of which are then further mineralized aerobically (Dos Santos et al. 2007; Stolz 2001). Many bacterial strains with azo-dye-decolorizing capacity have been isolated and identified in the past decades (Pearce et al. 2003; Saratale et al. 2011). However, most bacteria are sensitive to the high concentrations of salts in saline azo dye wastewaters due to plasmolysis and/or loss of activity of cell. Compared to the large numbers of studies on non-halophilic azo-dye-decolorizing bacteria, much fewer investigations on decolorization performances of halophilic or halotolerant organisms were available. Previously, Halomonas sp. GTW and Gracilibacillus sp. GTY capable of decolorizing azo dyes in the presence of up to 150 g L−1 NaCl were isolated and characterized in our lab (Guo et al. 2008a, b; Uddin et al. 2007). Another three Halomonas strains were also found to be able to reduce azo dye in the presence of ~200 g L−1 NaCl (Asad et al. 2007). In addition, Khalid et al. (2008) recently reported the decolorization of azo dyes at up to 60 g L−1 NaCl by Shewanella putrefaciens AS96, which was isolated from activated sewage sludge.
Shewanella strains are well-known for their remarkable respiratory versatility (Fredrickson et al. 2008). Besides oxygen, Shewanella can respire a diverse pool of inorganic and organic substrates (e.g., iron(III), manganese(III/IV), vanadium(V), chronium(VI), uranium(VI), technetium(VII), nitrate, nitrite, elemental sulfur, sulfite, thiosulfate, fumarate, trimethylamine N-oxide, dimethyl sulfoxide, etc.), many of which are heavy metals and environmental pollutants (Fredrickson et al. 2008; Hau and Gralnick 2007). Thus, the roles of Shewanella in biogeochemical circulation of metal oxides, potentials of exploiting it for the remediation, immobilization, and detoxification of various contaminants, and electricity generation in microbial fuel cells (MFCs) have attracted particular interests (Fredrickson et al. 2008; Hau and Gralnick 2007). Members of genus Shewanella are widely distributed in different habitats including soil, aquatic, sediments, etc. (Dikow 2011; Fredrickson et al. 2008). Although several studies on azo dye decolorization by Shewanella strains have been conducted, most of these strains were isolated from freshwater and terrestrial sources (Hong et al. 2007; Khalid et al. 2008; Liu et al. 2011; Pearce et al. 2006; Wu et al. 2009; Xu et al. 2007b, c). Little was known about the decolorization capacity of marine Shewanella strains.
In this study, anaerobic decolorization of amaranth by growing cells of five Shewanella strains was compared. Then the decolorization performances of two marine strains (Shewanella algae and Shewanella marisflavi) were studied in detail, which to our knowledge was for the first time and might provide more information for microbial treatment of saline wastewater containing azo dye.
Materials and methods
Chemicals
Azo dyes including amaranth, acid orange 7 (AO7), acid orange 52 (AO52), reactive red 120 (RR120), and direct blue 71 (DB71) (Fig. S1) were purchased from Sigma-Aldrich, TCI, or SCRC and used without further purification. All other chemicals used in this study were of analytical grade.
Bacterial strains and culture conditions
S. algae (1A02601) and type strains of S. marisflavi (1A02628), Shewanella decolorationis (1A02635), and S. putrefaciens (1A02627) were obtained from Marine Culture Collection of China (Xiamen, China). Shewanella oneidensis MR-1 was obtained from American Type Culture Collection (700550). Aerobic cultivation of these strains was performed in Luria–Bertani (LB) medium. Anaerobic decolorization studies with growing or suspended cells were conducted in modified MR2A medium (Fries et al. 1994), in which KNO3 was replaced with KCl to avoid the impacts of nitrate. Lactate (20 mM) was added as electron donor and the pH was adjusted to 7.0.
Effects of salt concentration on amaranth decolorization by growing Shewanella strains
Cells of the five Shewanella strains were pregrown overnight in LB and then respectively inoculated (1 %) into sterile serum bottles containing 100 mL deoxygenated modified MR2A medium supplemented with 0–70 g L−1 NaCl. One millimolar of amaranth was added to each vial under anaerobic conditions. Then the growth and decolorization performance of each strain were monitored at intervals.
Assay of azo dye decolorization under saline conditions by cell suspensions of S. algae and S. marisflavi
Cells of S. algae and S. marisflavi aerobically grown in LB overnight were harvested by centrifugation (10,000 × g, 10 min) and washed three times with deoxygenated phosphate buffer (20 mM, pH 7.0, filter-sterilized). The final wash was made in an anaerobic chamber. The harvested cells were then resuspended in modified MR2A medium and held in the anaerobic chamber before use in the following studies. The experimental systems were sterile 100 mL serum bottles containing 50 mL deoxygenated modified MR2A medium supplemented with 50 g L−1 NaCl, 20 mM lactate as electron donor, and around 200 μM amaranth, AO7, AO52, RR120, or DB71. Decolorization of a mixture of three azo dyes, amaranth, AO52, and DB71, was also investigated. Each of them was added at about 200 μM, respectively. Decolorization studies were started by addition of cells to achieve a cell concentration of 0.36 g L−1.
In the presence of 50 g L−1 NaCl, the effects of different organic substances (formate, acetate, salicylate, citrate, pyruvate, glucose, sucrose, and glycerin), cell concentrations (0.04 to 0.72 g L−1), and initial amaranth concentrations (0.1–2.0 mM) on decolorization were studied. The impacts of different salt species on amaranth decolorization by the two strains were investigated by adding 10–100 g L−1 NaCl, 10–100 g L−1 Na2SO4, and 85–680 mg L−1 NaNO3, respectively.
All the anaerobic decolorization experiments were carried out in an anaerobic chamber at 30 °C. After cell inoculation, samples were periodically taken with sterile needle and syringe and analyzed as described below. Abiotic controls without inoculation of cells and biotic controls that contained no lactate or amaranth were performed. All treatments and controls were run in triplicate.
Decolorization of textile effluent
Real dye wastewater obtained from a local textile dyeing plant was also applied to test decolorization capacities of the two strains. Composition of textile wastewater was as follows: pH = 8.9; total suspended solids, 84 mg L−1; total dissolved solids, 6 g L−1; dissolved oxygen concentration, 2 mg L−1; NH3-N, 220 mg L−1; Cl−, 30 g L−1; and chemical oxygen demand (COD), 6,994 mg L−1. The original textile effluent was filtered by using filter paper and autoclaved. Thereafter, the effluent was used in sequential anaerobic–aerobic incubation with 0.36 g L−1 S. algae or S. marisflavi in serum bottles and flasks, respectively. Controls without inoculum were also performed. Samples were taken at intervals to monitor the removal of color and COD.
Cell extracts preparation and enzyme assays
S. algae and S. marisflavi cells were grown overnight in LB media supplemented with different concentrations of NaCl (0–70 g L−1). Cells were harvested by centrifugation at 8,000 × g for 15 min and washed twice with 20 mM sodium phosphate buffer (pH 7.0). The washed pellets were then resuspended in 20 mL of the same buffer and lysed by freezing and thawing followed by sonication (225 W, at 4 °C for 30 min, Ultrasonic processor CPX 750, USA). Cell debris was removed by centrifugation (60,000 × g, at 4 °C for 30 min). The supernatant was stored at 4 °C and used as cell extracts in the following studies.
The azoreductase, laccase, and NADH-dichlorophenol indophenol (DCIP) reductase activities of cell extracts of strains cultured at different salinities were studied. The reaction mixture (2 mL) for azoreductase assay contained 20 mM phosphate buffer (pH 7.0), 40 μM NADH, 50 μM amaranth, and 0.1 mL of cell extracts. Azoreductase activity was determined spectrophotometrically by monitoring the removal of amaranth at 520 nm (ε = 22.6 mM−1 cm−1). The 2-mL reaction mixture for laccase activity determination contained 1 mM 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt in 100 mM sodium acetate buffer (pH 4.5) and 0.1 mL of cell extracts. Laccase activity was measured via monitoring the increase in absorbance at 420 nm (ε = 36.0 mM−1 cm−1) (Saratale et al. 2009). The NADH-DCIP activity was assayed by monitoring the decrease in absorbance at 590 nm (ε = 19.0 mM−1 cm−1) for a 2-mL reaction mixture containing 50 μM DCIP, 50 mM phosphate buffer (pH 7.4), 50 μM NADH, and 0.1 mL of cell extracts (Salokhe and Govindwar 1999). One unit of enzyme activity was calculated as the amount of substrate consumed or product generated per milligram protein per minute. Protein concentration of the cell extracts was measured according to the Bradford (1976) procedure, using bovine serum albumin as a standard.
Analytical methods
To monitor the decolorization process, samples taken from the experimental system were centrifuged at 10,000 × g for 10 min to remove the cells. The absorbance of the supernatant was measured at maximum wavelengths of respective azo dyes (λ max = 520 nm of amaranth, λ max = 484 nm of AO7, λ max = 465 nm of AO52, λ max = 513 nm of RR120, and λ max = 582 nm of DB71). The decolorization efficiency was expressed as color removal (%) = C i − C r/C i × 100 %, where C i and C r were initial and residual dye concentrations, respectively. Dye mixture decolorization processes were monitored according to the method reported previously (Harazono and Nakamura 2005; Liu et al. 2007). The growth of cells was monitored through protein determination (Bazylinski et al. 2000). COD was measured as per APHA (1998) standard protocol.
The decolorization products of AO52 were analyzed using a high-performance liquid chromatography (HPLC; Shimadzu LC-20AT, Japan) with a Hypersil ODS-2 column (4.6 × 250 mm) and UV detector (detection wavelength of 245 nm). Ammonium acetate solution (10 mM, pH 4.0) and methanol (50:50, v/v) were used as eluent at a flow rate of 0.5 mL min−1.
Fourier transform infrared spectroscopy (FTIR) was also applied for analysis of AO52 and its decolorization products. After complete decolorization, cells were removed through centrifugation (10,000 × g, 10 min). The supernatant was extracted by equal volume of ethyl acetate and dried by anhydrous Na2SO4 in a rotary evaporator. The obtained samples were ground with KBr powder and pressed to form a uniform disk, which was then measured with an FTIR (Nicolet-20DXB, Nicolet Co., USA).
Phytotoxicity studies
Phytotoxicity tests were performed to assess the toxicity of the untreated and treated dye (Saratale et al. 2010). The decolorization metabolites of amaranth extracted by ethyl acetate were dried and dissolved in sterile distilled water. Seeds of Phaselus mungo and Oryza sativa (ten seeds of each) were watered separately per day with 10 mL sample of amaranth (1,000 mg L−1) or metabolites obtained after its decolorization. Control set was carried out using sterile distilled water at the same time. Germination as well as lengths of plumule and radical were recorded after a week.
Results
Effects of NaCl on amaranth decolorization by growing Shewanella strains
Amaranth decolorization by five different Shewanella strains was investigated in the presence of 0–70 g L−1 NaCl (Fig. S2). The presence of NaCl significantly restrained the decolorization performance of S. oneidensis MR-1 and S. decolorationis. In the absence of NaCl, MR-1 removed 98.4 % amaranth in 24 h. However, 31–109 h was required by MR-1 to reach similar decolorization efficiency in the presence of 10–50 g L−1 NaCl. Only 42.5 and 32.2 % amaranth were reduced in 109 h in the presence of 60 and 70 g L−1 NaCl, respectively.
Amaranth was almost completely decolorized in 24 h by S. decolorationis under non-saline conditions or in the presence of 10 g L−1 NaCl. In the same period, only 66.6, 27.0, 13.7, and 4.2 % amaranth was removed in the presence of 20–50 g L−1 NaCl, respectively. Neglectable decolorization was observed when the salinity was higher than 60 g L−1. Around 29, 37, and 54 h were taken by S. decolorationis to realize complete removal of amaranth in the presence of 20–40 g L−1 NaCl, respectively. Decolorization efficiencies of 72.9, 36.2, and 12.7 % were reached in 86 h in the presence of 50–70 g L−1 NaCl, respectively.
Complete removal of amaranth in ~75 h was observed for S. putrefaciens in the absence or presence of 10–20 g L−1 NaCl. However, a further increase of NaCl concentration from 30 to 70 g L−1 resulted in decrease of its decolorization capacity. Over 120 h was needed to realize complete color removal when NaCl concentration was higher than 40 g L−1.
In contrast, slightly saline conditions were found to be favorable for amaranth decolorization by the other two Shewanella strains. Without the addition of NaCl, 32 h was required by S. marisflavi to reduce over 98 % amaranth. When 10–30 g L−1 NaCl was added, similar decolorization level was reached in around 24 h. When the NaCl concentration was enhanced to 40–50 g L−1, complete color removal could still be observed in around 50 h. The presence of 20 g L−1 NaCl was most favorable for amaranth decolorization by S. algae, which removed over 95 % amaranth in 36 h. The decolorization efficiencies observed in the presence of 10 and 30–40 g L−1 NaCl were similar with that obtained under non-saline conditions. Complete decolorization could still be achieved in 72 h when the salinity was increased to 50 g L−1.
The variation of protein concentrations in decolorization systems was monitored. Although both S. algae and S. marisflavi could grow during decolorization in the presence of 0–50 g L−1 NaCl (data not shown), their optimal growth was observed when the salt concentration was 20 g L−1. In the presence of 20 g L−1 NaCl, the protein concentration was increased 41.7 % for S. algae to 55 mg L−1 and 27.5 % for S. marisflavi to 49 mg L−1 in 80 h. Cell growth ceased when over 95 % amaranth was reduced (data not shown). Higher decolorization capability generally correlated with higher biomass increase. No change of protein concentration was observed with systems containing 60–70 g L−1 NaCl in 70 h. Similar trends of cell growth were also observed for the rest Shewanella strains (data not shown).
Azo dye decolorization by S. algae and S. marisflavi cell suspensions under saline conditions
As shown in Fig. 1, besides amaranth, the other four azo dyes (200 μM) could also be decolorized by cells of S. algae and S. marisflavi in the presence of 50 g L−1 NaCl. In 6 h, around 21.1 % DB71, 32.6 % AO7, 42.3 % RR120, 49.1 % AO52, and 97.5 % amaranth were decolorized by S. algae and about 19.7 % DB71, 25.9 % AO7, 29.2 % RR120, 32.7 % AO52, and 53.4 % amaranth were reduced by S. marisflavi, respectively. The decolorization of dye mixture containing amaranth, AO52, and DB71 (each at 200 μM) was also investigated. Both of the two strains could decolorize dye mixture. All the three dyes could be reduced simultaneously (data not shown). Decolorization efficiencies of around 47.8 and 32.1 % were observed for S. algae and S. marisflavi in 12 h, respectively.
Decolorization of azo dyes with different structures (DB71, AO7, RR120, AO52, and amaranth) by suspended cells of S. algae and S. marisflavi under saline conditions. The experimental systems were consisted of 50 mL cell suspensions (0.36 g L−1) in deoxygenated modified MR2A containing 50 g L−1 NaCl, 20 mM lactate and around 200 μM each dye, respectively. Data represent averages from triplicate assays, with error bars showing the standard deviation
The decolorization performance under saline conditions (50 g L−1 NaCl) was improved by increase of cell concentration (Fig. 2). When less than 0.04 g L−1 S. algae or S. marisflavi cells were present in the system, no obvious decolorization of 200 μM amaranth could be detected in 12 h. About 60.0 and 24.9 % amaranth were removed in 12 h in the presence of 0.18 g L−1 cells of S. algae or S. marisflavi, respectively. In the presence of 0.36 g L−1 cells, 62.2 % amaranth was decolorized by S. marisflavi in 12 h, whereas almost complete removal of amaranth by S. algae was observed in 6 h. Further increases of cell concentrations to 0.54 and 0.72 g L−1 resulted in over 95 % decolorization of amaranth in less than 4 h by both strains.
Effects of biomass concentration on the decolorization of amaranth by S. algae (solid) and S. marisflavi (open) under saline conditions. Series of concentrations (0–0.72 g L−1) of S. algae and S. marisflavi cells were applied to reduce 200 μM amaranth in the presence of 50 g L−1 NaCl in 12 h. Data represent averages from triplicate assays, with error bars showing the standard deviation
Complete removal of different concentrations of amaranth (up to 2 mM) could be achieved in 34 h by S. algae and 52 h by S. marisflavi, respectively (data not shown). In addition, the relationship between specific decolorization rates of the two strains and dye concentration could be described with Michaelis–Menton kinetics (Fig. 3). The values of kinetic constants K m and V max were estimated to be 0.3 mM and 7.0 μmol mg cell−1 h−1 for S. algae and 0.3 mM and 5.5 μmol mg cell−1 h−1 for S. marisflavi, respectively.
Effects of dye concentration on specific amaranth decolorization rates of S. algae and S. marisflavi under saline conditions. Amaranth concentration was varied from 0.1 to 2.0 mM whereas NaCl concentration was held at 50 g L−1. Data represent averages from triplicate assays, with error bars showing the standard deviation
Lactate and the other eight organic substances were tested to function as electron donor for amaranth decolorization by S. algae and S. marisflavi. No decolorization was detected for both strains in the absence of externally added electron donor. Amaranth was completely removed by S. algae in 5 h in the presence of 20 mM lactate or formate. For the rest organic substances studied, 54.6, 51.3, 44.6, 41.1, 24.5, 18.3, and 9.0 % amaranth were reduced in 11 h in the presence of glucose, pyruvate, acetate, glycerin, sucrose, salicylate, and citrate, respectively. Over 98 % of 200 μM amaranth was decolorized by S. marisflavi in 24 h when lactate or formate was used as electron donor. Decolorization efficiencies of 76.6, 39.6, 35.8, 29.1, and 20.9 % were obtained in 24 h when pyruvate, acetate, glucose, glycerin, and sucrose were applied as electron donors, respectively. However, no obvious amaranth decolorization occurred in the presence of citrate or salicylate.
Effects of different salt species on amaranth decolorization by S. algae and S. marisflavi were compared. In the absence of externally added salts, both S. algae and S. marisflavi could decolorize over 97 % amaranth (200 μM) in 4 h (data not shown). In the presence of 10 and 30 g L−1 NaCl, both S. algae and S. marisflavi could remove over 97 % amaranth in 4 h. In the presence of 50 g L−1 NaCl, 97.2 % amaranth was decolorized by S. algae in 5 h, whereas only 59.2 % amaranth was removed by S. marisflavi in 12 h. When the NaCl concentration was raised to 80 g L−1, around 72.5 and 50.3 % amaranth were removed by S. algae and S. marisflavi in 12 h, respectively. A further increase of NaCl concentration to 100 g L−1 continued to lower the decolorization efficiencies to 57.4 and 33.4 % for S. algae and S. marisflavi, respectively (Fig. 4a).
Effects of different concentrations of NaCl (a), Na2SO4 (b), and NaNO3 (c) on amaranth decolorization by S. algae (solid) and S. marisflavi (open). Decolorization of 200 μM amaranth by S. algae and S. marisflavi was monitored in the presence of 10–100 g L−1 NaCl, 10–100 g L−1 Na2SO4 L−1, and 85–680 mg L−1 NaNO3, respectively. Data represent averages from triplicate assays, with error bars showing the standard deviation
Less inhibition on decolorization was observed when NaCl was replaced with Na2SO4 (Fig. 4b). Similar to the effects of NaCl concentration, 10–30 g L−1 Na2SO4 had almost no inhibition on decolorization capacities of both S. algae and S. marisflavi. Although inhibition on decolorization was found (especially for S. marisflavi) in the presence of 50 g L−1 Na2SO4, both strains could still remove over 97 % amaranth in 5 h. In the presence of 80 g L−1 Na2SO4, 96.8 % amaranth was reduced by S. algae in 8 h, whereas only 73.5 % amaranth was removed by S. marisflavi in 12 h. In the presence of 100 g L−1 Na2SO4, 61.7 and 38.5 % amaranth were decolorized by S. algae and S. marisflavi in 12 h, respectively.
The decolorization was restrained more strongly by nitrate (Fig. 4c). Lower concentrations of nitrate caused similar inhibiting effects on decolorization performances of S. algae and S. marisflavi. In the presence of 85 and 255 mg L−1 NaNO3, S. algae and S. marisflavi could decolorize over 97 % amaranth in 5 and 7–8 h, respectively. When 425 mg L−1 NaNO3 was present in the media, S. algae reduced 97.7 % amaranth in 10 h while S. marisflavi removed only 49.2 % amaranth in 12 h. A further increase of nitrate concentration to 680 mg L−1 resulted in 43.9 % decolorization by S. algae and only 7.0 % decolorization by S. marisflavi in 12 h, respectively.
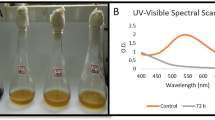
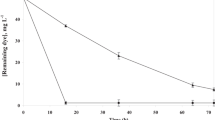
Under anaerobic conditions, both strains could effectively decolorize real textile effluents (Fig. S3). S. algae reached over 87.8 % decolorization in 48 h, whereas S. marisflavi removed nearly 80 % color of the effluents in 72 h. No more decolorization occurred under subsequent aerobic conditions. Only 9.5 and 5.9 % COD were removed by S. algae and S. marisflavi in 96 h of anaerobic incubation, respectively. However, during the following aerobic incubation, around 52.7 and 46.8 % COD were removed by S. algae and S. marisflavi in 264 h, respectively.
Enzymatic activities in cell extracts
Activities of azoreductase, laccase, and NADH-DCIP reductase in cell extracts of S. algae and S. marisflavi cultured under different salinities were studied (Fig. 5). When cells were cultured in the absence of NaCl, azoreductase activities in cell extracts of S. algae and S. marisflavi were 0.3 and 0.08 μmol mg protein−1 min−1, respectively. Lower salinities stimulated the azoreductase activities of Shewanella strains, and the highest azoreductase activities (0.42 and 0.19 μmol mg protein−1 min−1 for S. algae and S. marisflavi, respectively) were obtained from cells cultured in the presence of 20 g L−1 NaCl. Further increases of salinities resulted in gradually decreasing azoreductase activities. When the salt concentration was higher than 50 g L−1, the azoreductase activities of both strains were lower than those of cells cultivated under non-saline conditions.
S. algae and S. marisflavi cells grown in the presence of 30 g L−1 NaCl demonstrated the highest NADH-DCIP reductase activities, which were 21.5 and 23.3 % higher than those of cells grown in the absence of NaCl, respectively. Salinities higher than 30 g L−1 led to descending of NADH-DCIP reductase activities of both strains. When the NaCl concentration was higher than 40 g L−1, the NADH-DCIP reductase activities of S. algae and S. marisflavi were lower than those observed from strains cultured in the absence of NaCl.
The highest laccase activities were observed in S. algae and S. marisflavi cells cultivated in the presence of 30 g L−1 NaCl and were 83.7 and 48.5 % higher than those of cells grown under non-saline conditions, respectively. When the NaCl concentration in the media was raised to 50 g L−1 or even higher, the laccase activities of both strains were lower than those of cells grown under non-saline conditions.
Products analysis of AO52 decolorization
The decolorization products of AO52 by S. algae and S. marisflavi were analyzed with HPLC. For both strains, the peak for AO52 with a retention time (R t) of 10.4 min disappeared after decolorization whereas the other two peaks corresponding to N,N-dimethyl-p-phenylenediamine (R t = 5.1 min) and 4-aminobenzenesulfonic acid (R t = 8.9 min) were detected (Fig. S4).
The AO52 reduction and aromatic amines production were also confirmed by FTIR spectra analysis (Fig. S5). FTIR spectra of AO52 displayed peak at 2,903 cm−1 for asymmetric –CH3 stretching vibrations; peaks at 1,437 and 1421 cm−1 for asymmetric C = C–H in plane C–H bend; peaks at 1,006, 944, and 846 cm−1 for ring vibrations; and peak at 816 cm−1 for disubstituted benzene ring, which confirmed the aromatic nature of the dye. The peak at 1,608 cm−1 for N = N stretch and peaks at 1,201 and 1,121 cm−1 for –C–N confirmed the azo nature of the dye. Peaks at 698, 624, and 574 cm−1 for –C–S– stretching vibrations and peak at 1,367 cm−1 for S = O stretching vibrations confirmed the sulfonic nature of dye (Parshetti et al. 2010).
For the decolorization products, the FTIR spectra displayed peak at 3,337 cm−1 for N–H bend. The peak at 1,608 cm−1, which was derived from N = N, disappeared after the decolorization. An absorbance peak appeared at 1,520 cm−1 was associated with the C = C stretching vibration of aromatic rings. In addition, a new peak for the in-plane bending vibration of N–H emerged at 1,627 cm−1. The peaks at 3,337 and 819 cm−1 for the stretching vibration and plane bending vibration of primary amine N–H, respectively, became sharper in the reduction products, indicating the formation of amines (Cai et al. 2012).
Phytotoxicity assessment
Phytotoxicity of amaranth decolorization products was studied with seeds of P. mungo and O. sativa (Fig. S6). Compared to those measured under control conditions, the plumule and radical lengths of P. mungo and O. sativa seeds were shorter when treated with amaranth or decolorization metabolites. However, the plumule and radical lengths of P. mungo seeds treated with decolorization metabolites were 9.5–16.2 and 18.9–31.9 % longer than those of seeds treated with amaranth, respectively. Similarly, the plumule and radical lengths of O. sativa seeds treated with decolorization metabolites were 9.4–22.8 and 21.5–33.2 % longer than those of seeds incubated with untreated dye, respectively. On the other hand, compared to the 100 % germination found in control tests using distilled water, amaranth decreased the germination of both plant seeds to 90 %. When seeds were incubated with decolorization metabolites, the germination rate of O. sativa remained at 90 % whereas that of P. mungo was increased to 100 % (data not shown).
Discussion
Many bacterial species have been reported to be capable of decolorizing azo dye. Nonetheless, a limiting factor for the development and application of biological decolorization processes has been the sensitivity of many bacteria to high concentrations of salts accompanied with dye wastewater (Amoozegar et al. 2010). Dilution of saline effluent before biological treatment generally increased the volumes of wastewater. Therefore, isolation and characterization of halophilic or halotolerant bacterial strains that could thrive and function under saline conditions has attracted many interests recently (Amoozegar et al. 2010; Asad et al. 2007). Shewanella has a reputation for its metabolic and respiratory diversity and great potentials for biodegradation of environmental pollutants. In addition, members of genus Shewanella were believed to have marine origins. Many of them were isolated from marine and brackish environments (Hau and Gralnick 2007) and found to be able to thrive at salt concentrations up to 60 g L−1 (Holt et al. 2005; Yoon et al. 2004), which may guarantee their functions under saline conditions. Huang et al. (2010) recently reported that S. marisflavi EP1 could generate power when ionic strength of electrolyte in MFC was as high as ~1.5 M (80 g L−1 NaCl). Thus, it was surprising to find that all currently available studies on azo dye reduction by Shewanella employed isolates from sources of freshwater, soil, or activated sludge (Cai et al. 2012; Hong et al. 2007; Khalid et al. 2008; Liu et al. 2011; Pearce et al. 2006; Wu et al. 2009; Xu et al. 2007b, c). Here, for the first time, we studied azo dye decolorization activities of marine Shewanella strains.
By comparing the decolorization performance of growing cells of different Shewanella strains, it was found that the presence of certain amounts of NaCl was advantageous for marine Shewanella strains like S. algae and S. marisflavi to decolorize amaranth. The optimal NaCl concentration for amaranth decolorization by growing S. algae cells was 20 g L−1. And similar decolorization efficiencies were obtained in 60 h for systems containing up to 40 g L−1 NaCl. The presence of 10–30 g L−1 NaCl resulted in the same decolorization level in 24 h by S. marisflavi, which showed slower decolorization under non-saline conditions. Similar with results of many previous studies, the type strain S. oneidensis MR-1 and the azo-dye-decolorizing S. decolorationis showed good color removal performances in the absence of NaCl. However, their decolorization capacities decreased with the increase of NaCl concentration. Khalid et al. (2008) recently isolated S. putrefaciens AS96 from activated sludge and reported its efficient decolorization capacity under saline conditions. Nevertheless, the poorest decolorization performance in our study was found with the type strain of S. putrefaciens, which reached no complete decolorization until 70 h and showed inhibited decolorization capacity at NaCl concentrations higher than 20 g L−1.
S. decolorationis was suggested to be capable of obtaining energy to support growth during dissimilatory azo dye reduction (Hong et al. 2007). Recently, Khalid et al. (2008) also observed biomass increase of S. putrefaciens AS96 during dye decolorization under saline conditions and indicated that the presence of certain concentrations of NaCl (5–30 g L−1) was helpful for cell growth. Amaranth removal in our study was also accompanied by growth of Shewanella strains. And higher decolorization efficiencies under optimal NaCl concentrations generally correlated with better cell growth. The ultimate protein concentrations in S. algae and S. marisflavi decolorization systems could reach up to 50 mg L−1, which was lower than that (over 100 mg L−1) reported in a recent study on growth-associated methyl orange decolorization by S. oneidensis MR-1 (Cai et al. 2012). It seems that growth during anaerobic azo dye decolorization is the common character shared by different Shewanella strains.
Studies on the effects of biomass concentration found that the decolorization performances of S. algae and S. marisflavi were improved through increase of cell concentration (up to 0.72 g L−1). No decolorization was observed under abiotic conditions, and little decolorization occurred in the presence of lower biomass concentrations. The reduction rate decreased with time at all cell concentrations, which might be due to substrate limitation and accumulation of salt stress.
S. algae always demonstrated higher decolorization efficiencies than S. marisflavi under saline conditions for all tested azo dyes. Amaranth and AO52 with simple structures and/or low molecular weight were generally easier to decolorize whereas RR120 possessing more complex structure and DB51 having three azo bonds were reduced less efficiently. The inefficient reduction of AO7 possessing relatively simple structure might be due to its lower redox potential (−345 mV; Böttcher et al. 2010). Similar poor reduction of AO7 was also observed in previous decolorization studies with S. oneidensis MR-1 and Klebsiella oxytoca (Liu et al. 2011; Yu et al. 2011). Khalid et al. (2008) reported the complete or partial decolorization of azo dye mixture by S. putrefaciens AS96 and the other five bacterial strains isolated from activated sludge. Both S. algae and S. marisflavi were also capable of removing the color of dye mixture. Like results of single dye decolorization, S. algae demonstrated better decolorization capacity than S. marisflavi for dye mixture decolorization.
Yang et al. (2011) recently found that S. oneidensis MR-1 could not completely decolorize 200 mg L−1 of acid yellow 199 under microaerophilic and non-saline conditions, and there was a negative correlation between dye removal and initial dye concentration. In the presence of 50 g L−1 NaCl, S. algae and S. marisflavi could fully remove as high as 2 mM amaranth. The relationship between decolorization rates of S. algae and S. marisflavi and amaranth concentration followed the Michaelis–Menton kinetics. The same K m value was found with the two strains whereas the V max of S. algae was almost 1.3-fold higher than that of S. marisflavi, corresponding with the better decolorization capacity of S. algae. However, previous studies on azo dye decolorization by S. putrefaciens AS96 under saline conditions indicated an inverse linear relationship between the decolorization rate and dye concentration and attributed this to the inhibition of AS96 growth (Khalid et al. 2008). The two marine strains investigated here might be more proper for decolorization of higher concentrations of azo dyes.
A wide range of organic substances could act as electron donors for azo dye removal by S. algae and S. marisflavi under saline conditions. Formate and lactate have long been recognized as optimal choices of electron donor for Shewanella strains to reduce azo dyes under non-saline conditions (Hong et al. 2007; Pearce et al. 2006). The most efficient decolorization of amaranth by S. algae and S. marisflavi under saline conditions was also observed when lactate and formate were supplied as electron donors. In addition, other organic substances including glucose, pyruvate, acetate, glycerin, and sucrose could also support amaranth decolorization by the two strains under saline conditions. Although the presence of salicylate and citrate could still lead to less than 20 and 10 % decolorization of amaranth by S. algae in 11 h, no decolorization occurred when they were used for amaranth decolorization by S. marisflavi. While pyruvate was also a good electron donor for S. decolorationis S12 to reduce azo dyes, other organic compounds such as acetate, salicylate, glycerin, glucose, sucrose, and citrate could not be utilized by S12 as electron donor under anaerobic and non-saline conditions (Hong et al. 2007). On the other hand, glucose was reported to have strong inhibitory effects on the decolorization processes of S. putrefaciens AS96 under saline conditions (Khalid et al. 2008).
The presence of 10–30 g L−1 NaCl had almost no effect on the decolorization performances of cell suspensions of S. algae and S. marisflavi. Both strains could still decolorize certain amounts of amaranth when NaCl concentration was further increased from 50 g L−1 to as high as 100 g L−1. However, inhibitory effects on decolorization increased with the increase of NaCl concentration. Better decolorization performance was observed with S. algae, which always demonstrated over 20 % higher decolorization efficiencies than S. marisflavi in the presence of over 30 g L−1 NaCl. An inverse linear relationship between dye decolorization rate and NaCl concentration (5–60 g L−1) was also found for azo dye reduction by S. putrefaciens AS96. The reduced decolorization activity was attributed to the negative effects of NaCl on activities of bacteria (Khalid et al. 2008). Besides NaCl, Na2SO4 and NaNO3 are also typical salts used for dyeing and thus existed at high concentrations in colored textile wastewater (Carliell et al. 1998). Much stronger inhibition of amaranth reduction was detected with nitrate, which at very low concentrations (<1 g L−1) significantly restrained decolorization. The delay of the onset of decolorization by nitrate has been reported in studies of direct azo dye decolorization by S. putrefaciens AS96 and S. decolorationis S12 and humic acid-mediated amaranth reduction by S. oneidensis MR-1 (Hong et al. 2007; Khalid et al. 2008; Liu et al. 2011). The presence of 6 mM nitrate completely inhibited azo dye reduction by S. decolorationis S12 in 36 h (Hong et al. 2007). However, in the presence of 8 mM (680 mg L−1) NO −3 , S. marisflavi started to remove amaranth after a delay of 7 h, and S. algae could still decolorize over 40 % amaranth in 12 h, further indicating the suitability of marine Shewanella strains for azo dye decolorization under saline conditions. The redox potentials of amaranth and NO −3 are −250 and +360 mV, respectively. Therefore, besides causing common salt stress, nitrate might also be more preferred than amaranth to act as electron acceptor. Surprisingly, nitrate was not found to inhibit azo dye decolorization by Shewanella strain J18 143 (Pearce et al. 2006). On the other hand, SO 2−4 with a redox potential of −516 mV would not compete with amaranth for electrons and thus resulted in less inhibition. The presence of less than 30 g L−1 sulfate caused no inhibition of amaranth decolorization by S. algae and S. marisflavi. Although further increase of sulfate concentration resulted in gradually increased inhibition of decolorization, the two marine Shewanella strains were still capable of decolorizing around one- to two-thirds of 200 μM amaranth in the presence of 100 g L−1 Na2SO4 in 12 h.
Both S. algae and S. marisflavi could remove the color and COD in saline textile effluents through sequential anaerobic–aerobic incubation. Pseudomonas sp. SU-EBT demonstrated more than 90 % decolorization and 50 % COD removal of textile industry effluent (Telke et al. 2010). Alishewanella sp. KMK6 could effectively decolorize mixture of textile dyes under anoxic conditions and subsequently remove over 90 % COD under shaking conditions (Kolekar et al. 2012). The increase in COD reduction under aerobic conditions might be due to further degradation of dye intermediates (Kolekar et al. 2012). These observations suggested that the two Shewanella strains might be potential strains for the treatment of textile industry effluent.
In order to get additional insight into salinity effects on decolorization, azoreductase, laccase, and NADH-DCIP reductase activities in cell extracts of S. algae and S. marisflavi grown under different salinities were assayed. In accordance with the decolorization results of cell suspensions, cell extracts of S. algae grown at all salinities generally possessed higher enzyme activities over those of S. marisflavi. Moreover, the presence of lower NaCl concentrations (20–30 g L−1) stimulated enzyme activities of cell extracts of S. algae and S. marisflavi. During investigation of reactive black 5 decolorization by S. oneidensis WL-7, instead of azoreductase activity, Wu et al. (2009) only identified laccase and NADH-DCIP reductase activities from cell extracts of WL-7 and suggested that laccase was responsible for the decolorization process. In addition, azoreductase and NADH-DCIP reductase activities of cell extracts of S. oneidensis MR-1 were indicated to be involved in methyl orange and acid yellow 199 decolorization (Yang et al. 2011). Kolekar and Kodam (2011) recently found that activities of azoreductase and NADH-DCIP reductase in cell extracts of Alishewanella sp. KMK6 were increased after decolorization.
HPLC analysis of AO52 reduction products by S. algae and S. marisflavi only detected two new peaks, which were identified as N,N-dimethyl-p-phenylenediamine and 4-aminobenzenesulfonic acid, respectively. In addition, FTIR analysis of decolorization products also confirmed the breakage of azo bond and generation of aromatic amines. The results were consistent with those of recent studies on AO52 decolorization by S. oneidensis MR-1 and Kocuria rosea MTCC 1532 (Cai et al. 2012; Parshetti et al. 2010). Azo dye removal by S. algae and S. marisflavi was mainly carried out through reductive pathway, and laccase might not be functional in cellular decolorization. Similarly, a study on azo dye decolorization by Shewanella strain J18 143 suggested that color removal by this strain was a result of microbially mediated reduction of the chromophore in the dye molecules (Pearce et al. 2006). Data of HPLC analysis indicated that S. decolorationis S12 removed amaranth through reductive mechanisms (Hong et al. 2007). Khalid et al. (2008) also proposed reductive mechanisms for the decolorization of azo dyes by S. putrefaciens AS96 under saline conditions.
Seed germination and plant growth bioassays are most common techniques used to evaluate phytotoxicity of toxicants. Data of phytotoxicity assessment indicated that decolorization metabolites of amaranth by S. algae and S. marisflavi were less toxic than the dye itself. Thus, the decolorization process resulted in partial detoxification of the dye. Ghodake et al. (2011) also found that the decolorization products of amaranth by Acinetobacter calcoaceticus were less toxic than the original dye.
In summary, azo dye reduction under saline conditions by Shewanella strains were investigated in this study. Azo dye decolorization might be common capability shared by different Shewanella strains, among which marine ones like S. algae and S. marisflavi could be better choices for treatment of dye-polluted saline wastewaters. Shewanella strains can be cultured easily and have been reported to aerobically mineralize aromatic amines (Khalid et al. 2008; Xu et al. 2007c). Thus, further studies are underway to set up combined anaerobic–aerobic bioreactors applying Shewanella strains to completely degrade azo dyes present in saline wastewaters.
References
Amoozegar MA, Hajighasemi M, Hamedi J, Asad S, Ventosa A (2010) Azo dye decolorization by halophilic and halotolerant microorganisms. Ann Microbiol 61:217–230
APHA (1998) Standard method for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Asad S, Amoozegar MA, Pourbabaee AA, Sarbolouki MN, Dastgheib SMM (2007) Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour Technol 98:2082–2088
Bazylinski DA, Dean AJ, Schüler D, Philips EJ, Lovley DR (2000) N2-dependent growth and nitrogenase activity in the metal-metabolizing bacteria, Geobacter and Magnetospirillum species. Environ Microbiol 2:266–273
Boer CG, Obici L, Souza CG, Peralta RM (2004) Decolourization of synthetic dyes by solid state cultures of Lentinula (Lentinus) edodes producing manganese peroxidase as the main lignolytic enzyme. Bioresour Technol 94:107–112
Böttcher H, Mahltig B, Sarsour J, Stegmaier T (2010) Qualitative investigations of the photocatalytic dye destruction by TiO2-coated polyester fabrics. J Sol-Gel Sci Technol 55:177–185
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown MA, DeVito SC (1993) Predicting azo dye toxicity. Crit Rev Environ Sci Technol 23:249–324
Cai P, Xiao X, He Y, Li W, Chu J, Wu C, He M, Zhang Z, Sheng G, Lam MHW, Xu F, Yu H (2012) Anaerobic biodecolorization mechanism of methyl orange by Shewanella oneidensis MR-1. Appl Microbiol Biotechnol 93:1769–1776
Carliell CM, Barclay SJ, Shaw C, Wheatley AD, Buckley CA (1998) The effect of salts used in textile dyeing on microbial decolourisation of a reactive azo dye. Environ Technol 19:1133–1137
Dikow RB (2011) Genome-level homology and phylogeny of Shewanella (Gammaproteobacteria: Interomonadales: Shewanellaceae). BMC Genomics 12:237–250
Dos Santos AB, Cervantes FJ, Van Lier JB (2007) Review paper on current technologies for decolourization of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 98:2369–2385
Dubrow SF, Boardman GD, Michelsen DL (1996) Chemical pretreatment and aerobic–anaerobic degradation of textile dye wastewater. In: Reife A, Freeman HS (eds) Environmental chemistry of dyes and pigments. Wiley, New York, pp 75–102
EPA (1997) Profile of the textile industry. Environmental Protection Agency, Washington, DC
Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM (2008) Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603
Fries MR, Zhou J, Chee-Sanford J, Tiedje JM (1994) Isolation, characterization, and distribution of denitrifying toluene degraders from a variety of habitats. Appl Environ Microbiol 60:2802–2810
Ghodake G, Jadhav U, Tamboli D, Kagalkar A, Govindwar S (2011) Decolorization of textile dyes and degradation of mono-azo dye amaranth by Acinetobacter calcoaceticus NCIM 2890. Indian J Microbiol 51:501–508
Guo J, Zhou J, Wang D, Tian C, Wang P, Uddin SM (2008a) A novel moderately halophilic bacterium for decolorizing azo dye under high salt condition. Biodegradation 19:15–19
Guo J, Zhou J, Wang D, Yang J, Li Z (2008b) The new incorporation bio-treatment technology of bromoamine acid and azo dyes wastewaters under high-salt conditions. Biodegradation 19:93–98
Harazono K, Nakamura K (2005) Decolorization of mixtures of different reactive textile dyes by the white-rot basidiomycete Phanerochaete sordida and inhibitory effect of polyvinyl alcohol. Chemosphere 59:63–68
Hau HH, Gralnick JA (2007) Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol 61:237–258
Holt HM, Gahrn-Hansen B, Bruun B (2005) Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect 11:347–352
Hong Y, Xu M, Guo J, Xu Z, Chen X, Sun G (2007) Respiration and growth of Shewanella decolorationis S12 with an azo compound as sole electron acceptor. Appl Environ Microbiol 73:64–72
Huang J, Sun B, Zhang X (2010) Electricity generation at high ionic strength in microbial fuel cell by a newly isolated Shewanella marisflavi EP1. Appl Microbiol Biotechnol 85:1141–1149
Khalid A, Arshad M, Crowley DE (2008) Decolorization of azo dyes by Shewanella sp. under saline conditions. Appl Microbiol Biotechnol 79:1053–1059
Kolekar YM, Kodam KM (2011) Decolorization of textile dyes by Alishewanella sp. KMK6. Appl Microbiol Biotechnol. doi:10.1007/s00253-011-3698-0
Kolekar YM, Konde PD, Markad VL, Kulkarni SV, Chaudhari AU, Kodam KM (2012) Effective bioremoval and detoxification of textile dye mixture by Alishewanella sp. KMK6. Appl Microbiol Biotechnol. doi:10.1007/s00253-012-3983-6
Liu G, Zhou J, Qu Y, Ma X (2007) Decolorization of sulfonated azo dyes with two photosynthetic bacterial strains and a genetically engineered Escherichia coli strain. World J Microbiol Biotechnol 23:931–937
Liu G, Zhou J, Wang J, Wang X, Jin R, Lv H (2011) Decolorization of azo dyes by Shewanella oneidensis MR-1 in the presence of humic acids. Appl Microbiol Biotechnol 91:417–424
Ogugbue CJ, Sawidis T, Oranusi NA (2010) Evaluation of colour removal in synthetic saline wastewater containing azo dyes using an immobilized halotolerant cell system. Ecol Eng 37:2056–2060
Parshetti GK, Telke AA, Kalyani DC, Govindwar SP (2010) Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J Hazard Mater 176:503–509
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigm 58:179–196
Pearce CI, Christie R, Boothman C, von Canstein H, Guthrie JT, Lloyd JR (2006) Reactive azo dye reduction by Shewanella strain J18 143. Biotechnol Bioeng 95:692–703
Salokhe MD, Govindwar SP (1999) Effect of carbon source on the biotransformation enzymes in Serratia marcescens. World J Microbiol Biotechnol 15:259–263
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2009) Decolorization and biodegradation of textile dye navy blue HER by Trichosporon beigelii NCIM-3326. J Hazard Mater 166:1421–1428
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2010) Decolorization and biodegradation of reactive dyes and dye wastewater by a developed bacterial consortium. Biodegradation 21:999–1015
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Engrs 42:138–157
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80
Telke AA, Joshi SM, Jadhav SU, Tamboli DP, Govindwar SP (2010) Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT Biodegradation 21:283–296
Uddin MS, Zhou J, Qu Y, Guo J, Wang P, Zhao L (2007) Biodecolorization of azo dyes acid red B under high salinity conditions. Bull Environ Contam Toxicol 79:440–444
Wu J, Kim KS, Sung NC, Kim CH, Lee YC (2009) Isolation and characterization of Shewanella oneidensis WL-7 capable of decolorizing azo dye reactive black 5. J Gen Appl Microbiol 55:51–55
Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H (2007a) Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal microflora. Appl Environ Microbiol 73:7759–7762
Xu M, Guo J, Kong X, Chen X, Sun G (2007b) Fe(III)-enhanced azo reduction by Shewanella decolorationis S12. Appl Microbiol Biotechnol 74:1342–1349
Xu M, Guo J, Sun G (2007c) Biodegradation of textile azo dye by Shewanella decolorationis S12 under microaerophilic conditions. Appl Microbiol Biotechnol 76:719–726
Yang YY, Du LN, Wang G, Jia XM, Zhao YH (2011) The decolorisation and mechanism of Shewanella oneidensis MR-1 for methyl orange and acid yellow 199 under microaerophilic conditions. Water Sci Technol 63:956–963
Yoon JH, Yeo SH, Kim IG, Oh TK (2004) Shewanella marisflavi sp. nov. and Shewanella aquimarina sp. nov., slightly halophilic organisms isolated from sea water of the Yellow Sea in Korea. Int J Syst Evol Microbiol 54:2347–2352
Yu L, Li WW, Lam MHW, Yu HQ, Wu C (2011) Isolation and characterization of a Klebsiella oxytoca strain for simultaneous azo-dye anaerobic reduction and bio-hydrogen production. Appl Microbiol Biotechnol 95:255–262. doi:10.1007/s00253-011-3688-2
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (nos. 51008044 and 21077019) and China Postdoctoral Science Foundation (no. 201104596).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1057 kb)
Rights and permissions
About this article
Cite this article
Liu, G., Zhou, J., Meng, X. et al. Decolorization of azo dyes by marine Shewanella strains under saline conditions. Appl Microbiol Biotechnol 97, 4187–4197 (2013). https://doi.org/10.1007/s00253-012-4216-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4216-8