Abstract
Artemisinin is an efficient anti-malarial drug and it possesses biological activity against a wide range of cancers. The combined application of two different elicitors can be an efficient way to increase the production of secondary metabolite in plant cell cultures. The results of coronatine (Cor) pretreatment and three concentrations of sorbitol were assessed on the growth, biochemical traits, expression of artemisinin biosynthetic genes, and artemisinin production in Artemisia annua cell suspension culture (CSC). After pretreating CSC with 0.05 µM Cor [on the 14th day (three days before the stationary phase) for 48 h], liquid medium in the culture flasks was decanted and replaced with fresh medium (containing 30 g/L sucrose) plus or minus sorbitol at selected concentrations (0, 20, 30, and 40 g/L) on day 16th (one day before the stationary phase). The sorbitol treatment enhanced the contents of malondialdehyde (MDA) and hydrogen peroxide (H2O2) and resulted in oxidative stress. Cor-pretreatment increased the activity of antioxidant enzymes and consequently it reduced H2O2 content and oxidative stress which resulted in decreased MDA content and better growth. The application of Cor plus sorbitol resulted in a dramatic enhancement in the expression of artemisinin biosynthetic genes and artemisinin production at all concentrations. The expression levels of artemisinin biosynthetic genes (about 7.66, 8.67, 8.67, and 8.33-fold in ADS, CYP71AV1, ALDH1, and DBR2 genes, respectively at 4 h after sorbitol treatment) and artemisinin production (9.33 mg/L, 8-fold) peaked at 30 g/L sorbitol plus Cor and decreased at 40 g/L sorbitol, probably because of higher oxidative stress.
Keymessage
The simultaneous application of Cor and sorbitol resulted in a dramatic enhancement in the expression of artemisinin biosynthetic genes and artemisinin production owing to a synergistic or potentiating result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artemisinin is an efficient anti-malarial drug and it possesses biological activity against a wide range of cancers (Efferth 2009, 2017). Some Artemisia species produce artemisinin which is a sesquiterpene lactone (Ranjbar et al. 2015; Hamidi et al. 2018; Salehi et al. 2018a, b). The main source of artemisinin is Artemisia annua where the artemisinin production is subjected to environmental and seasonal changes. Plant tissue and cell cultures are the viable renewable resource of plant natural products, but a very low amount of secondary metabolites (SMs) is synthesized in cultures compared with those in intact plants (Oksman-Caldentey and Inzé 2004; Naik and Al-Khayri 2016; Salehi et al. 2017, 2018c, 2019a, b). Phyton Biotech (https://phytonbiotech.com/) has received $400,000 in 2017 to find out if it can manufacture artemisinin directly by fermenting cells from A. annua itself. Plants synthesize SMs from primary metabolites for the defense aim (Naik and Al-Khayri 2016). Elicitor is a stress agent that increases the SM production in a particular tissue, organs, and cells (Naik and Al-Khayri 2016). Elicitor induces stress which results in the activation of several defense-related genes or inactivation of non-defense-related genes, transient phosphorylation/dephosphorylation of proteins, and enzyme expression (Narayani and Srivastava 2017). The depth science of the metabolic response to elicitation in plant cells, as well as a good comprehension of the mechanisms responsible for these results, can result in the cost-effective and sustainable commercial production of plant SMs (Ramirez-Estrada et al. 2016). Even overexpressing the biosynthetic pathway key genes in plant cells still require elicitation for high production of a related SM (Naik and Al-Khayri 2016; Ramirez-Estrada et al. 2016). Hence, choosing the most efficient elicitor for enhancing SM biosynthesis in plant cell cultures is important. In the exponential growth phase of plant cell cultures, the primary metabolite precursors are needed for biomass formation and therefore, many metabolites are produced at low contents, or not all. The induction of SM production from primary compounds is more efficient in the stationary growth phase (Cusido et al. 2002; Malik et al. 2011; Ramirez-Estrada et al. 2016). The artemisinin production has been outstandingly increased in A. annua cell suspension cultures (CSC) by treatment with methyl jasmonate (Baldi and Dixit 2008), potassium nitrate (KNO3, Keng et al. 2010), β-cyclodextrins and methyl jasmonate (MeJA, Durante et al. 2011). Moreover, increased artemisinin production has been gotten in A. annua hairy root cultures by treating with GA3 (Smith et al. 1997), Colletotrichum mycelia (Wang et al. 2001), fungal cerebroside and nitric oxide (Wang et al. 2009), oligogalacturonides (Zhang et al. 2010), and elicitor derived from Verticillium dahlia (Wang et al. 2000). The studies showed that osmotic stress is effective to stimulate the production of the diverse SMs in the plant cell cultures (Do and Cormier 1990; Zhang et al. 1995; Kim et al. 2001; Wu et al. 2005; Shi et al. 2007; Liu and Cheng 2008; Naik and Al-Khayri 2016; Hussein and Aqlan 2011; Sarmadi et al. 2018). The osmotic stress can be induced by osmotic agents such as sucrose, glucose and polyols (sugar alcohols) in plant cell cultures to increase SM production (Kim et al. 2001; Shi et al. 2007; Hussein and Aqlan 2011; Sarmadi et al. 2018). Sorbitol (C6H14O6, non-metabolic sugar) raise osmotic potential in the medium only and were not applied as nutrient sources in plant cell cultures, therefore it was applied as an osmoticum for increasing medium osmolality and developing osmotic stress in the plant CSCs. Reduction in tissues water absorption lead to growth decrement and physiological changes (Akula and Ravishankar 2011). Coronatine (Cor) functions as a molecular imitator of the isoleucine-conjugated form of jasmonic acid (JA–Ile, Katsir et al. 2008), while it is more stable and less toxic than MeJA (Onrubia et al. 2013), and as a result, possesses a similar action mechanism to the elicitor MeJa. Interestingly, cultures treated with Cor are usually more productive when compared with higher concentrations of MeJa in the same culture conditions (Ramirez-Estrada et al. 2016). Cor usually activates the production of SMs in the plant cell cultures at concentrations lower compared with that of MeJa (Onrubia et al. 2013; Ramirez-Estrada et al. 2016). In addition to increasing the SM production, Cor can alleviate biotic and abiotic stress in plants (Zare Dehabadi et al. 2013; Ceylan et al. 2013; Zhou et al. 2015; Ahmad et al. 2016). The simultaneous application of two different elicitors has been shown to be more efficient owing to a synergistic or potentiating result such as stimulation of artemisinin production by combined cerebroside and nitric oxide elicitation in A. annua hairy roots (Wang et al. 2009), significant increase of artemisinin yields in A. annua CSC elicited by combination of β-cyclodextrin and methyl jasmonate (MeJA, Durante et al. 2011), the highest artemisinin content in A. annua hairy root culture elicited by combination of MeJA and cell homogenate of Piriformospora indica (Ahlawat et al. 2014), effective enhancement of total tanshinone by combined use of osmotic stress (sorbitol) and yeast elicitor in Salvia miltiorrhiza Bunge hairy-root cultures (Shi et al. 2007); the highest levels of taxol in Taxus media and Taxus globosa CSC by combined use of Cor and β-cyclodextrin (Ramirez-Estrada et al. 2015), dramatic enhancement of taxol production in Corylus avellana CSC elicited by salicylic acid pretreatment and ultrasound stress (Rezaei et al. 2011), and significant effect of combined use of salicylic acid and osmotic stress on production of taxane in the callus culture of Taxus baccata (Sarmadi et al. 2018). To the best of our knowledge, there are no published studies on the influence of Cor, osmotic stress, and the synergistic result of Cor and osmotic stress on artemisinin production in A. annua CSC. The current study was carried out to assess the results of Cor pretreatment and different concentrations of sorbitol on the growth, biochemical traits, expression of artemisinin biosynthetic genes, and artemisinin production in A. annua CSC for the first time.
Materials and methods
Cell suspension cultures
Artemisia annua cv. Anamed which is regarded as a high artemisinin cultivar (Salehi et al. 2018a, b) was used for the establishment of CSC, employing the procedure described by Baldi and Dixit (2008) with slight modifications. Callus was developed from aseptically germinated seedlings and maintained on MS medium, supplemented with NAA and Kin (0.5 mg/L of each) as growth regulators and solidified with 8 g/L agar agar. The CSC was developed with cultivating 5 g fresh callus into 250 mL flasks, containing 100 mL of liquid MS media with 30 g/L sucrose and 0.1 mg/L of each of NAA and Kin. The flasks were incubated on a rotary shaker (125 rpm) at 25 ± 2 °C with a photoperiod of 16/8 h light/dark cycle. The CSCs were then subcultured until the cells reached homogeneity.
Elicitation treatments
About 1 ± 0.1 g of cells (fresh mass) was placed to 100 mL flasks, containing 30 mL of the cell culture medium. At the preliminary experiment, three concentrations (0.01, 0.05, and 0.5 µM) of Cor elicitor were added to the CSC on the 14th day (three days before the stationary phase). High artemisinin content and no effect on biomass formation was detected on 0.05 µM Cor at 48 h after elicitation and therefore, this concentration was selected as Cor pretreatment. Experimental treatments included control, Cor, sorbitol, and Cor × sorbitol treatments. The CSC pretreated with 0.05 µM of Cor on the 14th day (three days before the stationary phase) for 48 h. For osmotic stress treatment, the liquid medium in the culture flasks was decanted and replaced with fresh medium, containing 30 g/L sucrose plus or minus sorbitol at selected concentrations (0, 20, 30, and 40 g/L) on the 16th day (one day before the stationary phase). The cell growth, hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents, enzyme activities, and artemisinin content were measured on the 19th day (according to the preliminary test, the highest amount of artemisinin was obtained on the 19th day). The expression of artemisinin biosynthesis genes was determined at 30 min, 4 h, and 24 h after treating with sorbitol.
Measurement of cell growth
The cell growth was determined by measuring the dry cell weight (DCW). In brief, separation of biomass from medium was done by filtration. Removing the residual medium was done by washing with distilled water. Then biomass was lyophilized to constant weight, using a vacuum-freeze drier.
H2O2 determination
H2O2 content was assessed employing the procedure described by Alexieva et al. (2001). In brief, addition of 500 µL of 0.1% trichloroacetic acid (TCA) freeze-dried cells extract supernatant was done to 500 µL of phosphate buffer (100 mM, pH 7.0) and 2000 µL of KI (1 M). The reaction was made for 1 h in darkness and absorbance recorded at 390 nm. The H2O2 concentration was measured, using a H2O2 standard curve.
Lipid peroxidation assay
The lipid peroxidation level was measured using determination of MDA amount generated by the thiobarbituric acid (TBA) reaction (Heath and Packer 1968). Homogenization of 0.3 g freeze-dried cells was done in 1500 µL of 0.1% TCA and then centrifuged at 10,000 ×g for 5 min. Addition of 4 mL of 0.5% TBA in 20% TCA was done to 1000 µL of supernatant. Heating of mixture at 95 °C for 30 min, cooling rapidly and centrifuging (at 10,000 ×g for 15 min) were done. The absorbance at 600 nm and 532 nm was subtracted and then MDA content was computed employing the extinction coefficient of 155/mM cm.
Determination of enzyme activities
Antioxidant enzymes was extracted by homogenizing 0.5 g of freeze-dried cells in 2.5 mL of sodium phosphate buffer (50 mM, pH 7), and then centrifuging at 10,000 ×g and 4 °C for 15 min (Gapińska et al. 2008). Sodium phosphate buffer contained 1 mM Ethylenediaminetetracetic acid (EDTA) and 1% Polyvinylpyrrolidone (PVP-40).
Ascorbate peroxidase (EC 1.11.1.11)
Ascorbate peroxidase (APX) activity was assayed employing the procedure described by Nakano and Asada (1981). The reaction mixture contained 50 mM K-phosphate buffer (pH 7), 0.5 mM ascorbate, 0.1 mM EDTA Na2, 0.1 mM H2O2 and 0.1 mL of enzyme extract in a final assay volume of 1 mL. The reduction in absorbance at 290 nm was recorded and enzyme activity was computed using an extinction coefficient of 2.8/mM cm.
Glutathione reductase (EC 1.6.4.2)
Glutathione reductase (GR) activity was assayed according to the method of Foyer and Halliwell (1976). The assay medium contained 25 mM Na-phosphate buffer (pH 7.8), 0.5 mM oxidized form of glutathione (GSSG), and 0.12 mM NADPH and 0.1 mL enzyme extract in a final assay volume of 1 mL. NADPH oxidation was followed at 340 nm. Activity was computed, using the extinction coefficient of NADPH (6.2/mM cm). One unit of GR was defined as 1 mmol/mL GSSG reduced per min.
Superoxide dismutase (EC 1.15.1.1)
The activity of superoxide dismutase (SOD) was assessed using the procedure depicted by Giannopolitis and Ries (1977). The reaction was constructed using the mixture of potassium phosphate buffer (50 mM, pH 7.8), EDTA (0.1 mM), 75 µL nitroblue-tetrazolium (NBT), methionine (13 mM), 2 µM riboflavin and 100 µL of enzyme extract and it was stirred and put 50 cm below a light bank (eight fluorescent lamps 15 W) for 10 min. Finally, absorbance was recorded at 560 nm. One unit of SOD activity was described as enzyme content that inhibited 50% of NBT photoreduction.
Quantification of artemisinin
After cell separation from CSC, the cell samples were frozen in liquid nitrogen and lyophilized overnight. 50 mg lyophilized cell powder were extracted with 4 ml n-hexane for 16 h under magnetic stirring, then for 15 min in an ultrasonic water bath. The extract was centrifuged at 4000 ×g for 10 min, and the supernatant was removed and placed in new tubes. The pellet was resuspended in 1 ml n-hexane and centrifuged again for 10 min at 4000 ×g. This second supernatant was added to the first, and the pellet discarded (Caretto et al. 2011). Extracts were then dried under vacuum. Within 24 h, samples were reconstituted in 1 ml of acetonitrile, filtered through pre-wetted 0.2 µm (25 mm) nylon Millex-GN filters (Millipore Corporation, Bedford, MA), connected to disposable 3-ml syringes (Salehi et al. 2018a, b). The artemisinin content of the extracts was determined by an HPLC (high-performance liquid chromatography) system (Waters, USA), equipped with a C18 column (NUCLEODUR 100-5 C18 ec, 250 mm × 4.6 mm, China) and detection was conducted at 210 nm wavelength. The acetonitrile:water 65:35% (v/v) was used as a mobile phase with 1 mL/min flow rate (Lapkin et al. 2009; Salehi et al. 2018a, b). Artemisinin was identified by comparison with artemisinin standard. Artemisinin content was determined, using calibration curve of artemisinin standard (Sigma).
Real-time RT-PCR
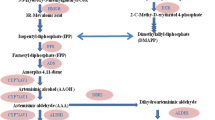
Relative expression of artemisinin biosynthetic genes including amorpha-4,11-diene synthase (ADS), amorphadiene-12-hydroxylase (CYP), aldehyde dehydrogenase 1 (ALDH1), artemisinic aldehyde Δ11(13) reductase (DBR2) and dihydroartemisinic aldehyde reductase (RED1, Fig. 1) were assessed. Total RNA extraction, genomic DNA removal, cDNA synthesis, and qPCR were done; employing the procedure described by Salehi et al. 2018a, b. β-Actin was used as reference genes. The qPCR primers were obtained from Salehi et al. (2018a). Amplicon efficiencies of all primer pairs were computed with cDNA serial dilutions using this formula: E = 10−1/slope−1. Relative expression levels were calculated using the (1 + E)−ΔΔCT method (Pfaffi 2001). The transcription levels of five artemisinin biosynthesis genes under elicitation conditions including Cor, sorbitol and Cor + sorbitol conditions were compared relative to control condition, which was chosen as the reference condition.
Summary of artemisinin biosynthesis pathway. Aa-ADS amorpha-4,11-diene synthase, Aa-CYP71AV1 amorphadiene-12-hydroxylase, Aa-CPR cytochrome P450 reductase, Aa-ADH1 alcohol dehydrogenase 1, Aa-ALDH1 aldehyde dehydrogenase 1, Aa-DBR2 artemisinic aldehyde Δ11(13) reductase, Aa-RED1 dihydroartemisinic aldehyde reductase (Salehi et al. 2018a)
Statistical analysis
The study was conducted, using a factorial experiment based on a complete randomized block design (CRBD) with three replications. The data were tested for the normality and then analyses of variances were done, using PROC GLM of SAS (SAS Institute 2002). Mean comparisons were conducted using Fisher’s least significant differences (LSDs) at 0.01 probability levels. Also, the standard error (SE) was calculated.
Results and discussion
Growth changes
DCW (g) declined significantly (p < 0.01) as the concentration of sorbitol increased, the least cell weight was observed in the medium containing 40 g/L sorbitol (1.99 g/L, Fig. 2). Adding sorbitol in the culture medium increased osmotic stress and caused cell degradation and consequently cell growth decrement. Also, other researchers reported that intracellular water and weight of plant calli decreased with increasing osmotic stress (Tholakalabavi et al. 1994; Akula and Ravishankar 2011; Sarmadi et al. 2018). Cor pretreatment had no significant effect on DCW on the sorbitol-free medium (Cor + 0 sorbitol, Fig. 2), while it increased DCW at all sorbitol concentration (Fig. 2). Cor can alleviate abiotic stress in plants (Zare Dehabadi et al. 2013; Ceylan et al. 2013; Zhou et al. 2015; Ahmad et al. 2016). A Cor pretreatment increased the relative growth rate of chickpea roots under PEG-induced osmotic stress, heat stress, and combined stresses (Ceylan et al. 2013) and fresh mass of Ocimum basilicum under arsenic treatment (Zare Dehabadi et al. 2013). More cell growth after Cor pretreatment suggest an increased tolerance to osmotic stress induced by sorbitol.
H2O2 and MDA content
MDA and H2O2 levels are indicators of ROS mediated destruction to cell membranes and oxidative stress, respectively under stressful and elicitor conditions (Hossain et al. 2015; You and Chan 2015; Sarmadi et al. 2018). In the current study, sorbitol significantly (p < 0.01) enhanced the H2O2 and MDA contents of the cells (Fig. 3). The highest contents of H2O2 (14.32 µmol/g FW) and MDA (583.33 nmol/g FW) were detected under 40 g/L sorbitol (Fig. 3). After the Cor pretreatment, less contents of H2O2 and MDA were observed under all sorbitol concentrations, while Cor had no effect on H2O2 and MDA contents in the sorbitol-free medium (Cor + 0 sorbitol, Fig. 3).
Effects of Cor pretreatment and different concentrations of sorbitol treatments on H2O2 (a) and MDA (b) content of A. annua cells on 19th day of culture cycle. Error bars represent SE (n = 3). Means followed by the same letter are not significantly different according to LSD at 0.01 probability level
Environmental stresses and elicitors enhance the free radicals and reactive oxygen species (ROS) levels and cause lipid peroxidation and bio-membrane degradation (Wang et al. 2008; Hossain et al. 2015; You and Chan 2015). The level of membrane damage directly depends on the oxidative stress intensity and the ROS level. In the current study, H2O2 level increased under sorbitol treatment and consequently MDA content increased (Fig. 3). Cor pretreatment reduced H2O2 and MDA levels indicating Cor can stabilize and protect cell membranes. This result is consistent with those of Wang et al. (2008), Zare Dehabadi et al. (2013) and Ceylan et al. (2013) reporting a Cor-induced reduction of H2O2 and MDA levels under drought stress, arsenic toxicity, and combined osmotic and heat Stresses, respectively.
Antioxidant enzyme activity
The activity of GR, APX, and SOD enzymes increased under all sorbitol treatments compared with those in control (Fig. 4). Increasing the antioxidant activities is a stress tolerance mechanism for preventing oxidative damage (Wang et al. 2008; Zare Dehabadi et al. 2013; Ceylan et al. 2013; Hossain et al. 2015; You and Chan 2015). The main mechanism for detoxifying the cells from free radicals is the enzymatic antioxidant system which functions for adapting and surviving the plant under stress conditions (Hossain et al. 2015; You and Chan 2015). The first and most important enzyme in the detoxifying the cells from ROS is SOD which converts radical superoxide (O2) to H2O2 in the chloroplasts, mitochondria and cytosol and therefore it plays a fundamental role in the cell defense mechanisms against radical hydroxyl (OH) formation (You and Chan 2015). In the next step, enzymes such as GR and APX scavenge the produced H2O2 (Hossain et al. 2015; You and Chan 2015). SOD enzyme had more activity under 30 g/L sorbitol treatment compared with 40 g/L sorbitol treatment (Fig. 4c). The activity of APX enzyme did not show a significant difference in 30 and 40 g/L sorbitol treatments (Fig. 4b). GR enzyme had higher activity under 40 g/L sorbitol treatment compared with 30 g/L sorbitol treatment (Fig. 4a). The higher activity of SOD antioxidant enzyme under 30 g/L sorbitol compared with that under 40 g/L sorbitol (Fig. 4c) indicates that its efficiency is higher in mild stress compared to sever stress. In severe stress, free radicals are generated more quickly than they can be scavenged, and thus the destruction of enzymes will be more. The less activity of SOD enzyme under 40 g/L sorbitol compared with that under 30 g/L sorbitol (Fig. 4c) may be due to less tolerance of A. annua cells under high level of oxidative stress. Considering that SOD enzyme is the first enzyme in detoxification of cells from ROS and less activity of SOD under 40 g/L sorbitol compared with that under 30 g/L sorbitol (Fig. 4c), it seems that 40 g/L sorbitol is not proper elicitor for enhancing SM production.
Effects of Cor pretreatment and different concentrations of sorbitol treatments on the antioxidant enzyme activities of A. annua cells on 19th day of culture cycle. GR (a), APX (b), and SOD (c). Error bars represent SE (n = 3). Means followed by the same letter are not significantly different according to LSD at 0.01 probability level
Cor pretreatment enhanced the activity of three studied antioxidant enzymes under different sorbitol concentration (Fig. 4). The antioxidant system neutralizes the impact of oxidative stress in plants and researchers showed that the exogenous use of Cor in stress conditions improves the antioxidant enzyme activities (Wang et al. 2008; Zare Dehabadi et al. 2013; Ceylan et al. 2013). Sarmadi et al. (2018) showed that salicylic acid pretreatment enhanced antioxidant enzyme activities under different glucose levels in a T. baccata callus culture.
Artemisinin content
The artemisinin content was significantly higher in all sorbitol concentrations compared with that in the control, having a peak at 30 g/L sorbitol and declining at 40 g/L (Fig. 5). Previous studies reported that SM production increased in plant cell and tissue cultures elicited by sucrose, mannitol and sorbitol (Suzuki 1995; Zhang et al. 1996; Kim et al. 2001; Shi et al. 2007; Namdeo et al. 2007; Hussein and Aqlan 2011; Sarmadi et al. 2018). The SM production decreases under severe stress due to increased oxidative degradation (Sarmadi et al. 2018). The Cor pretreatment enhanced artemisinin content in CSC (Fig. 5). Cor increased both SM production and expression of SM biosynthetic genes in plant CSCs (Ramirez-Estrada et al. 2016). The application of Cor plus sorbitol resulted in a dramatic enhancement in artemisinin content. The highest content of artemisinin (9.33 mg/L, 8-fold, Fig. 5) was observed with the combination of Cor and 30 g/L sorbitol. In addition to increasing the SM gene expression, Cor alleviate abiotic stress (Fig. 3) and resulted in more cell growth (Fig. 2), which can lead to higher artemisinin production per liter. The simultaneous application of Cor and osmotic stress was more efficient to produce SM owing to a synergistic or potentiating result. Researchers reported that simultaneous application of two different elicitors is more efficient owing to a synergistic or potentiating result (Shi et al. 2007; Wang et al. 2009; Durante et al. 2011; Rezaei et al. 2011; Ahlawat et al. 2014; Ramirez-Estrada et al. 2015; Sarmadi et al. 2018).
Effects of Cor pretreatment and different concentrations of sorbitol treatments on artemisinin production in A. annua L. cell suspension culture on 19th day of culture cycle. Error bars represent SE (n = 3). Means followed by the same letter are not significantly different according to LSD at 0.01 probability level
Gene expression
The qPCR method was used to understand the possible results of the Cor or/and sorbitol treatments on the expression of artemisinin biosynthetic genes (Fig. 1) starting from 30 min up to 24 h. The expression of RED1 gene was not significantly influenced by Cor or/and sorbitol treatments and therefore the results were not presented. The expression levels of ADS, CYP71AV1, ALDH1 and DBR2 genes increased under 20 and 30 g/L sorbitol treatments compared with those in control at all times (Fig. 6). The low content of H2O2 as a signaling molecule could modify the expression of SM genes (Sarmadi et al. 2018). The osmotic stress induced by low concentrations of sorbitol triggered oxidative cascade which enhances expression of artemisinin biosynthetic genes (Fig. 6). The application of Cor plus sorbitol resulted in a dramatic enhancement in the expression of ADS, CYP71AV1, ALDH1 and DBR2 genes at all concentrations (Fig. 6). The expression of these genes peaked at 30 g/L sorbitol and decreased at 40 g/L sorbitol (Fig. 6), probably because of higher oxidative stress.
Effects of Cor pretreatment and different concentrations of sorbitol treatments on relative expression of ADS (a), CYP71AV1 (b), ALDH1 (c), and DBR2 (d) genes of A. annua cells at 30 min (a1, b1, c1, and d1), 4 h (a2, b2, c2, and d2), and 24 h (a3, b3, c3, and d3) after sorbitol elicitation. Error bars represent SE (n = 3). Means followed by the same letter are not significantly different according to LSD at 0.01 probability level
Results indicated that expression of ADS, CYP71AV1, ALDH1 and DBR2 genes were enhanced about 7.66, 8.67, 8.67, and 8.33-fold, respectively at 4 h after Cor + 30 g/L sorbitol joint treatments (Fig. 6). Sorbitol up-regulated DBR2 gene, while Cor had no effect on DBR2 gene expression (Fig. 6d). Cor alleviated oxidative stress (Fig. 3) by enhancing antioxidant enzyme activities (Fig. 4) and possibly, other physiological mechanisms. Furthermore, Cor increased the expression of ADS, CYP71AV1, and ALDH1 genes but did not affect the expression of the DBR2 gene (Fig. 6). The oxidative stress had a positive result on the expression of ADS, CYP71AV1, ALDH1, and especially DBR2 genes.
The relative turnover potential of the artemisinin biosynthetic enzymes can be computed, using the application of relative transcript levels [(1 + E)−∆∆CT method, Pfaffi 2001] in combination with kinetic data [(1 + E)−ΔΔCT × Kcat (S−1), Olofsson et al. 2011; Salehi et al. 2018a]. For this estimate, it was presumed that the number of enzyme active sites was proportional to the transcription rate and that the enzymes were acting at substrate saturation with an optimal NADPH/NADP+ ratio (Olofsson et al. 2011; Salehi et al. 2018a). In this condition, the kcat-value is a well indicator of the conversion of substrate to product. The highest conversion potential of the ADS (0.14) and ALDH1 [(34.17, on dihydroartemisinic aldehyde, DHAA) and (6.66, on artemisinic aldehyde, AA)] was observed in Cor + sorbitol treatment followed by Cor treatment and the highest conversion potential of DBR2 (6.01) was observed in Cor + sorbitol treatment followed by sorbitol treatment (Table 1). In Cor + sorbitol, the highest relative turnover potential of ADS, ALDH1, and DBR2 was observed. The Cor + sorbitol treatment was more efficient owing to this synergistic result. The relative turnover of ALDH1 and DBR2 are nearly equal in control, sorbitol, and Cor + sorbitol treatments, while the potential conversion capacity of ALDH1 (3.49) was 2.9-fold than that of DBR2 (1.21) in Cor treatment (Table 1). Aa-DBR2 and Aa-ALDH1 are acting on the same pool of intermediate. Aa-DBR2 enzyme converts AA into DHAA which is a precursor of artemisinin, while Aa-ALDH1 catalyzed the transformation of AA into artemisinic acid which is a precursor of arteannuin B (Fig. 1). Since, the relative turnover of Aa-ALDH1 is higher than Aa-DBR2 under Cor treatment. Hence, it was deduced that artemisinic acid/arteannuin B was produced more compared with artemisinin under Cor treatment.
Conclusion
The effects of Cor and sorbitol treatments on A. annua CSC are studied for the first time. Sorbitol treatment enhanced the membrane lipid peroxidation (Fig. 3b) through inducing oxidative stress and increasing H2O2 level (Fig. 3a). The low oxidative stress (low content of H2O2 as a signaling molecule) increased the expression of ADS, CYP71AV1, ALDH1, and especially DBR2 genes (Fig. 6). Also, artemisinin content (Fig. 5) and antioxidant enzyme activities (Fig. 4) increased under sorbitol treatment, which is a physiological defense response to osmotic stress. Pretreatment of A. annua cells with Cor increased antioxidant enzyme activity (Fig. 4) which regulates the ROS rate and increased the cell tolerance to sorbitol stress; resulting in increased cell biomass (Fig. 2). Moreover, Cor enhanced the expression of ADS, CYP71AV1, and ALDH1 genes but did not affect the expression of the DBR2 gene (Fig. 6). Uppalapati et al. (2005) showed that Cor enhances proteinase inhibitors and as a result enzymes involved in the SM biosynthesis pathway would possess a more continuous activity in cell cultures treated with this elicitor, resulting in higher SM contents. In Cor + sorbitol, the highest relative turnover potential of ADS, ALDH1, and DBR2 was observed (Table 1). Also, relative turnover of ALDH1 and DBR2 are nearly equal in Cor + sorbitol treatment (Table 1). The simultaneous application of Cor and osmotic stress was more efficient to produce SM owing to a synergistic or potentiating result.
Abbreviations
- AA:
-
Artemisinic aldehyde
- ADS:
-
Amorpha-4, 11-diene synthase
- ALDH1:
-
Aldehyde dehydrogenase 1
- APX:
-
Ascorbate peroxidase
- Cor:
-
Coronatine
- CSC:
-
Cell suspension culture
- CYP71AV1:
-
Amorphadiene-12-hydroxylase
- DBR2:
-
Artemisinic aldehyde Δ11(13) reductase
- DCW:
-
Dry cell weight
- DHAA:
-
Dihydroartemisinic aldehyde
- EDTA:
-
Ethylenediaminetetracetic acid
- GA3:
-
Gibberellic acid
- GR:
-
Glutathione reductase
- H2O2 :
-
Hydrogen peroxide
- HPLC:
-
High-performance liquid chromatography
- Kin:
-
Kinetin
- LSD:
-
Least significant difference
- MDA:
-
Malondialdehyde
- MeJA:
-
Methyl jasmonate
- NAA:
-
1-Naphthaleneacetic acid
- PVP-40:
-
Polyvinylpyrrolidone
- RED1:
-
Dihydroartemisinic aldehyde reductase
- ROS:
-
Reactive oxygen species
- SE:
-
Standard error
- SM:
-
Secondary metabolite
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Ahlawat S, Saxena P, Alam P, Wajid S, Abdin MZ (2014) Modulation of artemisinin biosynthesis by elicitors, inhibitor, and precursor in hairy root cultures of Artemisia annua L. J Plant Interact 9:811–824. https://doi.org/10.1080/17429145.2014.949885
Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M, Kazi AM, Gucel S (2016) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813. https://doi.org/10.3389/fpls.2016.00813
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant signal behav 6:1720–1731. https://doi.org/10.4161/psb.6.11.17613
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x
Baldi A, Dixit V (2008) Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour Technol 99:4609–4614. https://doi.org/10.1016/j.biortech.2007.06.061
Caretto S, Quarta A, Durante M, Nisi R, De Paolis A, Blando F, Mita G (2011) Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol 13:51–58. https://doi.org/10.1111/j.1438-8677.2009.00306.x
Ceylan HA, Türkan I, Sekmen AH (2013) Effect of coronatine on antioxidant enzyme response of chickpea roots to combination of PEG-induced osmotic stress and heat stress. J Plant Growth Regul 32:2–82. https://doi.org/10.1007/s00344-012-9277-5
Cusidó RM, Palazón J, Bonfill M, Navia-Osorio A, Morales C, Piñol MT (2002) Improved paclitaxel and baccatin III production in suspension cultures of Taxus media. Biotechnol Prog 18:418–423. https://doi.org/10.1021/bp0101583
Do CB, Cormier F (1990) Accumulation of anthocyanins enhanced by a high osmotic potential in grape (Vitis vinifera L.) cell suspensions. Plant Cell Rep 9:143–146. https://doi.org/10.1007/BF00232091
Durante M, Caretto S, Quarta A, De Paolis A, Nisi R, Mita G (2011) β-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl Microbiol Biotechnol 90:905–1913. https://doi.org/10.1007/s00253-011-3232-4
Efferth T (2009) Artemisinin: a versatile weapon from traditional Chinese medicine. In: Ramawat KG (ed) Herbal drugs: ethnomedicine to modern medicine. Springer, Heidelberg, pp 173–194
Efferth T (2017) From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol 46:65–83. https://doi.org/10.1016/j.semcancer.2017.02.009
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/BF00386001
Gapińska M, Skłodowska M, Gabara B (2008) Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol Plant 30:11. https://doi.org/10.1007/s11738-007-0072-z
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Hamidi F, Karimzadeh G, Rashidi Monfared S, Salehi M (2018) Assessment of Iranian endemic Artemisia khorassanica: karyological, genome size, and gene expressions involved in artemisinin production. Turk J Biol 42:329–340. https://doi.org/10.3906/biy-1802-86
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LSP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Hussein EA, Aqlan EM (2011) Effect of mannitol and sodium chloride on some total secondary metabolites of fenugreek calli cultured in vitro. Plant Tissue Cult Biotechnol 21:35–43. https://doi.org/10.3329/ptcb.v21i1.9561
Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105:7100–7105. https://doi.org/10.1073/pnas.0802332105
Keng CL, Singaram N, Lim BP (2010) Production of artemisinin from cell suspension culture of Artemisia annua L. Asia Pac J Mol Biol Biotechnol 18:139–141
Kim SI, Choi HK, Kim JH, Lee HS, Hong SS (2001) Effect of osmotic pressure on paclitaxel production in suspension cell cultures of Taxus chinensis. Enzyme Microb Technol 28:202–209. https://doi.org/10.1016/S0141-0229(00)00292-1
Lapkin AA, Walker A, Sullivan N, Khambay B, Mlambo B, Chemat S (2009) Development of HPLC analytical protocols for quantification of artemisinin in biomass and extracts. J Pharm Biomed Anal 4:908–915. https://doi.org/10.1016/j.jpba.2009.01.025
Liu CZ, Cheng XY (2008) Enhancement of phenylethanoid glycosides biosynthesis in cell cultures of Cistanche deserticola by osmotic stress. Plant Cell Rep 27:357–362. https://doi.org/10.1007/s00299-007-0443-3
Malik S, Cusido RM, Mirjalili MH, Moyano E, Palazon J, Bonfill M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46:23–34. https://doi.org/10.1016/j.procbio.2010.09.004
Naik PM, Al-Khayri JM (2016) Impact of abiotic elicitors on in vitro production of plant secondary metabolites: a review. J Adv Res Biotech 1:7. https://doi.org/10.15226/2475-4714/1/2/00102
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1:69–79
Narayani M, Srivastava S (2017) Elicitation: a stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem Rev 16:1227–1252. https://doi.org/10.1007/s11101-017-9534-0
Oksman-Caldentey KM, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440. https://doi.org/10.1016/j.tplants.2004.07.006
Olofsson L, Engström A, Lundgren A, Brodelius PE (2011) Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol 11:45. https://doi.org/10.1186/1471-2229-11-45
Onrubia M, Moyano E, Bonfill M, Cusidó RM, Goossens A, Palazón J (2013)) Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol 170:211–219. https://doi.org/10.1016/j.jplph.2012.09.004
Pfaffi MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. https://doi.org/10.1093/nar/29.9.e45
Picaud S, Olofsson L, Brodelius M, Brodelius PE (2005) Expression, purification, and characterization of recombinant amorpha-4, 11-diene synthase from Artemisia annua L. Arch Biochem Biophys 436:215–226. https://doi.org/10.1016/j.abb.2005.02.012
Ramirez-Estrada K, Osuna L, Moyano E, Bonfill M, Tapia N, Cusido RM, Palazon J (2015) Changes in gene transcription and taxane production in elicited cell cultures of Taxus × media and Taxus globosa. Phytochemistry 117:174–184. https://doi.org/10.1016/j.phytochem.2015.06.013
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó R, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21:182–205. https://doi.org/10.3390/molecules21020182
Ranjbar M, Naghavi MR, Alizadeh H, Soltanloo H (2015) Expression of artemisinin biosynthesis genes in eight Artemisia species at three developmental stages. Ind Crops Prod 76:836–843. https://doi.org/10.1016/j.indcrop.2015.07.077
Rezaei A, Ghanati F, Behmanesh M, Mokhtari-Dizaji M (2011) Ultrasound-potentiated salicylic acid-induced physiological effects and production of taxol in hazelnut (Corylus avellana L.) cell culture. Ultrasound Med Biol 37:1938–1947. https://doi.org/10.1016/j.ultrasmedbio.2011.06.013
Rydén AM, Ruyter-Spira C, Quax WJ, Osada H, Muranaka T, Kayser O, Bouwmeester H (2010) The molecular cloning of dihydroartemisinic aldehyde reductase and its implication in artemisinin biosynthesis in Artemisia annua. Planta Med 76:1778–1783. https://doi.org/10.1055/s-0030-1249930
Salehi M, Moieni A, Safaie N (2017) A novel medium for enhancing callus growth of hazel (Corylus avellana L.). Sci Rep 7:15598. https://doi.org/10.1038/s41598-017-15703-z
Salehi M, Karimzadeh G, Naghavi MR, Naghdi Badi H, Monfared SR (2018a) Expression of artemisinin biosynthesis and trichome formation genes in five Artemisia species. Ind Crops Prod 112:130–140. https://doi.org/10.1016/j.indcrop.2017.11.002
Salehi M, Karimzadeh G, Naghavi MR, Naghdi Badi HN, Monfared SR (2018b) Expression of key genes affecting artemisinin content in five Artemisia species. Sci Rep 8:12659. https://doi.org/10.1038/s41598-018-31079-0
Salehi M, Moieni A, Safaie N (2018c) Elicitors derived from hazel (Corylus avellana L.) cell suspension culture enhance growth and paclitaxel production of Epicoccum nigrum. Sci Rep 8:12053. https://doi.org/10.1038/s41598-017-15703-z
Salehi M, Moieni A, Safaie N, Farhadi S (2019a) Elicitors derived from endophytic fungi Chaetomium globosum and Paraconiothyrium brasiliense enhance paclitaxel production in Corylus avellana cell suspension culture. Plant Cell Tissue Organ Cult 136:161–171. https://doi.org/10.1007/s11240-018-1503-9
Salehi M, Moieni A, Safaie N, Farhadi S (2019b) New synergistic co-culture of Corylus avellana cells and Epicoccum nigrum for paclitaxel production. J Ind Microbiol Biotechnol 1–11. https://doi.org/10.1007/s10295-019-02148-8
Sarmadi M, Karimi N, Palazón J, Ghassempour A, Mirjalili MH (2018) The effects of salicylic acid and glucose on biochemical traits and taxane production in a Taxus baccata callus culture. Plant Physiol Biochem 132:271–280. https://doi.org/10.1016/j.plaphy.2018.09.013
SAS Institute (2002) SAS/STAT user’s guide. SAS Institute, Inc, Cary
Shi M, Kwok K, Wu JY (2007) Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy root culture by hyperosmotic stress and yeast elicitor. Biotechnol Appl Biochem 46:191–196. https://doi.org/10.1042/BA20060147
Smith TC, Weathers PJ, Cheetham RD (1997) Effects of gibberellic acid on hairy root cultures of Artemisia annua: growth and artemisinin production. In Vitro Cell Dev Biol Plant 33:75–79. https://doi.org/10.1007/s11627-997-0044-4
Suzuki M (1995) Enhancement of anthocyanin accumulation by high osmotic stress and low pH in grape cells (Vitis hybrids). J Plant Physiol 147:152–155. https://doi.org/10.1016/S0176-1617(11)81428-8
Teoh KH, Polichuk DR, Reed DW, Covello PS (2009) Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany 87:635–642. https://doi.org/10.1139/B09-032
Tholakalabavi A, Zwiazek JJ, Thorpe TA (1994) Effect of mannitol and glucose-induced osmotic stress on growth, water relations, and solute composition of cell suspension cultures of poplar (Populus deltoides var. Occidentalis) in relation to anthocyanin accumulation. In Vitro Cell Dev Biol Plant 30:164–170. https://doi.org/10.1007/BF02632208
Uppalapati SR, Ayoubi P, Weng H, Palmer DA, Mitchell RE, Jones W, Bender CL (2005) The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J 42:201–217. https://doi.org/10.1111/j.1365-313X.2005.02366.x
Wang H, Ye H, Li G, Liu B, Zhong K (2000) Effects of fungal elicitors on cell growth and artemisinin accumulation in hairy root cultures of Artemisia annua. Acta Bot Sin 42:905–909
Wang JW, Zhang Z, Tan RX (2001) Stimulation of artemisinin production in Artemisia annua hairy roots by the elicitor from the endophytic Colletotrichum sp. Biotechnol Lett 23:857–860. https://doi.org/10.1023/A:1010535001943
Wang B, Li Z, Eneji AE, Tian X, Zhai Z, Li J, Duan L (2008) Effects of coronatine on growth, gas exchange traits, chlorophyll content, antioxidant enzymes and lipid peroxidation in maize (Zea mays L.) seedlings under simulated drought stress. Plant Prod Sci 11:283–290. https://doi.org/10.1626/pps.11.283
Wang JW, Zheng LP, Zhang B, Zou T (2009) Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl Microbiol Biotechnol 85:285–292. https://doi.org/10.1007/s00253-009-2090-9
Wu JY, Wong K, Ho KP, Zhou LG (2005) Enhancement of saponin production in Panax ginseng cell culture by osmotic stress and nutrient feeding. Enzyme Microb Technol 36:133–138. https://doi.org/10.1016/j.enzmictec.2004.07.010
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092. https://doi.org/10.3389/fpls.2015.01092
Zare Dehabadi S, Shoushtari A, Asrar Z (2013) Modulation of arsenic toxicity-induced oxidative damage by coronatine pretreatment in sweet basil (Ocimum basilicum) seedlings. Botany 91: 442–448. https://doi.org/10.1139/cjb-2012-0296
Zhang YH, Zhong JJ, Yu JT (1995) Effect of osmotic pressure on cell growth and production of ginseng saponin and polysaccharide in suspension cultures of Panax notoginseng. Biotechnol Lett 17:1347–1350. https://doi.org/10.1007/BF00189224
Zhang YH, Zhong JJ, Yu JT (1996) Enhancement of ginseng saponin production in suspension cultures of Panax notoginseng: manipulation of medium sucrose. J Biotechnol 51:49–56. https://doi.org/10.1016/0168-1656(96)01560-X
Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJ, Ross AR, Covello PS (2008) The molecular cloning of artemisinic aldehyde ∆11 (13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283:21501–22150. https://doi.org/10.1074/jbc.M803090200
Zhang B, Zou T, Lu YH, Wang JW (2010) Stimulation of artemisinin biosynthesis in Artemisia annua hairy roots by oligogalacturonides. Afr J Biotechnol 9:3437–3442. https://doi.org/10.4314/ajb.v9i23
Zhou Y, Zhang M, Li J, Li Z, Tian X, Duan L (2015) Phytotoxin coronatine enhances heat tolerance via maintaining photosynthetic performance in wheat based on electrophoresis and TOF-MS analysis. Sci Rep 5:13870. https://doi.org/10.1038/srep13870
Acknowledgements
Authors gratefully acknowledge the support provided for this survey by the Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Maryam Salehi designed and performed experiments and prepared the manuscript under the joint supervision of Assoc. prof. G. Karimzadeh and Prof. M. R. Naghavi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Ali R. Alan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salehi, M., Karimzadeh, G. & Naghavi, M.R. Synergistic effect of coronatine and sorbitol on artemisinin production in cell suspension culture of Artemisia annua L. cv. Anamed. Plant Cell Tiss Organ Cult 137, 587–597 (2019). https://doi.org/10.1007/s11240-019-01593-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01593-8