Abstract
Artemisinin is a sesquiterpene antimalarial compound produced, though at low levels (0.1–1% dry weight), in Artemisia annua in which it accumulates in the glandular trichomes of the plant. Due to its antimalarial properties and short supply, efforts are being made to improve our understanding of artemisinin biosynthesis and its production. Native β-cyclodextrins, as well as the chemically modified heptakis(2,6-di-O-methyl)-β-cyclodextrin (DIMEB) and 2-hydroxypropyl-β-cyclodextrins, were added to the culture medium of A. annua suspension cultures, and their effects on artemisinin production were analysed. The effects of a joint cyclodextrin and methyl jasmonate treatment were also investigated. Fifty millimolar DIMEB, as well as a combination of 50 mM DIMEB and 100 μM methyl jasmonate, was highly effective in increasing the artemisinin levels in the culture medium. The observed artemisinin level (27 μmol g−1 dry weight) was about 300-fold higher than that observed in untreated suspensions. The influence of β-cyclodextrins and methyl jasmonate on the expression of artemisinin biosynthetic genes was also investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
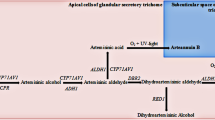
Artemisinin is a plant secondary metabolite with an isoprenoid structure produced by Artemisia annua L. (Asteraceae family), an annual herb native to Asia. The plant has been used for many centuries in traditional Chinese medicine for the treatment of fever. The antimalarial properties of A. annua extracts were discovered in China in 1972, and artemisinin was identified as the active principle (Liao 2009). Artemisinin is a sesquiterpene trioxane lactone containing an endoperoxide bridge essential for its activity against the malarial agents Plasmodium falciparum and Plasmodium vivax (Ferreira et al. 1997). Recently, artemisinin has been recommended to be used in the form of artemisinin-based combination therapies against drug-resistant and cerebral malaria-causing strains of P. falciparum (Newton and White 1999). Artemisinin is produced by the aerial parts of the plant and accumulated in the leaf glandular trichomes (Duke and Paul 1993; Olsson et al. 2009). Unfortunately, the production of artemisinin by the plant is very low (0.1–1% on a dry-weight basis) and its chemical synthesis is very difficult and expensive. In recent years many efforts have been made to improve artemisinin production and several studies have been made to identify genes and enzymes involved in artemisinin biosynthesis (Weathers et al. 2006; Covello 2008). The A. annua ADS gene, encoding amorpha-4,11-diene synthase (ADS) enzyme, involved in the first step of artemisinin biosynthesis, has been cloned (Mercke et al. 2000; Wallaart et al. 2001). Other downstream genes of the artemisinin biosynthetic pathway have also been cloned. These include CYP71AV1, which encodes a cytochrome P450 that catalyzes two oxidation reaction steps of amorpha-4,11-diene to artemisinic aldehyde (Teoh et al. 2006); DBR2, which encodes a double-bond reductase involved in the conversion of artemisinic aldehyde to dihydroartemisinic aldehyde (Zhang et al. 2008) and ALDH1, encoding an aldehyde dehydrogenase involved in the production of dihydroartemisinic acid that can be converted to artemisinin (Teoh et al. 2009). An alternative route leads instead to artemisinic acid and arteannuin B (Brown and Sy 2007). The elucidation of the artemisinin biosynthetic pathway (Fig. 1) and the knowledge of its regulatory mechanisms are essential for improving artemisinin production either in plants or genetically engineered microorganisms (Ro et al. 2006; Zeng et al. 2008a; Tsuruta et al. 2009; Zhang et al. 2010).
Artemisinin biosynthetic pathway adapted from Arsenault et al. (2010). ADS amorphadiene 4,11-diene synthase, CYP cytochrome P450 monoxygenase, CPR cytochrome P450 reductase, DBR2 artemisinic aldehyde Δ11(13) reductase, Aldh1 aldehyde dehydrogenase
A. annua cell and tissue cultures have been explored for the production of artemisinin, although the yields obtained are not commercially attractive (Liu et al. 2006; Covello 2008). Nevertheless, this approach is fundamental to identify chemical and molecular factors that could have a role in artemisinin biosynthesis (Baldi and Dixit 2008; Wang et al. 2009). We have recently established A. annua cell cultures that are able to produce artemisinin and to respond to the elicitor effect of methyl jasmonate (MeJA). Some artemisinin produced by these cultures was also found in the culture medium (Caretto et al. 2011). Artemisinin has poor aqueous solubility, and its solubility can be improved by cyclodextrins (CDs, Usuda et al. 2000). Cyclodextrins are non-reducing cyclic oligomers of 1,4-α-d-linked glucose units, derived from starch by the action of microbial enzyme cyclodextrin glycosyl transferase. The most common CDs are α-, β- and γ-CDs, which are formed by six, seven and eight glucose units, respectively. CDs possess a cone shape with a lipophilic cavity and a hydrophilic exterior. The hydrophobic central cavity can form inclusion complexes with guest molecules of low molecular weight (Szejtli 1982, 2004). CDs have, therefore, received considerable attention as complexing agents in pharmaceutical, cosmetics and food industries to increase the water solubility of various compounds, such as drugs, vitamins and food dyes (Loftsson and Brewster 1996). Among the natural CDs, β-CDs, in particular, are widely used, since their cavity size is suitable for a wide variety of guest molecules with molecular weights ranging from 200 to 800 g mol−1 (Waleczek et al. 2003). Chemically modified β-CDs (alkylated, esterified, glycosylated or substituted), such as heptakis(2,6-di-O-methyl)-β-cyclodextrin (DIMEB) and 2-hydroxypropyl-β-CD (HYPROB), are even more soluble than native β-CDs and are consequently preferred. The capability of cyclodextrins to form host–guest inclusion complexes with artemisinin has already been reported in several studies using pure artemisinin and various β-CDs (Wong and Yuen 2001; Illapakurthy et al. 2003; Marconi et al. 2004; Ansari et al. 2009). The addition of β-CDs to plant cell cultures to improve the production of various secondary metabolites has been described. Moreover, β-CDs were reported to act as genuine elicitors of resveratrol biosynthesis in grapevine (Vitis vinifera) in vitro cell suspension cultures (Bru et al. 2006; Zamboni et al. 2006). A synergistic effect of β-CDs and MeJA on resveratrol biosynthesis has also been reported (Lijavetzky et al. 2008).
In this work we have evaluated the ability of DIMEB, HYPROB and native β-CDs to enhance the production of artemisinin in A. annua suspension cell cultures also treated with MeJA. The expression levels of genes of the artemisinin biosynthetic pathway have also been assayed.
Materials and methods
A. annua cell cultures
A. annua suspension cell cultures were established and maintained as described previously (Caretto et al. 2011). Briefly, suspension cultures were maintained in MS medium (Murashige and Skoog 1962) supplemented with 2 mg l−1 of 2,4-dichlorophenoxyacetic acid and 0.15 mg l−1 6-benzylaminopurine (G6 medium). Cultures were incubated on a rotary shaker (120 rpm) at 25 °C under continuous fluorescent white light (125 μmol photons m−2 s−1) and were subcultivated every 35 days in 500-ml Erlenmeyer flasks by transferring 15 ml of the 35-day-old suspensions into 85 ml fresh G6 medium. Growth of suspension cultures was monitored by measuring dry weight during the culture cycle. Cell viability was assayed using the fluorescein diacetate staining method (Wildholm 1972).
Treatments of A. annua cell cultures and artemisinin determination
Fifteen-day-old suspension cultures were centrifuged at 300×g for 10 min, and medium was discarded. Cells (2.5 g fresh weight) were transferred to a 100-ml Erlenmeyer flask containing 10 ml fresh G6 liquid medium (control) or 10 ml G6 medium supplemented with β-CDs and/or MeJA (Sigma, St. Louis, MO, USA). DIMEB and HYPROB were used at concentrations of 5, 10 or 50 mM, while native β-CDs were used at 5 or 10 mM due to their lower solubility (solubility limit in water at 25 °C is 18 mM). Methyl jasmonate was added to G6 medium at 100 μM concentration. Suspension cultures were incubated on a rotary shaker (120 rpm) in continuous light conditions at 25 °C for various time intervals (30 min; 4 h; 1, 2, 3, 4 and 7 days). At the end of the treatment, suspensions were filtered under vacuum using Miracloth filters (Calbiochem, Los Angeles, CA) and the medium harvested. Cells were washed three times (5 min each) with 150 ml total fresh G6 medium. Cells were frozen and lyophilized overnight (Labconco, Kansas City, MO, USA). Lyophilized cell samples (50 mg) were extracted with 4 ml of methanol for 16 h under magnetic stirring, then for 15 min in an ultrasonic water bath (L&R SweepZone Technology). The extracts were centrifuged at 4,000×g for 10 min, and the supernatant was removed and placed in new tubes. The pellet was extracted again with 4 ml of methanol for 2 h under magnetic stirring; then, after centrifugation for 10 min at 4,000×g, this second supernatant was added to the first and dried under vacuum. Dried samples were redissolved in 1 ml of methanol.
Artemisinin was determined by HPLC analysis of the Q260 derivative, as previously reported (Caretto et al. 2011) and according to Smith et al. (1997). Briefly, samples (100 μl) were derivatized by the addition of 200 μl 60 mM NaOH, incubated at 45 °C for 30 min and after cooling at room temperature, acidified with acetic acid (62.5 mM final concentration). Artemisinin standard (Sigma) was derivatized as described above and used to prepare standard curves for quantification. HPLC analyses were carried out using an Agilent 1100 Series HPLC system equipped with pre-column, Guard, Ultrasphere ODS (Beckmann, 0.46 × 4.5 cm, 5 μm particle size) and a C18 Ultrasphere ODS column (Beckmann, 0.46 × 25 cm, 5 μm particle size). The mobile phase was methanol: sodium phosphate buffer pH 7.0 (55:45 v/v) at 1 ml min−1 constant flow rate, 35 °C column temperature and 260 nm wavelength for detection. The injection volume was 20 μl. Artemisinin identity was confirmed by spectrum analysis of putative peaks and LC-MS analysis according to Wang et al. (2005, data not shown).
Expression analysis of artemisinin biosynthetic genes
Suspension cultures were filtered, frozen in liquid nitrogen lyophilized and ground to a powder. RNA was isolated using SV Total RNA Isolation System (Promega s.r.l., Milan, Italy). cDNAs were obtained starting from 1 μg total RNA and using random primers and the ImProm-II Reverse Transcription System (Promega), according to the manufacturer's instructions.
Primers and probes used for real-time PCR experiments are listed in Table S1 and were all purchased from PRIMM srl (Milan, Italy). The probes were labelled at the 5′-end with 6-carboxy-fluorescein and at the 3′-end with tetramethylrhodamine. Amplification conditions and transcript levels were quantified as previously described (Caretto et al. 2011). Briefly, transcripts were quantified using the comparative quantitation module as described in the ABI 7500 Sequence Detection System (User Bulletin 2, Applied Biosystems), based on the \( 2^{{ - \Delta \Delta C}} _{{\text{T}}} \) method (Livak and Schmittgen 2001). The relative expression was normalized against ubiquitin and calculated using the untreated samples as a calibrator, whose expression was arbitrarily set to one.
Statistical analysis
Results are presented as the mean value ± standard deviation of three independent replicated experiments. Data were analysed statistically by two-way ANOVA, followed by Tukey HSD post-hoc tests, using SigmaStat software Version 3.1 (SPSS Inc., Chicago, IL, USA). Significance level was set at 5%.
Results
CDs and MeJA do not affect cell growth
Exponentially growing A. annua suspension cultures were transferred for various exposure time intervals (1, 2, 3, 4 and 7 days) into G6 medium containing various concentrations of different β-CDs (5, 10 and 50 mM for DIMEB and HYPROB, 10 mM in the case of native β-CDs). In addition, on the basis of preliminary results and other reports in different plant species (Komaraiah et al. 2003; Lijavetzky et al. 2008), A. annua cell cultures were subjected to 100 μM MeJA or to a joint treatment of MeJA and β-CDs to investigate any possible synergistic effect.
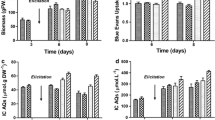
Cell growth during the period of treatment was monitored, and results indicated that β-CDs or MeJA, as well as the joint treatment, had no negative effects on the growth of the cultures. Figure 2 shows the results obtained when 50 mM DIMEB and/or 100 μM MeJA were used. Similar results were also observed using lower concentrations (5 or 10 mM DIMEB) or different β-CDs (HYPROB and native β-CDs, not shown). The viability assay, carried out using the fluorescein diacetate staining method (Wildholm 1972), confirmed that β-CDs and MeJA treatments did not affect the viability of the cultures (not shown).
Artemisinin in the culture medium
On the basis of preliminary results obtained using different concentrations of the various β-CDs (not shown), A. annua suspension cultures were incubated with 50 mM chemically modified β-CDs (DIMEB and HYPROB) or 10 mM native β-CDs at various time intervals. The addition of 100 μM MeJA was also assayed. When analysing the medium of suspension cultures subjected to DIMEB or DIMEB + MeJA joint treatment, artemisinin levels were significantly higher than the control (0.086 μmol g−1) soon after 1 day treatment, being 2.99 and 4.24 μmol g−1 DW, respectively. The maximum amount was observed in the 3-day-treated samples where 25.19 and 27.50 μmol g−1 DW were observed. After this time, the artemisinin content decreased and after 7 days, it was about 15% of the maximum value observed (Fig. 3).
Although less pronounced, the treatments with HYPROB and HYPROB + MEJA also increased artemisinin levels in the culture medium. The maximum values were observed in the 3-day-treated samples: 6.58 and 8.49 μmol g−1 DW in HYPROB and HYPROB + MeJA-treated suspensions, respectively (Fig. 4). In the case of samples treated with native β-CDs, an increase of artemisinin level was also observed; nevertheless, this increase was much lower than those observed for DIMEB and HYPROB-treated samples (not shown). No significant differences were observed between untreated and MeJA-treated cell cultures (Figs. 3 and 4), indicating that, in the experimental conditions used, mostly β-CDs were responsible for the observed increase of artemisinin levels in the culture medium. Moreover, DIMEB were more effective than HYPROB and native β-CDs.
To investigate whether β-CDs could protect artemisinin from its possible degradation in the culture medium, we added 0.4 mM exogenous artemisinin to the culture medium obtained from 15-day-old suspension cultures (or to the same medium supplemented with 50 mM DIMEB) and analysed the artemisinin content after 2, 4 and 7 days. The results indicated that the artemisinin molecule was stable in the medium, suggesting that cyclodextrins were not involved in preventing artemisinin degradation in the culture medium (not shown).
Intracellular artemisinin levels
Artemisinin levels were also measured in cell extracts of A. annua suspension cultures incubated in G6 medium or G6 supplemented with 50 mM DIMEB and/or MeJA for 1, 2, 3, 4 and 7 days (Fig. 5). In comparison with the untreated cultures, in both DIMEB and DIMEB + MeJA-treated cultures, artemisinin levels significantly increased and reached the maximum value (0.190 μmol g−1 DW) after 7 days (Fig. 5).
Expression of artemisinin biosynthetic genes
To verify possible effects of the β-CDs or/and MeJA treatments on the expression of artemisinin biosynthetic genes, quantitative real-time PCR experiments (qRT-PCR) were performed and the expression of ADS, CYP71AV1, CPR and DBR2 genes was analysed starting from 30 min up to 2 days. Moreover, the expression of AaWRKY1, a transcription factor recently reported to regulate the ADS gene (Ma et al. 2009), was also monitored. Results indicated that CYP71AV1 expression was enhanced about twofold after 4 h MeJA and DIMEB + MeJA joint treatments and then declined to values similar to those observed in the control untreated sample (Fig. 6). The expression of CPR gene was less or not at all affected by the treatments. As far as DBR2 gene expression is concerned, β-CDs induced an up-regulation of the gene between 30 min and 1 day. Moreover, as already observed in previous work (Caretto et al. 2011), it was never possible to detect the expression of the ADS gene in treated or untreated suspension cultures.
As far as AaWRKY1 is concerned, both MeJA and DIMEB induced a clear and early up-regulation of this gene soon after 30 min; after this time, the expression dropped to control levels.
Discussion
A. annua in vitro cultures have been explored as a possible alternative to whole plants for the production of the antimalarial compound artemisinin. This approach, however, is far from being commercially attractive due to the low yields of artemisinin so far obtained (Covello 2008). Nevertheless, in vitro cell cultures are a useful tool to study plant cell metabolism and make it possible to test the effects of different elicitors on the regulation of plant biosynthetic pathways and the production of specific metabolites.
In a previous work, we established A. annua suspension cultures and verified that they were able to produce artemisinin. It was interesting to note that small amounts of artemisinin were also found in the culture medium (Caretto et al. 2011). Artemisinin has poor aqueous solubility, and cyclodextrins have been shown to increase its solubility by forming host–guest inclusion complexes (Usuda et al. 2000; Wong and Yuen 2001; Illapakurthy et al. 2003; Marconi et al. 2004; Ansari et al. 2009). Furthermore, recently, β-CDs have been reported to elicit, synergistically with MeJA, the production of resveratrol and the expression of stilbenes biosynthetic genes in grapevine in vitro suspension cultures (Bru et al. 2006; Lijavetzky et al. 2008). On the basis of this information, we carried out a study to investigate whether β-CDs and MeJA treatments could have similar effects on the production of artemisinin in A. annua suspension cultures.
The results revealed an increase in artemisinin level in the medium of both β-CDs and β-CDs + MeJA-treated suspension cultures. The highest value was measured in the 3-day-treated samples with both chemically modified and native β-CDs. The effectiveness of the different β-CDs in increasing artemisinin production in A. annua cell cultures was as follows: DIMEB > HYPROB > native β-CDs. In the DIMEB-treated samples, artemisinin increases, ranging from 140 up to 300-fold compared to the control, were obtained. The results also revealed that in DIMEB or DIMEB + MeJA-treated suspension cultures, intracellular artemisinin levels significantly increased.
The ability of β-CDs to increase artemisinin production could be due to their ability to complex artemisinin and consequently reduce a possible negative feedback loop. Arsenault et al. (2010) reported that artemisinin indeed could regulate artemisinic acid levels in A. annua seedlings as a consequence of a putative negative feedback loop. Moreover, it has been reported that artemisinin is highly phytotoxic to A. annua itself (Duke et al. 1987) and for this reason, its compartmentalization in the subcuticular space of trichome apical cells is necessary (Olsson et al. 2009). The capability of β-CDs to form inclusion complexes with artemisinin could thus reduce its cytotoxic effects in CD-treated suspension cell cultures.
The different capability of β-CDs in increasing artemisinin levels could be explained on the basis of their different interactions with artemisinin. Nevertheless, possible elicitor effects of the various β-CDs used cannot be excluded at this stage. The ability of β-CDs to elicit resveratrol production in grapevine cell cultures was possibly due to their chemical similarity to pectic oligosaccharides released from the cell wall after fungal infection (Bru et al. 2006).
To study possible effects of β-CDs and MeJA on gene expression, we analysed the transcript levels of known artemisinin biosynthetic genes as well as the expression of AaWRKY1 in control and treated cell cultures. AaWRKY1 is a transcriptional regulation factor recently isolated and suggested to activate the expression of ADS as well as other artemisinin biosynthetic genes in A. annua plants (Ma et al. 2009). The results so far obtained in suspension cultures do not make it possible to assess any clear correlation between transcript accumulation of either AaWRKY1 and the artemisinin biosynthetic genes, or artemisinin levels. Further investigations are needed to clarify whether the increase of artemisinin production induced by β-CDs was the result of the enhancement of the artemisinin biosynthetic flux. An intriguing question is the undetected expression of ADS. As already reported in our previous study (Caretto et al. 2011), here we confirm that, in spite of the various experimental conditions tested, it was not possible to observe any expression of ADS gene. ADS is reported to be involved in A. annua plants in the first committed step in artemisinin biosynthesis (Bouwmeester et al. 1999). Although the inability to detect ADS could be due to the very low expression level of this gene in suspension cultures, nevertheless, the possibility that in A. annua plant cell cultures, other genes/enzymes could be involved in the biosynthesis of artemisinin cannot be completely excluded. On the other hand, the regulation of ADS is still under investigation, since it has not been completely understood whether it occurs at the transcriptional or translational level (Zeng et al. 2008b).
The results here reported confirm that the established A. annua suspension cultures can produce artemisinin and, what is of more interest, release it into the medium. Moreover, there was a remarkably high increase of artemisinin in the medium supplemented with DIMEB. These results are quite promising, as the artemisinin yields obtained are significantly higher than those previously obtained, or so far reported, using A. annua suspension cultures. Further analyses will help to understand better the mechanism by which β-CDs can improve the biotechnological production of artemisinin by A. annua cell cultures.
References
Ansari MT, Iqbal I, Sunderland VB (2009) The utility of cyclodextrins for enhancing oral bioavailability. Arch Pharm Res 32:155–165
Arsenault PR, Vail DR, Wobbe KK, Weathers PJ (2010) Effect of sugars on artemisinin production in Artemisia annua L.: transcription and metabolite measurements. Molecules 15:2302–2318
Baldi A, Dixit VK (2008) Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour Technol 99:4609–4614
Bouwmeester HJ, Wallaart TE, Janssen MH, van Loo B, Jansen BJ, Posthumus MA, Schmidt CO, De Kraker JW, König WA, Franssen MC (1999) Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry 52:843–854
Brown GD, Sy LK (2007) In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron 63:9548–9566
Bru R, Sellés S, Casado-Vela J, Belchi-Navarro S, Pedreno MA (2006) Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J Agric Food Chem 54:65–71
Caretto S, Quarta A, Durante M, Nisi R, De Paolis A, Blando F, Mita G (2011) Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol 13:51–58
Covello PS (2008) Making artemisinin. Phytochemistry 69:2881–2885
Duke SO, Paul RN (1993) Development and fine structure of the glandular trichomes of Artemisia annua L. Int J Plant Sci 154:107–118
Duke SO, Vaughn KC, Croom EM, Elsohly HN (1987) Artemisinin, a constituent of annual wormwood (Artemisia annua), is a selective phytotoxin. Weed Sci 35:499–505
Ferreira JFS, Simon JE, Janick J (1997) Artemisia annua: botany, horticulture and pharmacology. Horticultural Reviews 19:319–371
Illapakurthy AC, Sabnis YA, Avery BA, Avery MA, Wyandt CM (2003) Interaction of artemisinin and its related compounds with hydroxypropyl-beta-cyclodextrin in solution state: experimental and molecular-modeling studies. J Pharm Sci 92:649–655
Komaraiah P, Reddy GV, Srinivas Reddy P, Raghavendra AS, Ramakrishna SV, Reddanna P (2003) Enhanced production of antimicrobial sesquiterpenes and lipoxygenase metabolites in elicitor-treated hairy root cultures of Solanum tuberosum. Biotechnol Lett 25:593–597
Liao F (2009) Discovery of artemisinin (Qinghaosu). Molecules 14:5362–5366
Lijavetzky D, Almagro L, Belchi-Navarro S, Martinez-Zapater JM, Bru R, Pedreno A (2008) Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Notes 1:132
Liu C, Zhao Y, Wang Y (2006) Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biot 72:11–20
Livak KJ, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 25:402–408
Loftsson T, Brewster ME (1996) Pharmaceutical application of cyclodextrins. Drug solubilisation and stabilization. J Pharm Sci 85:1017–1025
Ma D, Pu G, Lei C, Ma L, Wang H, Guo Y, Chen J, Du Z, Wang H, Li G, Ye H, Liu B (2009) Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol 50:2146–21461
Marconi G, Monti S, Manoli F, Degli Esposti A, Mayer B (2004) A circular dichroism and structural study of the inclusion complex artemisinin-β-cyclodextrin. Chem Phys Lett 383:566–571
Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE (2000) Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381:173–180
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 51:473–497
Newton P, White N (1999) Malaria: new development in treatment and prevention. Annu Rev Med 50:179–192
Olsson ME, Olofsson LM, Lindahl A-L, Lundgren A, Brodelius M, Brodelius PE (2009) Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular trichomes of Artemisia annua L. Phytochemistry 70:1123–1128
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–9433
Smith TC, Wheathers PJ, Cheetham RD (1997) Effects of giberellic acid on hairy root cultures of Artemisia annua: growth and artemisinin production. Vitro Cell Dev B 33:75–79
Szejtli J (1982) Chemistry and preparation of cyclodextrins. In: Szejtli J (ed) Cyclodextrins and their inclusion complexes. Akadémiai Kiadò, Budapest, pp 17–40
Szejtli J (2004) Past, present, and future of cyclodextrins research. Pure Appl Chem 76:1825–1845
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580:1411–1416
Teoh KH, Polichuk DR, Reed DW, Covello PS (2009) Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany 87:635–642
Tsuruta H, Paddon JC, Eng D, Lenihan JR, Horning T, Anthony LC, Regentin R, Keasling JD, Renninger NS, Newman J (2009) High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS ONE 4(2):e4489. doi:https://doi.org/10.1371/journal.pone.0004489
Usuda M, Endo T, Nagase H, Tomono K, Ueda H (2000) Interaction of antimalarial agent artemisinin with cyclodextrins. Drug Dev Ind Pharm 26:613–619
Waleczek KJ, Cabral Marques HM, Hempel B, Schimdt PC (2003) Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur J Pharm Biopharm 55:247–251
Wallaart ET, Bouwmeester HJ, Hille J, Poppinga L, Maijers NC (2001) Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Wang M, Park C, Wu Q, Simon EJ (2005) Analysis of artemisinin in Artemisia annua L. by LC-MS with selected ion monitoring. J Agr Food Chem 53:7010–7013
Wang JW, Zheng LP, Zhang B, Zou T (2009) Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl Microbiol Biot 85:285–292
Weathers PJ, Elkholy S, Wobbe KK (2006) Artemisinin: the biosynthetic pathway and its regulation in Artemisia annua, a terpenoid-rich species. Vitro Cell Dev Biol Plant 42:309–317
Wildholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Wong JW, Yuen KH (2001) Improved oral bioavailability of artemisinin through inclusion complexation with β- and γ-cyclodextrins. Int J Pharm 227:177–185
Zamboni A, Vrhosvek U, Kassemeyer HH, Mattivi F, Velasco R (2006) Elicitor-induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis 45:63–68
Zeng Q, Qiu F, Yuan L (2008a) Production of artemisinin by genetically modified microbes. Biotechnol Lett 30:581–592
Zeng Q, Zhao C, Yin L, Yang R, Zeng X, Huang Y, Feng L, Yang X (2008b) Cloning of artemisinin biosynthetic cDNAs and novel ESTs and quantification of low temperature-induced gene overexpression. Sci China C Life Sci 51:232–244
Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJ, Ross AR, Covello PS (2008) The molecular cloning of artemisinic aldehyde Delta11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283:21501–21508
Zhang Y, Novak G, Reed DW, Covello P (2010) The production of artemisinin precursors in tobacco. Plant Biotechnol J. doi:https://doi.org/10.1111/j.1467-7652.2010.00556.x
Acknowledgments
The authors are grateful to Dr. Patrick Covello for the critical reading of the manuscript and helpful suggestions. Thanks are due to Prof. H. Caffery for the revision of the English manuscript, to Giovanni Colella and Leone D'Amico for their skillful technical assistance and to Dr. Leopoldo De Carlo for the LC-MS analyses. A. Quarta and M. Durante are supported by fellowships funded by Regione Puglia. This work was supported by Regione Puglia, Italy, Progetto strategico PS070.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 35 kb)
Rights and permissions
About this article
Cite this article
Durante, M., Caretto, S., Quarta, A. et al. β-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl Microbiol Biotechnol 90, 1905–1913 (2011). https://doi.org/10.1007/s00253-011-3232-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3232-4