Abstract
In heterologous protein production by filamentous fungi, target proteins are expressed as fusions with homologous secretory proteins, called carriers, for higher production yields. Although carrier fusion is thought to overcome the bottleneck in transcriptional and (post)translational processes during heterologous protein production, there is limited knowledge of its physiological effects on the host strain. In this study, we performed DNA microarray analysis by comparing gene expression patterns of two Aspergillus oryzae strains expressing either carrier- or non-carrier-fused bovine chymosin (CHY). When CHY was expressed as a fusion with α-amylase (AmyB), the production level increased by approximately 2-fold as compared with the non-carrier-fused CHY. DNA microarray analysis revealed that the carrier fusion significantly up-regulated many genes involved in endoplasmic reticulum (ER) protein-folding and secretion. Consistently, hacA transcripts were efficiently spliced in the strain expressing the carrier-fused CHY, indicating an unfolded protein response (UPR). The carrier-fused CHY was detected intracellularly without processing at the Kex2 cleavage site, which is likely recognized in the Golgi, and the carrier fusion delayed extracellular CHY production in the early growth phase as compared with the non-carrier-fused expression. Taken together, our data suggest a proposal that the carrier fusion temporarily accumulates the carrier-fused CHY in the ER and significantly induces UPR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillus oryzae is a filamentous fungus considered to be one of the preferred hosts for heterologous protein production because of its prominent protein secretion ability. It is also regarded as safe due to its long history of use in Japanese fermented foods. Genome sequencing of A. oryzae (Machida et al. 2005) has enabled comprehensive gene expression analysis (Kimura et al. 2008). Recent developments in host–vector systems and highly efficient gene disruption techniques (Jin et al. 2004; Maruyama and Kitamoto 2008) have facilitated the manipulation of many genes in A. oryzae for improving protein production. We previously reported an improvement in heterologous protein production by A. oryzae through multiple protease disruption (Jin et al. 2007; Yoon et al. 2009, 2011), disruption of the gene encoding a vacuolar protein sorting receptor (Yoon et al. 2010) and repression of α-amylase genes (Nemoto et al. 2009a).
The production levels of proteins from higher eukaryotes (animals and plants) by A. oryzae are generally lower than those of homologous (fungal) proteins. For the efficient production of proteins from higher eukaryotes, they are generally fused with carrier proteins such as α-amylase (AmyB) or glucoamylase (GlaA) (Tsuchiya et al. 1994; Nakajima et al. 2006; Ito et al. 2007; Jin et al. 2007). In Aspergillus niger, where glucoamylase (GlaA) is often used as a carrier, the carrier fusion is suggested to overcome transcriptional and (post)translational bottlenecks in heterologous protein production (Gouka et al. 1997). However, the mechanism by which the carrier fusion improves heterologous protein production has not been elucidated in detail.
The unfolded protein response (UPR) is an intracellular signaling network that transmits information concerning the overload of unfolded proteins in the endoplasmic reticulum (ER) (Ron and Walter 2007). This response is accompanied by the transcriptional activation of genes involved in ER protein-folding and secretion. Several analyses of transcriptional response related with UPR in heterologous protein production by filamentous fungi have been reported. The production of some heterologous proteins up-regulates the expression of genes encoding ER chaperones (Punt et al. 1998; Wang and Ward 2000; Wang et al. 2003). DNA microarray analysis has revealed that genes involved in ER protein-folding and secretion are up-regulated upon expression of non-carrier-fused chymosin (CHY) in Aspergillus nidulans (Sims et al. 2005) and carrier-fused tissue plasminogen activator (tPA) in A. niger (Guillemette et al. 2007). However, a direct comparative analysis between the overall transcriptional response to the expression of carrier- and non-carrier-fused heterologous proteins has not been reported.
In order to further understand the physiological effects of carrier fusions in heterologous protein production, we performed a transcriptional analysis with a whole-genome DNA microarray of A. oryzae. We used CHY as a model heterologous protein and examined the effects of a carrier fusion on UPR and production of the target protein into the culture supernatant.
Materials and methods
Strains, media and transformation
A. oryzae wild-type strain RIB40 was used as a DNA donor and A. oryzae strain niaD300 (niaD −; Minetoki et al. 1996) was used for CHY production. The transformants used in this study are listed in Table 1. Escherichia coli DH5α was used for DNA manipulation. DPY medium (2% dextrin, 1% polypeptone, 0.5% yeast extract, 0.5% KH2PO4, 0.05% MgSO4·7H2O, pH 5.5) and 5× DPY medium (10% dextrin, 5% polypeptone, 2.5% yeast extract, 0.5% KH2PO4, 0.05% MgSO4·7H2O, pH 5.5) were used to grow the A. oryzae strains. Czapek-Dox (CD) medium (0.3% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, 2% glucose, pH 5.5) was used for transformation. A. oryzae was transformed according to a previously reported method (Kitamoto 2002).
Plasmids for CHY production
The plasmids used for CHY expression were constructed using the MultiSite Gateway™ system (Invitrogen, Carlsbad, CA, USA) (Mabashi et al. 2006). All primers used in this study are listed in Table S1.
The plasmid pgAKCN (Nemoto et al. 2009b) was used for the expression of the carrier-fused CHY (Fig. 1). For the expression of the non-carrier-fused CHY, the proCHY gene was amplified with the primers sAmyB-prochymosin-F and prochymosin-cEC-R using pgAKCN as a template. A DNA fragment consisting of the amyB promoter and a region encoding the signal peptide of AmyB was amplified with the primers pAmyB-cEC-F and sAmyB-prochymosin-R, again using pgAKCN as a template. The two fragments were fused with the primers pAmyB-cEC-F and prochymosin-cEC-R, and introduced into pDONR221 (Invitrogen; for generation of the center entry clone) by the BP clonase reaction. The resultant center entry clone (pgEAaC) was mixed with the empty 5′ entry clone (pg5′non), the 3′ entry clone (pg3′TaN) containing the amyB terminator and niaD marker (Nemoto et al. 2009b), and the destination vector (pDEST™R4-R3; Invitrogen) for recombination using the LR clonase reaction. The resultant plasmid, pgACN, contains the proCHY gene with the sequence for the signal peptide of AmyB under the control of the amyB promoter along with the niaD marker (Fig. 1).
To construct the plasmid for expression of AmyB, the amyB promoter and its ORF were amplified with the primers pAmyB-cEC-F and pAmyB-ORFst-cEC-R using pgAKCN as template, and introduced into pDONR221 by the BP clonase reaction. The resultant center entry clone (pgEAA) was mixed with the empty 5′ entry clone (pg5′non), 3′ entry clone (pg3′TaN), and destination vector (pDEST™R4-R3) for recombination using the LR clonase reaction. The resultant plasmid, pgAMN, contained the amyB gene under the control of the amyB promoter along with the niaD marker (Fig. 1).
To construct the plasmid for the negative control, the plasmid pUNA containing the amyB promoter and terminator, and the niaD marker (Yamada et al. 1999) was used (Fig. 1).
To confirm integration of a single copy of the plasmid at the niaD locus, transformants were analyzed by Southern analysis (Fig. S1). Briefly, after electrophoresis the SalI-digested genomic DNA was transferred onto a Hybond N+ membrane (GE Healthcare, Amersham, Buckinghamshire, UK). The enhanced chemiluminescence (ECL) direct nucleic acid labeling and detection system (GE Healthcare) and luminescent image analyzer LAS-4000miniEPUV (Fuji Photo Film, Tokyo, Japan) were used for detection.
CHY production
Approximately 2 × 105 conidia of the CHY-expressing strains were inoculated into 20 ml of 5× DPY medium (Jin et al. 2007), and the culture was then incubated at 30 °C for 4 days with shaking. Alternatively, approximately 5 × 106 conidia of the CHY-expressing strains were inoculated into 100 ml of DPY medium, and the culture was then incubated at 30 °C with shaking. Four microliters of the culture supernatant was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a cellulose nitrate membrane Immobilon-NC (Millipore, Bedford, MA, USA) using a semi-dry blotting system (Nihon Eido, Tokyo, Japan). The membrane was immunostained using a polyclonal rabbit serum against CHY (Nordic Immunological Laboratories, Tilburg, Netherlands), anti-rabbit immunoglobulin G labeled with horseradish peroxidase (Vector Laboratories, Peterborough, UK), and the ECL Advance™ Western Blotting Detection kit (GE Healthcare). The immunostained membrane was analyzed with the luminescent image analyzer LAS-4000miniEPUV. Signal intensity was estimated using Science Lab 2005 Multi Gauge Ver3.0 (Fuji Photo Film).
The CHY activity was measured as described by Nemoto et al. (2009b). CHY production (mg/l) was calculated from a standard curve generated using authentic calf CHY (Sigma, St. Louis, MO, USA).
For the detection of intracellular CHY, mycelia grown in DPY medium were washed, frozen with liquid nitrogen, and then thoroughly ground into a powder using a Multi-beads shocker (Yasui Kikai, Osaka, Japan). Mycelial powder was suspended in extraction buffer (0.5 mM Tris–HCl (pH 7.5), 1 mM PMSF in methanol, 1% Protease inhibitor cocktail). Cell debris was removed by centrifugation at 2,000 × g at 4 °C for 10 min. The supernatants were then subjected to Western analysis using anti-CHY antibody.
DNA microarray analysis
Approximately 5 × 106 conidia were inoculated into 100 ml of DPY medium, and the culture was then shaken at 30 °C for 1 day. Mycelial pellets uniformly grown with a diameter of approximately 3 mm were harvested, frozen in liquid nitrogen and thoroughly ground into a powder using a Multi-beads shocker. Total RNA samples were extracted using the RNeasy Plant Mini Kit spin columns (Qiagen Sciences, Germantown, MD, USA) and dissolved in RNase-free water.
DNA microarray experiments were conducted according to the Affymetrix Gene Chip manual with an A. oryzae DNAchip personally designed by our laboratory with the assistance with Affymetrix (Santa Clara, CA, USA). The DNA microarray contains probe sets for 13,857 genes and 1,704 expressed sequence tags (ESTs) from A. oryzae strain RIB40 (Machida et al. 2005; Akao et al. 2007). The gene IDs registered in the A. oryzae genome database (http://nribf21.nrib.go.jp/CFGD/gnm.cgi?prj=02201&gnm=aor0-1) were referred. The ESTs selected for the DNA microarray do not overlap with the genes registered in the genome database (see A. oryzae EST database, http://nribf21.nrib.go.jp/EST2/index.html). Briefly, one cycle of cDNA synthesis was performed, and then biotin-labeled cRNA was amplified, followed by fragmentation. The fragmented cRNA was hybridized with the 25-mer oligonucleotide probe sets, each containing 16 probe pairs consisting of a perfect match probe (PM) and a mismatch probe (MM). After washing and staining, the probes were scanned with a GeneChip Scanner 3000 (Affymetrix).
Statistical analysis for signal intensities and “Detection p values” was performed with the algorithm of GeneChip Operating Software (GCOS) 1.4 (Affymetrix). Detection of the transcripts was assessed by “Detection Call” (present (P), absent (A), or marginal (M)) based on Detection p values. For normalization the trimmed mean signal intensities of the array was scaled to the target signal of 500 with the All Probe Sets scaling option.
Two RNA samples from independent cultures (flasks) were separately analyzed for each strain. The array data of the strains expressing the carrier- or non-carrier-fused CHY were compared with those of the negative control strain (transformed with pUNA; Fig. 1). The comparative analysis was performed by GCOS with four combinations of two independent experiments for each strain, which calculates “Signal Log Ratio” and “Change p values” to determine “Change Call” (increasing [I], marginally increasing [MI], no change [NC], marginally decreasing [MD] or decreasing [D]). Genes showing Change Calls “I” and “D” in all the four combinations were selected as up-regulated and down-regulated ones, respectively. Among up-regulated genes, those genes whose expression was detected (Detection Call = P) in the CHY-expressing strains were selected. Similarly, among down-regulated genes, those genes whose expression was detected in the negative control strain were selected. Fold changes converted from Signal Log Ratio in the four combinations of two independent experiments for each strain were averaged using Microsoft Excel. The genes showing up/down-regulation (fold change ≥1.5) in CHY-expressing strains compared with the negative control strain were selected. To clearly show the physiological effects of the heterologous protein expression on the host strain, genes showing signal intensities (after normalized scaling, see above) of more than 1,000 in at least one sample were selected. These genes were classified into functional categories designated by the Cluster of Orthologous Groups (COGs) according to the A. oryzae gene list of DOGAN (http://www.bio.nite.go.jp/dogan/MicroTop?GENOME_ID=ao).
Quantitative RT-PCR analysis
Quantitative RT-PCR analysis was performed as described previously (Kimura et al. 2008). Total RNA was prepared as described above, but was further treated with DNase (Qiagen Sciences) on a column and cDNA was synthesized using ReverTra-Ace reverse transcriptase (Toyobo, Osaka, Japan) with an oligo(dT)12–18 primer (Invitrogen). Real-time PCR was performed using LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics, Indianapolis, IN, USA) as instructed by the manufacturer. The primers used in this experiment are listed in Table S1. The expression of each gene was normalized to that of the β-actin gene.
Results
Increased CHY production by AmyB carrier fusion
In this study, bovine CHY was chosen as a model heterologous protein. CHY is secreted as proCHY and converts into mature CHY when the prosequence is autocatalytically cleaved at low pH (Pedersen et al. 1979). A. oryzae α-amylase (AmyB) was used for the carrier fusion because such fusions have been shown to be effective in heterologous protein production by A. oryzae (Nakajima et al. 2006; Ito et al. 2007; Jin et al. 2007; Nemoto et al. 2009b; Yoon et al. 2009).
For expression of the carrier-fused CHY, we used a previously constructed plasmid (Nemoto et al. 2009b) in which the amyB promoter and its structural gene are followed by the proCHY gene (Fig. 1; pgAKCN). The Kex2 cleavage site (−Lys-Arg–) was included upstream of the proCHY gene so that proCHY could be separated from the AmyB carrier (Nemoto et al. 2009b). For the non-carrier-fused construct, the amyB promoter and a region encoding the AmyB signal peptide were added to the proCHY gene (Fig. 1; pgACN). It was confirmed by Southern analysis that a single copy of the plasmid was integrated homologously at the niaD locus in the transformant (Fig. S1).
CHY production was performed using the cfCHY and nfCHY strains expressing carrier-fused and non-carrier-fused CHY, respectively. Western analysis with anti-CHY antibody detected mature CHY (approximately 35 kDa) in the culture supernatant of both strains, and additionally some proCHY (approximately 40 kDa) was present in the cfCHY culture supernatant (Fig. 2a). The average yield of the cfCHY strains was 42.2 mg/l, which was approximately 2-fold higher than the yield of the nfCHY strains (20.4 mg/l) (Fig. 2b). CHY was not detected in the host strain by Western analysis and enzyme activity assay (data not shown).
Extracellular production of CHY with and without the carrier fusion. a Western analysis of culture supernatants of the CHY-expressing strains grown for 4 days in 5× DPY liquid medium. Bands of approximately 35 and 40 kDa were detected using the anti-CHY antibody. Purified chymosin (Sigma) was used as a control. b CHY production level in the CHY-expressing strains. The amount of CHY produced (mg/l) was calculated by estimating the time required for the culture supernatant to clot a skim milk solution compared with the purified CHY standard. Three independent experiments were performed, and the mean values and standard errors are represented
Time-course analysis of CHY production
To investigate the effects of the carrier fusion on the CHY production, we performed time-course Western analysis of the culture supernatant in strains cfCHY7 and nfCHY3-1. On the first day, the extracellular production of CHY could be faintly observed in strain cfCHY7, while proCHY was easily detectable in the culture supernatant of strain nfCHY3-1 (Fig. 3a). However, by the third day CHY production had increased in strain cfCHY7, while it had decreased in the nfCHY3-1 strain. Delayed CHY detection in the culture supernatant of strain cfCHY7 raised the possibility of the intracellular accumulation of CHY. Western analysis of cell extracts showed that the AmyB-proCHY carrier-fusion protein was detectable in strain cfCHY7 on the first day, but had decreased by the third day (Fig. 3b). In RT-PCR analysis no apparent differences in CHY mRNA levels during the cultivation were found between strains cfCHY7 and nfCHY3-1 (data not shown).
Time-course analysis of CHY production. a CHY production in the culture supernatant. The strains expressing CHY (cfCHY7 and nfCHY3-1 strains for carrier-fused and non-carrier-fused CHY, respectively) were grown in DPY liquid medium and the culture supernatants were analyzed by Western analysis with anti-CHY antibody on days 1, 2 and 3. Filled and open arrowheads indicate proCHY and CHY, respectively. Similar results were obtained in five independent experiments as supported by quantification of the signal intensities of proCHY and CHY (data not shown). A representative picture is shown. b Western analysis of intracellular CHY. Mycelial protein extracts from the CHY-expressing strains on days 1, 2 and 3 were subjected to Western analysis using anti-CHY antibody. Carrier-fused CHY is indicated by the asterisk. Filled and open arrowheads indicate proCHY and CHY, respectively
DNA microarray analysis of the strains expressing carrier-fused or non-carrier-fused CHY
The CHY-expressing strains were subjected to DNA microarray analysis using probe sets designed with A. oryzae genes and ESTs (see Material and methods). Gene expression levels of strains cfCHY7 and nfCHY3-1 were compared with those of the negative control strain UNA5-1 (Table 1), which was made by transformation with the plasmid containing the amyB promoter and terminator (pUNA; Fig. 1). It was confirmed by Southern analysis that a single copy of the plasmid was integrated homologously at the niaD locus in strain UNA5-1 (Fig. S1). DNA microarray analysis was performed using RNAs extracted from the 1-day culture because RT-PCR analysis revealed that the expression levels of CHY gene in both strains nfCHY3-1 and nfCHY7 were the most abundant on the first day and decreased by the third day (data not shown). As we considered that genes with higher expression levels would mainly contribute to physiological responses to the heterologous protein expression, genes with signal intensities more than 1,000 in at least one sample (see Materials and methods) were selected from up/down regulated genes.
According to the DNA microarray data, strain cfCHY7 showed up/down-regulation (≥1.5-fold) in many more genes and ESTs than strain nfCHY3-1 (Fig. 4). Six out of ten up-regulated and five out of 14 down-regulated genes/ESTs in strain nfCHY3-1 were also up- and down-regulated in strain cfCHY7, respectively. However, three of the genes up-regulated in strain cfCHY7 showed down-regulation in strain nfCHY3-1 (Fig. 4).
Venn diagrams showing the number of overlapping and nonoverlapping up/down-regulated (≥1.5-fold) genes and ESTs in the CHY-expressing strains (strains cfCHY7 and nfCHY3-1 for carrier-fused and non-carrier-fused CHY, respectively) as compared with the control strain UNA5-1. Note that the ESTs selected for the DNA microarray do not overlap with the genes registered in the genome database (see A. oryzae EST database, http://nribf21.nrib.go.jp/EST2/index.html)
The up/down-regulated genes (≥1.5-fold) in the strains expressing CHY were classified into COGs based on the A. oryzae genome database DOGAN (http://www.bio.nite.go.jp/dogan/MicroTop?GENOME_ID=ao) (Table 2). Strain cfCHY7 showed increased expression of many genes categorized into the [O] (posttranslational modification, protein turnover, chaperones), and [U] (intracellular trafficking, secretion, and vesicular transport) COGs. These up-regulated genes are involved in protein folding, glycosylation, translocation/signal peptidase complex, proteolytic degradation, and vesicle trafficking/transport (Table S2). Most of the genes belonging to groups [O] and [U] were slightly up-regulated in strain nfCHY3-1 (Table S2), suggesting that similar physiological responses occurred as in strain cfCHY7, albeit more weakly. The other genes/ESTs showing up/down-regulation in cfCHY7 and nfCHY3-1 strains are listed in Tables S3, S4, S5, and S6.
Induction of the unfolded protein response by expression of carrier-fused CHY
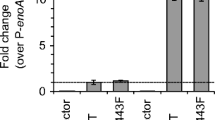
DNA microarray analysis suggested that the UPR was significantly induced in strain cfCHY7. In order to confirm the validity of the DNA microarray analysis, quantitative RT-PCR analysis was performed. We selected three UPR-induced genes: bipA (AO080503000248) (van Gemeren et al. 1997; Kasuya et al. 1999), clxA (AO080515000303) (Wang et al. 2003; Watanabe et al. 2007) and pdiA (Lee et al. 1996; Ngiam et al. 2000). Expression of these genes was increased by several folds in strain cfCHY7 as compared with strain UNA5-1 (Fig. 5a). However, the expression levels of the genes were only slightly increased in strain nfCHY3-1 as compared with strain UNA5-1. In order to eliminate the possibility that expression of the extra copy of the amyB gene was enough to induce UPR, strain Amy3-1 (Table 1), containing the plasmid for expression of AmyB alone (pgAMN; Fig. 1), was included in the analysis. It was confirmed by Southern analysis that a single copy of the plasmid was integrated homologously at the niaD locus in this strain (Fig. S1). In strain Amy3-1, increased α-amylase activity was detected in the culture supernatant (data not shown), but the expression levels of the ER chaperone/foldase genes were not significantly different from the negative control (Fig. 5a).
UPR induction in the A. oryzae strain expressing the carrier-fused CHY. a Quantitative RT-PCR analysis of the genes up-regulated by UPR. The total RNA from the 1-day culture was analyzed by quantitative RT-PCR. The quantity of transcripts was estimated using the β-actin gene transcripts as a reference for each strain (UNA5-1: negative control, nfCHY3-1: expressing the non-carrier-fused CHY, cfCHY7: expressing the carrier-fused CHY, Amy3-1: extra-copy of amyB). The values of strain UNA5-1 were adjusted to 1, and the relative transcript ratios are shown. The mean values and standard errors based on three independent experiments are represented. b Splicing of the unconventional intron (20 nt) in the hacA transcripts. The total RNA from the 1-day culture was analyzed by RT-PCR and subjected to electrophoresis. Filled and open arrowheads indicate nonspliced and spliced fragments of the hacA transcripts, respectively
We confirmed that the 20 nt unconventional intron in the A. oryzae hacA transcripts was efficiently spliced during treatment with UPR-inducing reagents (DTT and tunicamycin) (data not shown). This treatment resulted in the expression of the activated form of HacA, a transcription factor required for up-regulation of the ER chaperone/foldase genes upon UPR (Mulder et al. 2006). RT-PCR analysis revealed that the spliced hacA transcripts were abundantly found in strain cfCHY7 (Fig. 5b). In DNA microarray data expression level of hacA gene was not significantly altered in strains cfCHY7 and nfCHY3-1 (data not shown). Taken together, we concluded that the carrier fusion in CHY production significantly induced UPR.
Discussion
Carrier fusions are known to increase the production levels of heterologous proteins in filamentous fungi (Tsuchiya et al. 1994; Nakajima et al. 2006; Ito et al. 2007; Jin et al. 2007). In order to investigate the physiological effects of a carrier fusion on the host strain, we performed DNA microarray analysis by expressing carrier- and non-carrier-fused CHY in A. oryzae. In this study, the AmyB carrier fusion improved CHY production as compared with the expression of the non-carrier-fused CHY (Fig. 2). This result is in agreement with a previous report showing that CHY production was improved through a fusion with glucoamylase (GlaA) in A. oryzae (Tsuchiya et al. 1994). DNA microarray analysis of the strain expressing the carrier-fused CHY revealed significant up-regulation of the genes involved in ER protein-folding and secretion (Tables 2 and S2, and Fig. 5a). This includes genes encoding ER chaperones and foldases (bipA, pdiA, clxA, etc.), which are known to be up-regulated by UPR (Mulder et al. 2006). In contrast, these genes were not significantly up-regulated in strains nfCHY3-1 and Amy3-1 (Fig. 5a). It was reported in A. niger that the transcription factor HacA, required for UPR, binds the sequence 5′-CAN(G/A)NTGT/GCCT-3′ in the promoter regions of these ER chaperone/foldase genes (Mulder et al. 2006). Splicing in the unconventional 20 nt intron of the hacA transcripts could be clearly observed in A. oryzae strain cfCHY7 (Fig. 5b). Therefore, our results indicate that the carrier fusion used for CHY production significantly induced UPR.
Our finding is the first to demonstrate that UPR is significantly induced by a carrier fusion for heterologous protein production in filamentous fungi. tPA is another heterologous protein which is known to induce UPR in filamentous fungi. It has been produced by a carrier fusion (Wang and Ward 2000; Wang et al. 2003; Arvas et al. 2006; Guillemette et al. 2007), but the transcriptional response to the expression of non-carrier-fused tPA has not been reported. Most of the genes whose expression was altered in the strain expressing the carrier-fused CHY appear to be shared by the A. niger tPA-expressing strain (Guillemette et al. 2007). The shared up-regulated genes include those involved in ER protein-folding and secretion (Table S2). The down-regulated genes in the strains expressing CHY and tPA include some of the ergosterol biosynthetic genes (Table S4; Guillemette et al. 2007), suggesting their physiological relevance in the ER stress response.
One can generally assume that it is difficult to properly fold heterologous proteins in the ER without a carrier fusion, resulting in degradation such as ERAD (Endoplasmic Reticulum Associated Degradation). Hence, it had been predicted that the expression of non-carrier-fused CHY would induce UPR to a much greater extent than that of carrier-fused CHY. Our analysis gave the unexpected result that UPR was significantly induced by the carrier fusion (Table S2 and Fig. 5), similar to the report that the clxA gene was up-regulated by expressing CHY fused to another carrier, glucoamylase (GlaA) in A. niger (Wang et al. 2003). Moreover, the genes involved in ERAD (e.g., AO080509000069 and AO080538000111 for E3 ubiquitin ligase Hrd1p and ubiquitin conjugating enzyme 1 Ubc6p, respectively) were also up-regulated (Table S2), which raises the assumption that some portion of the carrier-fused CHY may be degraded. On the other hand, Sims et al. (2005) reported that expression of non-carrier-fused CHY in A. nidulans up-regulated the genes encoding ER chaperones and foldases. Indeed, DNA microarray (Table S2) and quantitative RT-PCR (Fig. 5a) analyses suggested that the ER chaperone/foldase genes were slightly up-regulated in strain nfCHY3-1. Therefore, we cannot exclude the possibility that the expression of non-carrier-fused CHY induces UPR to a lesser extent than that of carrier-fused CHY.
One question was raised concerning the carrier fusion improvement of CHY production yields, even though UPR was significantly induced. In quantitative RT-PCR analysis carrier fusion seemed to increase the CHY transcripts only slightly (1.4-fold) (Fig. S2). However, it is presumed that this small alteration in mRNA levels of the CHY gene is not enough to explain the remarkable induction of UPR by the carrier fusion. Strain nfCHY3-1 produced (pro)CHY in the culture supernatant earlier than strain cfCHY7 (Fig. 3a), but did not significantly induce UPR (Fig. 5). This suggests no retardation of the secretory processes, which may reflect an efficient exit of the non-carrier-fused CHY from the ER. In contrast, the intracellular detection of the carrier-fused CHY (Fig. 3b) suggests that the fusion protein is temporarily retained in the ER without separation at the Kex2 cleavable sequence by Kex2-like protease (KexB) (Jalving et al. 2000; Punt et al. 2003), whose S. cerevisiae homolog Kex2p resides in the Golgi (Bryant and Boyd 1993). Very recently, we reported that AmyB-mDsRed (red fluorescent protein) fusion protein was observed in the ER besides at the hyphal tip, and then delivered to cell walls and septa (Kimura et al. 2011). This assumption of the temporary retention in the ER is in accordance with the fact that expression of the carrier-fused CHY significantly induced UPR (Fig. 5). Upon UPR induction by the carrier fusion, many genes involved in the ER protein-folding machinery were up-regulated (Table S2) that would help properly fold the carrier-fused CHY and release it from the ER. Furthermore, it was reported that forced UPR induction by overexpressing the activated form of hacA cDNA improves the heterologous protein production in filamentous fungi (Valkonen et al. 2003; Nakajima et al. 2006). Taken together, these results suggest that UPR is an important aspect for improving CHY production by enhancing the secretory capacity of the cell. Further molecular dissections of the ER protein-folding and secretory machinery would help resolve the bottlenecks in heterologous protein production.
References
Akao T, Sano M, Yamada O, Akeno T, Fujii K, Goto K, Ohashi-Kunihiro S, Takase K, Yasukawa-Watanabe M, Yamaguchi K, Kurihara Y, Maruyama J, Juvvadi PR, Tanaka A, Hata Y, Koyama Y, Yamaguchi S, Kitamoto N, Gomi K, Abe K, Takeuchi M, Kobayashi T, Horiuchi H, Kitamoto K, Kashiwagi Y, Machida M, Akita O (2007) Analysis of expressed sequence tags from the fungus Aspergillus oryzae cultured under different conditions. DNA Res 14:47–57
Arvas M, Pakula T, Lanthaler K, Saloheimo M, Valkonen M, Suortti T, Robson G, Penttilä M (2006) Common features and interesting differences in transcriptional responses to secretion stress in the fungi Trichoderma reesei and Saccharomyces cerevisiae. BMC Genomics 7:32
Bryant NJ, Boyd A (1993) Immunoisolation of Kex2p-containing organelles from yeast demonstrates colocalisation of three processing proteinases to a single Golgi compartment. J Cell Sci 106:815–822
Gouka RJ, Punt PJ, van den Hondel CA (1997) Glucoamylase gene fusions alleviate limitations for protein production in Aspergillus awamori at the transcriptional and (post) translational levels. Appl Environ Microbiol 63:488–497
Guillemette T, van Peij NN, Goosen T, Lanthaler K, Robson GD, van den Hondel CA, Stam H, Archer DB (2007) Genomic analysis of the secretion stress response in the enzyme-producing cell factory Aspergillus niger. BMC Genomics 8:158
Ito K, Asakura T, Morita Y, Nakajima K, Koizumi A, Shimizu-Ibuka A, Masuda K, Ishiguro M, Terada T, Maruyama J, Kitamoto K, Misaka T, Abe K (2007) Microbial production of sensory-active miraculin. Biochem Biophys Res Commun 360:407–411
Jalving R, van de Vondervoort PJ, Visser J, Schaap PJ (2000) Characterization of the kexin-like maturase of Aspergillus niger. Appl Environ Microbiol 66:363–336
Jin FJ, Maruyama J, Juvvadi PR, Arioka M, Kitamoto K (2004) Development of a novel quadruple auxotrophic host transformation system by argB gene disruption using adeA gene and exploiting adenine auxotrophy in Aspergillus oryzae. FEMS Microbiol Lett 239:79–85
Jin FJ, Watanabe T, Juvvadi PR, Maruyama J, Arioka M, Kitamoto K (2007) Double disruption of the proteinase genes, tppA and pepE, increases the production level of human lysozyme by Aspergillus oryzae. Appl Microbiol Biotechnol 76:1059–1068
Kasuya T, Nakajima H, Kitamoto K (1999) Cloning and characterization of the bipA gene encoding ER chaperone BiP from Aspergillus oryzae. J Biosci Bioeng 88:472–478
Kimura S, Maruyama J, Takeuchi M, Kitamoto K (2008) Monitoring global gene expression of proteases and improvement of human lysozyme production in nptB gene disruptant of Aspergillus oryzae. Biosci Biotechnol Biochem 72:499–502
Kimura S, Maruyama J, Kikuma T, Arioka M, Kitamoto K (2011) Autophagy delivers misfolded secretory proteins accumulated in endoplasmic reticulum to vacuoles in the filamentous fungus Aspergillus oryzae. Biochem Biophys Res Commun 406:464–470
Kitamoto K (2002) Molecular biology of the Koji molds. Adv Appl Microbiol 51:129–153
Lee BR, Yamada O, Kitamoto K, Takahashi K (1996) Cloning, characterization and overexpression of a gene (pdiA) encoding protein disulfide isomerase of Aspergillus oryzae. J Ferment Bioeng 82:538–543
Mabashi Y, Kikuma T, Maruyama J, Arioka M, Kitamoto K (2006) Development of a versatile expression plasmid construction system for Aspergillus oryzae and its application to visualization of mitochondria. Biosci Biotechnol Biochem 70:1882–1889
Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K, Arima T, Akita O, Kashiwagi Y, Abe K, Gomi K, Horiuchi H, Kitamoto K, Kobayashi T, Takeuchi M, Denning DW, Galagan JE, Nierman WC, Yu J, Archer DB, Bennett JW, Bhatnagar D, Cleveland TE, Fedorova ND, Gotoh O, Horikawa H, Hosoyama A, Ichinomiya M, Igarashi R, Iwashita K, Juvvadi PR, Kato M, Kato Y, Kin T, Kokubun A, Maeda H, Maeyama N, Maruyama J, Nagasaki H, Nakajima T, Oda K, Okada K, Paulsen I, Sakamoto K, Sawano T, Takahashi M, Takase K, Terabayashi Y, Wortman JR, Yamada O, Yamagata Y, Anazawa H, Hata Y, Koide Y, Komori T, Koyama Y, Minetoki T, Suharnan S, Tanaka A, Isono K, Kuhara S, Ogasawara N, Kikuchi H (2005) Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157–1161
Maruyama J, Kitamoto K (2008) Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (DeltaligD) in Aspergillus oryzae. Biotechnol Lett 30:1811–1817
Minetoki T, Nunokawa Y, Gomi K, Kitamoto K, Kumagai C, Tamura G (1996) Deletion analysis of promoter elements of the Aspergillus oryzae agdA gene encoding alpha-glucosidase. Curr Genet 30:432–438
Mulder HJ, Nikolaev I, Madrid SM (2006) HACA, the transcriptional activator of the unfolded protein response (UPR) in Aspergillus niger, binds to partly palindromic UPR elements of the consensus sequence 5′-CAN(G/A)NTGT/GCCT-3′. Fungal Genet Biol 43:560–572
Nakajima K, Asakura T, Maruyama J, Morita Y, Oike H, Shimizu-Ibuka A, Misaka T, Sorimachi H, Arai S, Kitamoto K, Abe K (2006) Extracellular production of neoculin, a sweet-tasting heterodimeric protein with taste-modifying activity, by Aspergillus oryzae. Appl Environ Microbiol 72:3716–3723
Nemoto T, Maruyama J, Kitamoto K (2009a) Improvement of heterologous protein production in Aspergillus oryzae by RNA interference of α-amylase genes. Biosci Biotech Biochem 73:2370–2373
Nemoto T, Watanabe T, Mizogami Y, Maruyama J, Kitamoto K (2009b) Isolation of Aspergillus oryzae mutants for heterologous protein production from a double proteinase gene disruptant. Appl Microbiol Biotechnol 82:1105–1114
Ngiam C, Jeenes DJ, Punt PJ, van den Hondel CA, Archer DB (2000) Characterization of a foldase, protein disulfide isomerase A, in the protein secretory pathway of Aspergillus niger. Appl Environ Microbiol 66:775–782
Pedersen VB, Christensen KA, Foltmann B (1979) Investigations on the activation of bovine prochymosin. Eur J Biochem 94:573–580
Punt PJ, van Gemeren IA, Drint-Kuijvenhoven J, Hessing JG, van Muijlwijk-Harteveld GM, Beijersbergen A, Verrips CT, van den Hondel CA (1998) Analysis of the role of the gene bipA, encoding the major endoplasmic reticulum chaperone protein in the secretion of homologous and heterologous proteins in black Aspergilli. Appl Microbiol Biotechnol 50:447–454
Punt PJ, Drint-Kuijvenhoven A, Lokman BC, Spencer JA, Jeenes D, Archer DA, van den Hondel CA (2003) The role of the Aspergillus niger furin-type protease gene in processing of fungal proproteins and fusion proteins. Evidence for alternative processing of recombinant (fusion-) proteins. J Biotechnol 106:23–32
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Sims AH, Gent ME, Lanthaler K, Dunn-Coleman NS, Oliver SG, Robson GD (2005) Transcriptome analysis of recombinant protein secretion by Aspergillus nidulans and the unfolded-protein response in vivo. Appl Environ Microbiol 71:2737–2747
Tsuchiya K, Nagashima T, Yamamoto Y, Gomi K, Kitamoto K, Kumagai C, Tamura G (1994) High level secretion of calf chymosin using a glucoamylase-prochymosin fusion gene in Aspergillus oryzae. Biosci Biotechnol Biochem 58:895–899
Valkonen M, Ward M, Wang H, Penttilä M, Saloheimo M (2003) Improvement of foreign-protein production in Aspergillus niger var. awamori by constitutive induction of the unfolded-protein response. Appl Environ Microbiol 69:6979–6986
van Gemeren IA, Punt PJ, Drint-Kuyvenhoven A, Broekhuijsen MP, van't Hoog A, Beijersbergen A, Verrips CT, van den Hondel CA (1997) The ER chaperone encoding bipA gene of black Aspergilli is induced by heat shock and unfolded proteins. Gene 198:43–52
Wang H, Ward M (2000) Molecular characterization of a PDI-related gene prpA in Aspergillus niger var. awamori. Curr Genet 37:57–64
Wang H, Entwistle J, Morlon E, Archer DB, Peberdy JF, Ward M, Jeenes DJ (2003) Isolation and characterisation of a calnexin homologue, clxA, from Aspergillus niger. Mol Genet Genomics 268:684–691
Watanabe T, Matsuo I, Maruyama J, Kitamoto K, Ito Y (2007) Identification and characterization of an intracellular lectin, calnexin, from Aspergillus oryzae using N-glycan-conjugated beads. Biosci Biotechnol Biochem 71:2688–2696
Yamada O, Lee BR, Gomi K, Iimura Y (1999) Cloning and functional analysis of the Aspergillus oryzae conidiation regulator gene brlA by its disruption and misscheduled expression. J Biosci Bioeng 87:424–429
Yoon J, Kimura S, Maruyama J, Kitamoto K (2009) Construction of quintuple protease gene disruptant for heterologous protein production in Aspergillus oryzae. Appl Microbiol Biotechnol 82:691–701
Yoon J, Aishan T, Maruyama J, Kitamoto K (2010) Enhanced production and secretion of heterologous proteins by the filamentous fungus Aspergillus oryzae via disruption of vacuolar protein sorting receptor gene Aovps10. Appl Environ Microbiol 76:5718–5727
Yoon J, Maruyama J, Kitamoto K (2011) Disruption of ten protease genes in the filamentous fungus Aspergillus oryzae highly improves production of heterologous proteins. Appl Microbiol Biotechnol 89:747–759
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (S) and a Grant-in-Aid for Scientific Research on Priority Areas “Applied Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 316 kb)
Rights and permissions
About this article
Cite this article
Ohno, A., Maruyama, Ji., Nemoto, T. et al. A carrier fusion significantly induces unfolded protein response in heterologous protein production by Aspergillus oryzae . Appl Microbiol Biotechnol 92, 1197–1206 (2011). https://doi.org/10.1007/s00253-011-3487-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3487-9