Abstract
Increasing the ionic strength of the electrolyte in a microbial fuel cell (MFC) can remarkably increase power output due to the reduction of internal resistance. However, only a few bacterial strains are capable of producing electricity at a very high ionic strength. In this report, we demonstrate a newly isolated strain EP1, belonging to Shewanella marisflavi based on polyphasic analysis, which could reduce Fe(III) and generate power at a high ionic strength of up to 1,488 mM (8% NaCl) using lactate as the electron donor. Using this bacterium, a measured maximum power density of 3.6 mW/m2 was achieved at an ionic strength of 291 mM. The maximum power density was increased by 167% to 9.6 mW/m2 when ionic strength was increased to 1,146 mM. However, further increasing the ionic strength to 1,488 mM resulted in a decrease in power density to 5.2 mW/m2. Quantification of the internal resistance distribution revealed that electrolyte resistance was greatly reduced from 1,178 to 50 Ω when ionic strength increased from 291 to 1,488 mM. These results indicate that isolation of specific bacterial strains can effectively improve power generation in some MFC applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) are devices that convert chemical energy directly from organic matter using electrochemically active bacteria as catalysts to generate electrical energy. Although the original concept was proposed early in the last century (Potter 1911), MFCs have received a great deal of attention only in the last decade (Kim et al. 1999; Wei and Zhang 2007). In an MFC, electrons released by microbes can be delivered to an anode and flow to a cathode through an external circuit operated under a load. Meanwhile, protons are generated in the anode and charges transfer across a proton exchange membrane (PEM), reacting with the electrons and oxygen on the cathode to form water. One of the most exciting applications of MFCs is their use as benthic unattended generators to power electrical detectors (Tender et al. 2002) or meteorological buoys (Tender et al. 2008) in remote locations, such as the bottom of the ocean or river. MFCs can also be developed as a promising tool to treat wastewater and simultaneously generate electricity.
In practice, the measured MFC voltage is substantially lower than the maximum thermodynamically attainable MFC voltage. This discrepancy, referred to as overvoltage or overpotential, is due to various potential losses inherent within the MFC that cause high internal resistance (Logan et al. 2006). Various factors affecting the performance of MFCs have been identified, including anodic and cathodic catalytic activities, substrate diffusion, and proton mass transfer (Kim et al. 2007). Of these factors, proton mass transfer is considered the main constraint to increasing the performance of MFCs as it causes high internal resistance dominantly captured into electrolyte resistance in single-chamber MFCs or into membrane resistance and electrolyte resistance in two-chamber MFCs (Fan et al. 2008). Removing the membrane, minimizing the electrode spacing, and increasing the ionic strength (IS) could efficiently reduce these losses caused mainly by proton mass transfer (Kim et al. 2007; Watanabe 2008). Increasing the solution ionic strength of electrolyte by adding NaCl (up to 400 mM) or KCl (up to 300 mM) could considerably increase power output (Liu et al. 2005; Oh and Logan 2006). Moreover, quantification of the internal resistance distribution of single-chamber MFCs shows that electrolyte resistance accounts for 36–78% of total resistance, which can be decreased from 208 to 85.9 Ω by increasing the phosphate buffer concentration from 50 to 200 mM (Fan et al. 2008). In previous work, however, the maximum ionic strength was maintained at relatively low levels due to the use of mixed cultures derived from freshwater and which cannot tolerate very high salt concentrations. Though MFCs using mixed cultures often achieve higher power output than those using pure cultures (Rabaey et al. 2004, 2005), a recently isolated bacterium can produce electricity at a higher power density than can mixed cultures in the same device (Xing et al. 2008). Furthermore, the pure culture can also serve as an appropriate model for electron transfer in MFCs to investigate the physiology of this process (Lanthier et al. 2008).
In this study, we report a newly isolated current-producing bacterium Shewanella marisflavi strain EP1 that could generate power at a high ionic strength in MFC. In addition, we also investigated the electrolyte resistance contribution to total internal resistance by varying the electrolyte ionic strength.
Materials and methods
Media and growth conditions
To enrich the electricity-producing bacteria in MFC, a sterile basal medium (BM) with 2% NaCl was employed as the routine culture medium (RCM). The basal medium (IS = 120 mM) consisted of (per liter): 1.3 g K2HPO4, 0.45 g KH2PO4, 1.19 g (NH4)2SO4, 0.25 g MgSO4, 0.05 g CaCl2, 0.02 g l-arginine HCl, 0.02 g l-glutamic acid, 0.02 g l-serine, 100 ml Wolfe’s minerals, and 25 mM HEPES as buffering agent. The initial pH of the culture medium was adjusted to 7.0. The cathodic electrolyte was devoid of the three amino acids and supplemented with 0.6 g/l sodium chloride to balance the osmotic pressure. Anoxic medium was prepared by boiling under N2 (99.999%) to remove dissolved oxygen and dispensed under N2 (99.999%) into anaerobic tubes or serum bottles (Bellco Glass, Inc., USA). Strain EP1 was routinely cultivated in RCM (2% NaCl) with l-lactate as the sole electron donor and carbon source at 25°C aerobically with shaking at 180 rpm or anaerobically static. Appropriate electron acceptors such as fumarate and ferric citrate were used in anoxic cultures.
Isolation of current-producing bacteria
Samples were collected from coastal sediments in Xiamen, China. The sediment was inoculated into the MFC anode chamber filled with RCM. Strain EP1 was isolated from one MFC fed with lactate operated for 2 months. Bacterial biofilm formed on graphite electrode was scraped with a sterile razor in an anaerobic glove box (Forma Anaerobic System, model 1029) and inoculated into anaerobic RCM using lactate as electron donor and ferric citrate as electron acceptor in anaerobic pressure tubes (Bellco Glass, Inc.). Bacterial isolation was performed by serial dilution of an enrichment culture on solid medium using strict anaerobic roll-tube technique (Hungate 1969). RCM supplemented with low-melting-point agarose (1.5%, Sigma) was employed as solid medium in the roll-tube method. In addition, 10 mM lactate and 50 mM ferric citrate were used as electron donor and acceptor, respectively. Red-brown single colonies were picked in glove box, and the isolation procedure was repeated at least three times until a pure culture was obtained. Once purified, the isolate was grown in RCM supplemented with lactate (20 mM) and ferric citrate (50 mM) or in Difco 2216 marine broth.
Polyphasic phylogenetic analysis, DNA–DNA hybridization, and morphology analysis
Cells from 10 ml culture grown on lactate and fumarate were collected by centrifugation. Genomic DNA was extracted from strain EP1 using E.Z.N.A.™ Bacterial DNA Kit (OMEGA, USA) following the manufacturer’s instructions. The 16S rRNA gene was amplified with ExTaq (TaKaRa, Dalian, China) using the 27f primer (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1525r primer (5′-AAGGAGGTGWTCCARCC-3′). PCR mixtures and amplification were performed as described elsewhere (Coates et al. 2001). The gyrB gene fragment was determined as reported previously (Yamamoto and Harayama 1995). PCR products were purified and ligated into vector pMD-18T (TaKaRa), and sequencing was performed by Invitrogen, Inc. China. The sequences obtained were aligned and compared with the GenBank using BLAST algorithms. DNA–DNA hybridization was performed as described previously (Ezaki et al. 1989). Hybridization of each sample was examined with five replications. The highest and lowest relatedness values for each sample were excluded, and the mean of the three remaining values was reported as the result.
Gram staining, cell morphology, and flagellum type were examined by light microscopy (Nikon, E600) and transmission electron microscopy using the exponentially growing cells. Growth at various NaCl concentrations was measured by examining Fe(III) reduction in anoxic BM with l-lactate and ferric citrate.
MFC construction and operation
Two-chamber MFCs (H type) were employed in this study. Reactors were assembled by joining two medium bottles with glass tubes having 26-mm outside diameter suitable for holding the PEM (Nafion 117, Dupont Co.) that was clamped between two glass tubes fitted with rubber gaskets. Each chamber was sealed with a silicon rubber fixed with a screw cap. Sampling ports sealed with butyl stoppers and aluminum crimps were added to the sides and top of each chamber, while electrodes were introduced from the top by feeding a wire through a butyl stopper in the sampling port. The volume of each chamber, with the electrode, was approximately 250 ml of the medium with a 50-ml headspace. The electrodes for fuel cells were 1×2×8-cm sticks of unpolished graphite (Grade GM42, Sanye Carbon Co., Ltd., Beijing). The distance between electrodes was approximately 18 cm. Electrodes were rinsed in 1 N NaOH and 1 N HCl to remove possible biomass and metal contamination and then soaked in deionization (DI) water for 1 day before testing. Corrosion-proof silicon rubber-coated wire was directly embedded into the hole drilled on the top side of the graphite stick, and holes were filled with conductive epoxy (type HE20; Epoxy Technology, USA) and sealed with insulated epoxy (type 730Black; Epoxy Technology). The PEM used in these reactors had a diameter of 2.2 cm (area, 3.8 cm2). The PEM was pretreated by boiling in DI water and H2O2 (30%) followed by 1 M H2SO4 and DI water, each for 1 h, and then stored in DI water before use. The anode chamber was filled with 250 ml anaerobic BM with various NaCl concentrations and continuously sparged with pure nitrogen to maintain anoxic conditions. The initial lactate concentrations for all tests were 10 mM. The cathode compartment was filled with 250-ml cathodic electrolyte into which air was continuously sparged to supply oxygen as the electron acceptor.
A set of MFCs was operated to investigate the effects of ionic strength by changing the salt concentration (1%, 2%, 4%, 6%, or 8%). At each ionic strength, MFCs were run for at least two batches for reproducible power generation. Polarization curves were obtained by gradually lowering external resistance from 19.2 kΩ to 100 Ω. For inoculation of MFC with one specific ionic strength, cells of strain EP1 were pre-grown in 100 ml anaerobic BM containing 25 mM lactate and 50 mM ferric citrate with corresponding ionic strength and collected by centrifugation and resuspended in 5 ml anaerobic BM. These cells were inoculated into the anode chamber using a syringe in the glove box. The device was removed from the glove box, and voltage across a 1,000 Ω resistor was measured using a multimeter. MFCs were operated in a temperature-controlled room (25°C), and both chambers were mixed using a magnetic stirrer. Potassium ferricyanide (50 mM) was used as the electron acceptor in the cathode chamber in some tests.
Analyses and calculations
Voltage was measured hourly with a precision multimeter and a data acquisition system (2000, Keithley, USA). Power (P) was calculated according to P = IV (I = V/R), where I (ampere) is current, V (volts) is voltage, and R (ohm) is external resistance. Power density normalized to anode area or reactor volume and Coulombic efficiency (CE) were calculated as previously described (Logan et al. 2006; Lanthier et al. 2008).
The distribution of internal resistance was calculated as described previously (Fan et al. 2008). Accordingly, Eq. 8 described in the reference could be simplified as follows:
where E (millivolts) is the external voltage; E b (millivolts) is the linear extrapolation open circuit voltage; R s (ohm) is the sum of anodic resistance, cathodic resistance, and membrane resistance; R e (ohm) is the electrolyte resistance; and I (milliampere) is the current.
Accordingly, the total resistance of MFC could be indicated as:
To calculate the power P (milliwatts), Eqs. 1 and 2 could be transformed into:
The SOLVER function in Microsoft Office Excel was employed, and the sum of squares of differences between the measured and calculated data was minimized by iterating the initial guess 1,000 times over a wide range of possible parameter values with a quasi-Newton search method.
Conductivity was measured using a conductivity meter (sensION™5, HACH). Fe(III) reduction was monitored by measuring the concentration of Fe(II), which was determined with the ferrozine assay (Phillips and Lovley 1987). Biomass from plankton, electrode, and PEM was collected as described previously (Bond and Lovley 2003), and protein concentration was measured using the BCA™ Protein Assay Kit (Thermo Scientific, USA) following the manufacturer’s instruction.
Statistical analysis
The numerical data from biologically independent experiments were analyzed by one-way ANOVA to calculate the mean and standard deviation of repeated assays. The comparison of differences among the groups was carried out by Student’s t test. Significance was defined as p < 0.05.
Nucleotide sequence accession number
The nucleotide sequences of the 16S rRNA gene and the gyrB gene fragment reported in this study were registered in the GenBank nucleotide sequence databases under accession numbers FJ589035 and FJ589038, respectively.
Results
Isolation and characterization of strain EP1
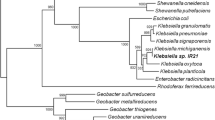
Several samples from the coastal sediments were inoculated into MFCs for enrichment of current-producing isolates. After serial transfers and dilutions to extinction, one isolate designated as strain EP1 was purified. The MFC inoculated with the pure culture of EP1 produced substantial current densities using the electrolyte containing 2% NaCl. Cells of strain EP1 were Gram-negative and rod-shaped with 0.3- to 1.0-μm width and 2.5- to 4.0-μm length and motile by means of a polar flagellum (data not shown). Strain EP1 could reduce nitrate, fumarate, ferric citrate, and Fe(III) oxide, with lactate as electron donor in anaerobic cultures. On the basis of 16S rRNA gene sequence analyses, strain EP1 showed high homologies with those of Shewanella species (Fig. 1). Strain EP1 exhibited its closest relatedness (99.9%) to the type strain S. marisflavi JCM 12192T. Because the 16S rDNA sequence lacks sufficient specificity for the differentiation of close relatives in this genus, the more rapidly evolving gyrB gene was also employed. The gyrB gene sequence of EP1 shared 98.1% similarity to that of S. marisflavi JCM 12192T (Fig. 1), which was far higher than the suggested species cutoff value of 90% for gyrB sequences in Shewanella genus (Venkateswaran et al. 1999). A further DNA–DNA hybridization assay revealed a high relatedness (91.7%) between the EP1 and S. marisflavi JCM 12192T (Table 1). On the basis of these phylogenetic and phenotypic characteristics, strain EP1 was classified as a member of species S. marisflavi. The new isolate was deposited in the China Center for Type Culture Collection (CCTCC) under accession number CCTCC M 209016.
Fe(III) reduction at various ionic concentrations
To better understand the physiology of Fe(III)-reducing bacteria in MFCs, anaerobic cultures with ferric iron as electron acceptor were often applied to investigate the growth conditions. Here, ferric citrate was used as the electron acceptor to test the growth of strain EP1 with lactate as electron donor at various ionic strengths. Cells pre-grown in 10 ml anaerobic RCM with fumarate and lactate were collected by centrifugation, washed twice, and resuspended in 1 ml anoxic RCM to inoculate the 100 ml ferric citrate culture. The Fe(II) production determined was shown in Fig. 2. It was found that no Fe(II) production was observed at 0% NaCl, demonstrating that salts were indispensable for the growth. With 8 mM lactate and 50 mM ferric citrate, the Fe(II) concentration eventually reached the maximum of 32 mM at 1%, 4%, and 6% NaCl. The results indicated that strain EP1 was able to completely utilize lactate as electron donor in the range of 1–6% NaCl, but not at 8%. In addition, differences of the time required for reaching the maximum Fe(III) reduction among various NaCl concentrations demonstrated that strain EP1 could grow well at 1%, 4%, and 6% NaCl and that its growth was slightly inhibited at 8% NaCl, presumably due to the excessively high ionic strength.
Fe(III) reduction at different ionic concentrations by strain EP1 with 8 mM l-lactate as electron donor and 50 mM ferric citrate as electron acceptor. The percentages on the right indicate the concentrations of NaCl. Each point represents the mean of triplicate cultures within ±1% standard deviations
Differential growth rates at different salt concentrations reflected the tolerance of strain EP1 for various ionic strengths or osmotic pressures. The growth of strain EP1 in such a wide range of NaCl concentrations (1–8%) made it an excellent instance for investigating electricity generation at various ionic strengths.
Current generation at different ionic strengths
Since strain EP1 could reduce Fe(III) at high salt concentrations, this strain was applied to better understand power generation at various ionic strengths. To initiate the growth of strain EP1 in MFC, the sterile anaerobic anode chamber with a certain ionic concentration was inoculated with 5 ml stationary phase culture of strain EP1 that pre-grew at the same ionic concentration with ferric citrate as the electron acceptor. All tests were performed with a 1,000 Ω fixed external resistance, and 10 mM lactate was provided as the electron donor with electrode as electron acceptor. The results showed that rates of current increase with 1–6% NaCl ranged from approximately 2.0 to 2.4 µA/h, whereas the rate with 8% NaCl was 1.2 µA/h (Fig. 3). These were consistent with the growth of strain EP1 using ferric citrate as electron acceptor in serum bottle cultures as described above.
Electricity generation by S. marisflavi EP1 at various ionic concentrations in the MFCs. Lactate was provided as an electron donor and graphite anode as the sole terminal electron acceptor. A 1,000 Ω resistor was applied as external circuit resistance in all tests. The percentages and CE on the top indicate the concentrations of NaCl and Coulombic efficiency, respectively
After a period of gradual increase, the current production eventually reached a plateau (Fig. 3). A maximum voltage of 134 mV was obtained using the electrolyte with a NaCl concentration of 1% (IS = 291 mM). With 10 mM lactate as electron donor and a fixed external resistance, the maximum voltage was increased gradually when the electrolyte ionic concentration was increased, reaching a maximum of 219 mV at 6% NaCl (IS = 1,146 mM). This maximum voltage was 63% larger than that using the electrolyte with a solution IS of 291 mM, and the corresponding maximum power density was increased by 167% from 3.6 to 9.6 mW/m2. However, further increasing the ionic strength (8% NaCl) led to a decrease of the maximum voltage and power density to 163 mV and 5.2 mW/m2, respectively. It was found that increasing the ionic strength of the electrolyte could increase the current by reducing the internal resistance of MFC, suggesting that bacterial growth was not greatly affected. However, the adverse effect of medium salinity on bacterial growth was obvious. Strain EP1 could not grow well at such a high ionic strength (1,488 mM).
To investigate the overall effects of high ionic strength on power generation, biomass recovered from three different parts of the anode chamber and the CE were determined. Substantial amounts of biomass were obtained from the anode chamber with higher quantities of planktonic biomass than those of electrode and PEM biomass at each ionic strength (Fig. 6). The total biomass in the anode chamber at 1%, 2%, 4%, and 6% NaCl were 8.6, 9.2, 8.7, and 8.3 mg, respectively. Biomass from plankton, electrode, and PEM at 8% NaCl all declined compared with those at 1–6% NaCl. The total biomass at 8% NaCl declined to only 4.1 mg. The CE values of MFCs at 1–6% NaCl were increased with ionic strength or the stable maximum current density (Fig. 3), reaching 54.6% at 4% NaCl and 57.2% at 6% NaCl. The CE at 8% NaCl decreased to 45.6% with the stable maximum current density decline.
Internal resistance distribution
To explore the effect of solution ionic strength on the internal resistance distribution and power output of MFCs using dissolved oxygen or ferricyanide as electron acceptors, polarization curves produced by strain EP1 were generated by varying the external resistance between the electrodes (Fig. 4). The total internal resistance R int in the two-chamber MFCs could be divided into anodic resistance, cathodic resistance, membrane resistance, and electrolyte resistance. During the tests with salt concentrations of 1–6%, bacterial growth was not affected by ionic concentration and the parameters of MFCs were unchanged. Therefore, anodic resistance, cathodic resistance, and membrane resistance were fixed. For the MFC with 8% NaCl, the anodic resistance might be significantly different since the catalytic activities of bacterial cells were depressed by the high ionic strength.
Comparison of the observed voltage (a) and power output (b) of air-cathode MFCs with different solution ionic strengths (1%, 2%, 4%, 6%, and 8% NaCl) and the ferricyanide-cathode condition with 2% and 6% NaCl (c). The calculated voltages and currents are shown as dash lines or solid lines. Insets indicate the correlation between the observed and the calculated data
When using Eq. 1 to fit the polarization data, those with actual current less than 0.03 mA were excluded since they were probably located in the activation loss-dominated region and departed away from the linear region of the polarization curve (Logan et al. 2006). E b, R s, R e, and R int were calculated using the SOLVER function, and the maximum value of the quadratic Eq. 3 (P max) was also derived. The results with corresponding ionic strengths were listed in Table 2.
According to the calculated values from Table 2, we reconstructed the polarization curves and power curves as shown in Fig. 4. Based on the functions of these curves, the calculated voltage and power data points fit very well the measured data points with correlation coefficients R 2 > 0.99 (n = 58). For the five air-cathode MFCs, electrolyte resistance ranged widely from 3% to 60%. Increasing the solution ionic strength could dramatically reduce electrolyte resistance within the IS range of 219 to 1,146 mM. However, further increasing IS to 1,488 mM only slightly decreased electrolyte resistance from 72 to 50 Ω. This was similar to the findings of a previous report in which internal resistance was only reduced from 83 to 79 Ω when IS was increased from 300 to 400 mM (Liu et al. 2005). By adding NaCl, the conductivity of the electrolyte nearly increased linearly, but electrolyte resistance was lowered scarcely, and the curve tended to become flat when the IS reached a higher value (Fig. 5). Moreover, the internal resistance increased from 861 to 1,551 Ω when the ionic strength increased from 1,146 to 1,488 mM. This phenomenon was reported in previous studies, and the internal resistance increased at high IS presumably due to the increase of anodic resistance caused by the limited bacterial activities at such an IS. Along with the decrease of electrolyte resistance (1–6% NaCl), on the other hand, the sum of anodic resistance, cathodic resistance, and membrane resistance contributed from 40% to 92%, although R s was unchanged in these tests. Using ferricyanide instead of air as cathodic electron acceptor, the calculated E b (787 mV) was much higher than 449 or 371 mV, and the corresponding R e, R s, or R int were all decreased. However, the percentage contribution to R int of each component was not changed distinctly. In addition, the relatively low internal resistance of MFCs with ferricyanide cathode also contributed to the higher power generation. The calculated maximum power of MFC with ferricyanide cathode and 6% NaCl was about one order of magnitude higher than that of MFC with air cathode and 1% NaCl, indicating that cathode and electrolyte resistances were the major factors limiting cell performance.
Discussion
For the similarities between the insoluble Fe(III) oxides and electrode, Shewanella putrefaciens that could breathe Fe(III) oxides was first thought to be able to reduce electrode to produce electricity in MFCs (Kim et al. 1999). From then on, various Shewanella strains were developed as electrogenic biocatalysts in MFCs, and intensive studies were conducted to understand the mechanism of electron transfer to electrode using Shewanella oneidensis MR-1 as a model microorganism (Lanthier et al. 2008). However, to our knowledge, S. marisflavi has not been reported as an electrogenic bacterium to generate power in MFCs. Our results demonstrate for the first time that a bacterium from S. marisflavi species can transfer electrons to an electrode to generate current at high ionic strength in an MFC. Although it has been demonstrated that the type strain of S. marisflavi can tolerate 8% NaCl in aerobic growth (Yoon et al. 2004), there is no evidence for its capacity to produce current at such an ionic strength in an MFC. Rhodopseudomonas palustris DX-1, for example, can produce very high current and power density, but the type strain of R. palustris cannot generate power under the same conditions (Xing et al. 2008).
In the present study, strain EP1 isolated from costal marine sediments could grow and produce electricity at a wide range of solution ionic strengths. Based on our knowledge, this is the first time electricity generation is demonstrated at such a high ionic strength (1,488 mM) by a pure culture. Strain EP1 produced 44 mA/m2 and 9.6 mW/m2 at the ionic strength of 1,146 mM, which was maintained at levels of power output comparable with those obtained from similar architected H-type MFCs with other species (Lanthier et al. 2008; Bond and Lovley 2003). Although the amount of power density was very small compared with those produced by different microorganisms in other MFC types (Xing et al. 2008; Ringeisen et al. 2006), low performance was mainly the result of the architecture of the MFC system but not the bacterium as this H-type MFC had high internal resistance, which could significantly affect the performance (Watanabe 2008).
Most of the biomass in the anode chamber was planktonic, indicating that strain EP1 may employ a similar strategy for growth with electrode as the electron acceptor as observed in S. oneidensis (Lanthier et al. 2008). Shewanella species are well known for their capacity to release electron shuttles out of cells, which help them transfer electrons to electrodes without direct contact (Marsili et al. 2008). The CEs obtained in this study (42–57%) are generally maintained at levels comparable with those using the same type of cell system (Lanthier et al. 2008). CE increased with current density at different ionic strengths. This pattern was also observed in a pure culture MFC (Xing et al. 2008). However, a high current density does not always infer a high CE. This is mainly caused by using MFCs with different configurations. MFC, in the absence of PEM, produced greater power density than MFC in the presence of PEM, but the CE remained very low (Liu and Logan 2004). The most likely reason for the low CE is the aerobic loss of substrate caused by the diffusion of oxygen from the cathode chamber. In the absence of PEM, oxygen easily diffuses to the anode and causes substantial loss of substrate. In the same MFC, oxygen diffusion rate is fixed, and the loss of substrate is in proportion to time. When the same amount of electrons is released, the longer time a low current runs for, the greater the loss of electrons and the lower the CE (Fig. 6).
The resistance distribution analysis shows that a low ionic strength may be a limiting factor for MFC power output and that increasing the solution ionic strength could significantly decrease electrolyte resistance and total resistance within the range of ionic strengths that the bacteria could tolerate. This indicates that the major contribution to total internal resistance was likely the electrolyte resistance when using a low solution ionic strength, and the major contribution could turn to the other resistances after the IS increased to a comparatively higher level. Through varying the area of PEM in a previous report (Fan et al. 2008), it was concluded that the main contribution to total internal resistance came from the membrane resistance component. However, the electrolyte resistance in our study was identified to contribute 60% of total internal resistance when the ionic strength of the electrolyte was maintained at a low level (1% NaCl). This discrepancy is presumably due to the different electrode material, MFC configurations, and biocatalysts. Our results suggest that electrolyte resistance could be another major contributor within internal resistance and that increasing ionic strength could greatly enhance the power output of two-chamber MFCs. With such a salt-tolerant strain applied at high ionic strength in the single-chamber MFC system, it is easy to deduce that internal resistance could be further reduced and power output could increase to a higher level. Our results also suggest that searching for and isolating novel microorganisms that are more halophilic and even acidophilic from various environments could be a new approach to improve performance in some MFCs that employ pure cultures.
References
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555
Coates JD, Bhupathiraju VK, Achenbach LA, McInerney MJ, Lovley DR (2001) Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int J Syst Evol Microbiol 51:581–588
Ezaki T, Hashimoto Y, Yabuuchi E (1989) Fluorometric deoxyribonucleic acid–deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol 39:224–229
Fan Y, Sharbrough E, Liu H (2008) Quantification of the internal resistance distribution of microbial fuel cells. Environ Sci Technol 42:8101–8107
Hungate R (1969) A roll tube method for cultivation of strict anaerobes. Methods Microbiol 3B:117–132
Kim BH, Kim HJ, Hyum MS, Park DH (1999) Direct electrode reaction of an Fe(III)-reducing bacterium, Shewanella putrefaciens. J Microbiol Biotechnol 9:127–131
Kim BH, Chang IS, Gadd GM (2007) Challenges in microbial fuel cell development and operation. Appl Microbiol Biotechnol 76:485–494
Lanthier M, Gregory KB, Lovley DR (2008) Growth with high planktonic biomass in Shewanella oneidensis fuel cells. FEMS Microbiol Lett 278:29–35
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Liu H, Cheng S, Logan BE (2005) Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ Sci Technol 39:5488–5493
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA 105:3968–3973
Oh S, Logan BE (2006) Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl Microbiol Biotechnol 70:162–169
Phillips EJP, Lovley DR (1987) Determination of Fe(III) and Fe(II) in oxalate extracts of sediment. Soil Sci Soc Am J 51:938–941
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc Lond B 84:260–276
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382
Rabaey K, Boon N, Hofte M, Verstraete W (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39:3401–3408
Ringeisen BR, Henderson E, Wu PK, Pietron J, Ray R, Little B, Biffinger JC, Jones-Meehan JM (2006) High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ Sci Technol 40:2629–2634
Tender LM, Reimers CE, Stecher HA, Holmes DE, Bond DR, Lowy DA, Piblobello K, Fertig S, Lovley DR (2002) Harnessing microbially generated power on the seafloor. Nat Biotechnol 20:821–825
Tender LM, Gray SA, Grovemanb E, Lowyc DA, Kauffmand P, Melhado J, Tyce RC, Flynn D, Petrecca R, Dobarro J (2008) The first demonstration of a microbial fuel cell as a viable power supply: powering a meteorological buoy. J Power Sources 179:571–575
Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson KH (1999) Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 49:705–724
Watanabe K (2008) Recent developments in microbial fuel cell technologies for sustainable bioenergy. J Biosci Bioeng 106:528–536
Wei D, Zhang X (2007) Current production by a deep-sea strain Shewanella sp. DS1. Curr Microbiol 55:497–500
Xing D, Zuo Y, Cheng S, Regan JM, Logan BE (2008) Electricity generation by Rhodopseudomonas palustris DX-1. Environ Sci Technol 42:4146–4151
Yamamoto S, Harayama S (1995) PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol 61:1104–1109
Yoon J-H, Yeo S-H, Kim I-G, Oh T-K (2004) Shewanella marisflavi sp. nov. and Shewanella aquimarina sp. nov., slightly halophilic organisms isolated from sea water of the Yellow Sea in Korea. Int J Syst Evol Microbiol 54:2347–2352
Acknowledgments
This work was supported by the China Ocean Mineral Resources R & D Association (DYXM-115-02-2-15 and DYXM-115-02-2-04) and the National Natural Science Foundation of China (40876070).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, J., Sun, B. & Zhang, X. Electricity generation at high ionic strength in microbial fuel cell by a newly isolated Shewanella marisflavi EP1. Appl Microbiol Biotechnol 85, 1141–1149 (2010). https://doi.org/10.1007/s00253-009-2259-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2259-2