Abstract
Lignocellulosic materials are the most abundant renewable organic resources (~200 billion tons annually) on earth that are readily available for conversion to ethanol and other value-added products, but they have not yet been tapped for the commercial production of fuel ethanol. The lignocellulosic substrates include woody substrates such as hardwood (birch and aspen, etc.) and softwood (spruce and pine, etc.), agro residues (wheat straw, sugarcane bagasse, corn stover, etc.), dedicated energy crops (switch grass, and Miscanthus etc.), weedy materials (Eicchornia crassipes, Lantana camara etc.), and municipal solid waste (food and kitchen waste, etc.). Despite the success achieved in the laboratory, there are limitations to success with lignocellulosic substrates on a commercial scale. The future of lignocellulosics is expected to lie in improvements of plant biomass, metabolic engineering of ethanol, and cellulolytic enzyme-producing microorganisms, fullest exploitation of weed materials, and process integration of the individual steps involved in bioethanol production. Issues related to the chemical composition of various weedy raw substrates for bioethanol formation, including chemical composition-based structural hydrolysis of the substrate, need special attention. This area could be opened up further by exploring genetically modified metabolic engineering routes in weedy materials and in biocatalysts that would make the production of bioethanol more efficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Producing second-generation ethanol from lignocellulosics such as agricultural and forestry residues, herbaceous and woody crops, weeds and waste paper, etc., has unique environmental, economic, and strategic benefits. The escalating demand for food, feed, and energy has raised several concerns about the potential use of food-based biofuels and their future sustainability, and global warming and energy security concerns have intensified the search for safe yet effective methods to commercially produce ethanol from other plants (Chandel et al. 2010a). Bioethanol is completely renewable in nature. Burning it releases carbon dioxide that is recycled back into plants, since plants use CO2 to synthesize cellulose during their photosynthesis cycle.
The wide variety of biomass is the backbone of biorefineries. The major types of biomass for ethanol production recognized to date are monoculture crops grown on fertile soils (such as sugarcane, corn, soya beans, oilseed rape, switch grass, willow, and hybrid poplar) (Farrell et al. 2006), waste biomass (such as straw, corn stover, and waste wood) (Kim and Dale 2004), and municipal solid waste (such as processed paper and newspaper; Kuhad et al. 2010). Another type of biomass is weedy cellulosics, viz. Eicchornia crassipes, Lantana camara, Prosopis juliflora, Saccharum spontaneum, Typha latifolia, Crofton, Chromolaena odorata, etc., which are promising and cheaper feedstocks for fuel ethanol production. These weedy cellulosic substrates do not require additional economic input as they grow on agriculturally degraded land or water bodies (Huber and Dale 2009).
No matter what plant it comes from, lignocellulosic biomass is composed of a complex mixture of cellulose, hemicellulose, and lignin (Fig. 1). After cellulose, hemicellulose is the fraction of the plant cell wall that has the most potential to serve as a source of bioethanol production (Chandel et al. 2007a; Kumar et al. 2008). The carbohydrate fractions of the plant cell wall can be converted into fermentable monomeric sugars through acidic and enzymatic (hemicellulase/cellulase) reactions, which have been exploited to produce ethanol, xylitol, and 2, 3-butanediol via microbial fermentation processes (Chandel et al. 2010b). The recalcitrance to saccharification is a one of the major limitations for conversion of lignocellulosic biomass to ethanol. The potential solution may lie in lignin modification, which could bypass the need for alkali or any microbial delignification step and thus facilitate the bioethanol process. In broader aspect, the future of biorefineries depends on low-input, high-diversity biomass feedstock that is rich in fermentable sugars and low in lignin (Tilman et al. 2007; Somerville et al. 2010). Basically, the bioconversion of lignocellulosics to ethanol includes three processes: (a) depolymerisation of structural polysaccharides into fermentable sugars via thermochemical and enzymatic routes, (b) fermentation of these sugars into ethanol, and (c) ethanol recovery (Fig. 2).
Molecular component of plant cell wall structure (Source: Rubin 2008, with permission and courtesy “Nature”)
For the long haul, it is necessary to understand the chemical compositions and structural hydrolysis of weedy substrates that are abundantly available on waste land for conversion to ethanol. This article aims to explore the chemical compositions of various weedy raw substrates for bioethanol formation. It will attempt to provide an in-depth understanding of the biotechnological aspects of lignocellulosic bioconversion from different biomass feedstocks in terms of the carbohydrate present in their cell walls, availability, feasible technologies for ethanol production, and new innovations involved in biorefineries.
Weedy lignocellulosic substrates: availability and chemical composition
Lignocellulosic biomass is an abundant renewable resource for the production of alternative biofuels, with 200 billion tons produced annually. It has a higher productivity rate per hectare than grains, oil seed, or sugars per unit of biomass produced (Kim and Dale 2004). Currently, the global yield of biomass crops, including woody and herbaceous crops grown in temperate and subtropical regions, varies from ~8 dry Mg/ha/year (for willow in Sweden) to 10–22 dry Mg/ha/year (for short rotation woody crops in the US). A conservative global biomass average would be ~10 dry Mg/ha/year, although some small-scale field trials have reported four times this level of biomass production (Perlack et al. 2005).
The production of bioethanol from agricultural residues and hays (wheat, barley, and triticale straws and barley, triticale, pearl millet, and sweet sorghum hays) is an attractive and feasible option (Kim and Dale 2004). Agro-residues are a very promising source of lignocellulosic feedstock for bioethanol production. Each source of biomass represents a technological challenge; the diversity of raw materials will allow the decentralization of fuel production with geopolitical, economic, and social benefits (Wyman 2007).
Plants using C4 photosynthesis tends to be productive in terms of fixing CO2 leading to increase photosynthesis, rapid growth even under extreme conditions such as drought and high temperatures. These plants can grow on marginal lands with high biomass density per unit area by using low nutrient and water needs (Rubin 2008). The photosynthesis reactions in C4 plants are the same as in C3. However, due to the dual carboxylase/oxygenase activity of RuBisCo in C3 plants, an amount of the substrate is oxidized rather than carboxylated. The oxidized substrate led to the loss of substrate and consumption of energy (i.e., photorespiration). In order to bypass the photorespiration, C4 plants developed a mechanism to efficiently deliver CO2 to the RuBisCO enzyme due to their specific leaf anatomy so called Kranz anatomy where chloroplasts exist, not only in the mesophyll cells in the outer part of their leaves but in the bundle sheath cells as well. C4 plants such as maize, sugarcane, sorghum, and millet efficiently fix CO2 during photosynthesis in turn storing high amount of carbohydrates.
The disadvantages of C4 plants are that they are rare in cold climates and unable to grow at temperatures less than 10°C. In these environments, trees (gymnosperms and angiosperms) that exclusively depend upon C3 photosynthesis are the only candidate species. The C3 group of potential energy crops includes trees such as poplar and eucalyptus that have relatively rapid growth potential in harsh conditions.
Perennial herbaceous energy crops make good feedstocks because they do not require annual reseeding once established, need fewer energy inputs (such as fertilizer and pesticides) than annual cropland, and can be grown on marginal croplands (Dien et al. 2005). They also have environmental benefits, including reduced soil erosion, enhanced carbon sequestration, and wildlife habitat (Lemus and Lai 2005). The major herbaceous energy crops that have been selected for bioethanol production in the US are switch grass (Panicum virgatum), miscanthus (Miscanthus spp. Anderss.), canary grass (Phalaris arundinacea), giant reed (Arundo donax L.), and alfalfa (Medicago sativa L.). They are considered to have energetic, economic, and environmental advantages over food crops for ethanol production (Hill et al. 2006). These dedicated energy crops have a fair amount of holocellulose (cellulose + hemicelluloses) in their cell walls, but their feedstock quality for livestock makes them unattractive options for fuel ethanol generation.
Major weedy substrates
Among the various forms of biomass (wood residues, agro residues, municipal solid wastes, and starchy substrates) available for ethanol production, weedy lignocelluloses seem to be the most promising as future biomass feedstock (Huber and Dale 2009). S. spontaneum (wild sugarcane) is a perennial weedy grass with deep roots and rhizomes that grows up to 4 m tall. It has worldwide distribution, extending across three geographic zones (the East Zone, Central Zone, and West Zone) and into other countries. In Asian countries like India, it has spread across millions of acres, often causing abandonment of fields. It can be an excellent biomass source for ethanol and cellulase production (Chandel et al. 2009b, 2010c; Scordia et al. 2010).
L. camara L. (Verbenaceae) is a noxious weed that can threaten land productivity, grazing for livestock, biodiversity, and consequently overall ecology. However, its luxuriant growth and vigorous survival give it potential economic value for utilization in value-added products such as ethanol (Pasha et al. 2007) and cellulose derivatives (Varshney et al. 2006). P. juliflora is a tree native to Mexico, South America, India, and the Caribbean that has become established as a weed in Asia, Australia, and elsewhere. It grows up to 12 m (39 ft) tall and has a trunk with a diameter of up to 1.2 m (3.9 ft), providing enough biomass for ethanol production (Gupta et al. 2009).
E. crassipes (water hyacinth) is a free-floating perennial aquatic plant native to tropical South America. The broad, thick, glossy, ovate leaves measure 10–20 cm across and float above the water surface. They have long, spongy, and bulbous stalks, and the plant may rise as much as 1 m above the surface of the water. The common water hyacinth is a vigorous grower that can double its population in 2 weeks. It is another potential biomass source for ethanol (Kumar et al. 2009a) and cellulase production (Sukumaran et al. 2009).
A perennial herbaceous plant, T. latifolia grows in temperate, subtropical, and tropical areas throughout the Northern Hemisphere. It grows in marshy areas and flowers in mid- to late summer. The plant is 1.5–3 m (5–10 ft) high and has 2–4 cm broad leaves. It may be a good carbon substrate for solid state fermentation to produce cellulase and ethanol (Chandel, unpublished data).
Eupatorium adenophorum (Crofton weed) is an erect, bushy, leafy, many-stemmed herbaceous perennial from Central America that grows to 2 m high. It is a highly invasive plant, forms dense stands, is tolerant of a wide range of conditions, and is common on roadsides and bush land edges and in wetlands. Zhao et al. (2007) studied pretreatment methods to enhance the enzymatic digestibility of this weed.
C. odorata (Siam weed or Christmas bush) is a species of flowering shrub native to North America, from Florida and Texas to Mexico and the Caribbean, and has been introduced to tropical Asia, West Africa, and parts of Australia. Recently, Zhao et al. (2010) explored its efficiency for ethanol production.
One of the most common noxious weeds, Parthenium (Asteraceae), is native to the tropical Americas and invades all disturbed land, including farms, pastures, and roadsides. The species Parthenium hysterophorus, also known as congress grass or gazar ghas, has become common in India, Australia, and parts of Africa and America (Everitt et al. 2007). As yet, there has been no report on ethanol production from this weed. We believe that harnessing it for biofuel use would be a legitimate application to promote a safe and clean environment.
Switch grass (P. virgatum) is a native tall prairie grass known for its rapid growth during the warm months to heights of 2–6 ft. Switch grass can be grown in most parts of the US, including swamplands, plains, and streams, and along the shores and interstate highways. It is self-seeding and resistant to many diseases and pests, and can produce high yields with low applications of fertilizer and other chemicals. It is also tolerant of poor soils, flooding, and drought; furthermore, it improves soil quality and prevents erosion due to its type of root system (Parrish and Fike 2009).
Miscanthus giganteus is another viable feedstock for cellulosic ethanol production. This species of grass is native to Asia and can grow up to 12 ft (3.7 m) tall with little water or fertilizer input. It is similar to switch grass with respect to cold and drought tolerance and water use efficiency (Ng et al. 2010). Miscanthus is commercially grown in the European Union as a combustible energy source.
Chemical composition
Lignocelluloses have three main components: cellulose, hemicelluloses, and lignin. Cellulose is the most abundant organic polymer on the earth, surpassing even starches and sugars; it is a homopolymer of sugars containing six carbon atoms linked together in a chain that constitutes the largest proportion of the plant cell wall. Hemicellulose is a heteropolymer consisting of xylose-linking compounds like arabinose, glucose, mannose, and other sugars through an acetyl chain (Chandel et al. 2010b). These compounds can be characterized as galactomannans, arabinoglucuronoxylans, or glucomannans based on their linkage with the main xylan backbone. Lignins are huge cross-linked jumbles of organic molecules that reinforce cellulose and hemicelluloses. They are complex, amorphous, three-dimensional polymers that have a phenyl propane structure (Rubin 2008; Fig. 1). Table 1 summarizes the basic cell wall composition of selected lignocellulosics. In general, hardwoods contain 18–25% lignin, 45–55% cellulose, and 24–40% hemicelluloses, while softwoods contain 25–35% lignin, 45–50% cellulose, and 5–35% hemicelluloses. Grasses normally contain 10–30% lignin, 25–40% cellulose, and 25–50% hemicelluloses (Betts et al. 1991). The structure and components of the cell walls of weeds are significantly different from those of most plant species, which may influence digestibility during the bioconversion process to bioethanol (Sarkar et al. 2009).
The critical parameters for selecting plants for fuel ethanol production include cell wall composition, growth rate, suitability for growth in different geographical regions, and resource–use efficiencies (Rubin 2008). Lignin and hemicelluloses differ in composition from species to species. Coniferous woods (gymnosperms) have a high proportion of mannose in their hemicelluloses, while deciduous wood species (angiosperms) have a high proportion of xylose (Sarkar et al. 2009). This complex composition can limit the potential of weedy substrates for use on an industrial scale. Hence, it is imperative to explore efficient and economical approaches to digesting complex chemicals.
Digestibility of weedy substrate
Pretreatment
Pretreatment is required to alter the macro- and microscopic size and structure of the biomass, as well as its submicroscopic chemical composition, so that the hydrolysis of the carbohydrate fraction to monomeric sugars can be achieved rapidly with greater yield (Kumar et al. 2009b). It solubilizes hemicellulose, reduces crystallinity, and increases the available surface area and pore volume of the substrate. In acid-catalyzed pretreatment, the hemicellulose layer is hydrolyzed, whereas in alkali-catalyzed treatment, a part of the lignin is removed and hemicelluloses are hydrolyzed using hemicellulases (Moiser et al. 2005; Kumar et al. 2009b). Various other types of pretreatment can be used, including mechanical, steam explosion, ammonia fiber explosion, and biological pretreatments (reviewed by Moiser et al. 2005).
A comparison of methods to assess the enzyme accessibility and hydrolysis of pretreated lignocellulosic substrates revealed one effective method for facilitating the enzymatic hydrolysis of a pretreated substrate (Chandra et al. 2009a). A lignocellulosic substrate of lodgepole pine chips was directly subjected to sulfite pretreatment to overcome the recalcitrance of lignocellulose pretreatment and then disk milled; the recovered cellulose substrate was quasi-simultaneously saccharified enzymatically and fermented into ethanol using commercial cellulases and Saccharomyces cerevisiae D5A (Zhu et al. 2010). Bak et al. (2010) proposed using rice straw that was fermented by the wood–rot fungus Dichomitus squalens as a biological pretreatment to increase the enzymatic digestibility of lignocellulose and promote cellulose hydrolysis. However, an efficient pretreatment process that can reduce the overall production cost of ethanol is still needed.
Removal of fermentation inhibitors from hemicellulosic hydrolysates
The acid hydrolysis of lignocellulosics releases xylose as the main sugar constituent in hydrolysates along with small fractions of arabinose, mannose, galactose, and glucose. Unfortunately, these hydrolysates also contain several fermentation inhibitors, such as furan derivatives from degradation of sugars, aliphatic acids released from hemicellulosic acetyl groups, phenolics from lignin, and metal traces if hydrolysis occurs in metal-based reactors. The compositional profile of hemicellulose hydrolysates depends upon the cell wall composition and the method employed for cell wall digestion (Chandel et al. 2007a, 2010b; Hahn-Hägerdal et al. 2007). These inhibitory compounds severely affect the fermentation performance of the biocatalyst used and reduce ethanol production. Several chemical, biological, and physical methods have been used to remove the inhibitors and increase the hydrolysate fermentability of lignocellulosic substrates (Chandel et al. 2007b). Parawira and Tekere (2010) reviewed the various physical, chemical, physico-chemical, and biotechnological strategies used for detoxification of lignocellulosic hydrolysates.
Enzymatic hydrolysis
After acid, alkaline, or fungal pretreatment, lignocellulosics can be saccharified enzymatically to obtain fermentable sugars. Microorganisms are potential sources of cellulases and hemicellulases, which can be used for the hydrolysis of pretreated lignocellulosics. Both bacteria and fungi are known to grow on these substrates in solid and submerged culture fermentation reactions. Table 2 summarizes the various lignocellulosics employed for cellulolytic enzyme production.
The enzyme cellulases act two orders of magnitude more slowly than other polysaccharidases. The action mechanism of cellulases needs to be deciphered at the molecular level. Studies must be done on mining of diversified cellulases and engineering proteins to make them penetrative. Enzymatic cocktails comprising cellulases, xylanases, mannanases, etc. are one option for efficient hydrolysis (Wilson 2009). The most important process improvement in the enzymatic hydrolysis of biomass was the introduction of simultaneous saccharification and fermentation, which has been improved to include the co-fermentation of multiple sugar substrates, and is now known as simultaneous saccharification and co-fermentation (Olofsson et al. 2010). Consolidated bioprocessing is another area of development, wherein the four steps—production of cellulases, enzymatic hydrolysis of lignocellulose, and conversion of hydrolysate (pentose and hexose) into ethanol—occur in a single step collectively (Lynd et al. 2005). The enzymatic hydrolysis in this process requires the use of cellulase, a multienzyme complex involving the synergistic action of endo-1,4-glucanase (EC 3.2.1.4), exo-alpha-1,4-glucanase (EC 3.2.1.91), and beta-glucosidase (EC 3.2.1.21). Cellobiose is a potent inhibitor of the cellulase enzyme. Beta-d-glucosidase thus provides a key catalytic activity for cellulase preparations and completes the saccharification of cellulose (Chandra et al. 2009b; Wilson 2009).
Bioethanol can be effectively economized by ensuring a maximum release of sugars from the pretreated substrate. Application of surfactants during enzymatic hydrolysis leads to an increase in the surface area of lignocellulosics and improves the yield of released sugars (Tabka et al. 2006). Nonionic surfactants like Tween-20 are more effective, and it is believed that surfactants change the nature of the substrate by increasing the available cellulose surface. The mechanism of surfactant activation may be due to their adsorption on hydrophobic surfaces composed of lignin fragments. Yang and Wyman (2006) reported that bovine serum albumin increased the surface area of pretreated corn stover and enhanced glucose yield (92%) at a loading of 7.5 FPU/g of cellulose. The enzymatic hydrolysis of lignocellulose can be limited by many factors, such as adsorption to surface areas, low fiber porosity, and low median pore size of fibers. Further limitations include the cellulase production, which is expensive and contributes significantly to the overall cost of saccharification.
Weedy substrate and microbial biosynthetic potential
Various sugars (pentose, hexose, and oligosaccharides) are derived from acid/enzymatic hydrolysis of the lignocellulosic sugar syrup derived from lignocellulosic biomass. The best-known alcohol-fermenting organisms, S. cerevisiae and Zymomonas mobilis, are capable of fermenting only hexose sugars and sucrose into ethanol. However, pentose-fermenting microorganisms such as Pichia stipitis, Candida shehatae, and Pachysolen tannophilus can produce ethanol from a variety of lignocellulosic substrates (Hahn-Hagerdaal et al. 2007). Anaerobic bacteria are able to ferment xylose, but are inhibited by high sugar and alcohol concentrations, producing excess byproducts that virtually lower the ethanol yield (Desai et al. 2004). Filamentous fungi are limited by their generation time, which would affect their overall ethanol yield on an industrial scale.
Microbial metabolic engineering
The production of alternative fuels can be enhanced by manipulating the metabolic intermediates in microbes that are mostly recognized in the cellular glycolysis pathway. Many native microorganisms have a distinct genetic system that is required for the synthesis of petroleum substitutes. However, these organisms lack a traditional usage that is economical and require genetic manipulation for industrial use. Metabolic engineering has played a pivotal role in the improvement of ethanol-producing microorganisms. Specific gene alteration was not possible through classical methods of genetic strain improvement, but industrial biotechnology can now provide pathways that extend the spectrum of usable industrial media (e.g., lignocellulosic hydrolysates) and enable the production of compounds not naturally formed by microorganisms.
Metabolic engineering of cellulase-producing microorganisms
Recombinant DNA technology offers significant potential for improving various aspects of lignocellulolytic enzymes to construct “synthetic” designer enzymes for specific applications. It may also be possible to fuse different lignocellulolytic genes or sections of genes from different organisms to produce novel chimeric proteins or enzymes with altered properties (Kumar et al. 2008).
With the advent of new biotechnologies and bioinformatics tools, searching for novel enzymes via metagenomic approaches may significantly contribute to their future economical production from renewable resources. Metagenomic analysis of the Nasutitermes hindgut reveals a rich diversity of bacterial genes encoding hitherto unknown glycosyl hydrolases. These enzymes constitute over 100 families of proteins that can break the glycosidic bonds between carbohydrates or between carbohydrate and non-carbohydrate entities (Warnecke et al. 2007). Later, Brulc et al. (2009) revealed forage-specific glycoside hydrolases that could be used in biofuel production through gene-centric metagenomics of the fiber-adherent bovine rumen microbiome. Alriksson et al. (2009) developed a recombinant Aspergillus niger strain expressing the Hypocrea jecorina endoglucanase Cel7B when grown on spent hydrolysates (stillage) from sugarcane bagasse and spruce wood. A. niger D15 [egI] displayed higher endoglucanase activity (2,100 nkat/ml) in the spent hydrolysates.
Martinez et al. (2008) performed a gene sequence analysis of the powerful cellulolytic fungus H. jecorina (Trichoderma reesei). Li et al. (2008a) compared function-based metagenome screening and sequence-based metagenome data mining as methods for discovering unusual enzymes related to the glycosyl hydrolase family from natural resources for degradation of recalcitrant lignocelluloses. Thermostable endocellulase (CelDR) was successfully cloned from a thermostable Bacillus subtillus and expressed into Escherichia coli BL21 (DE3), which showed almost three times the activity (0.78 U/ml).
Arrays of enzymes such as beta-glucosidases, endoglucanases, and cellobiohydrolases produced by T. reesei (Kumar et al. 2008; Martinez et al. 2008) and laccases and lignin peroxidases from white rot fungus (Larsson et al. 2001) were expressed in yeasts for enzymes and ethanol simultaneously. To identify new and useful enzymatic functions, researchers isolated a handful of microorganisms such as Z. mobilis, Clostridium phytofermentans, and Clostridium thermocellum and attempted to characterize their relative capacity for genetic manipulation and lignocellulosic conversion into ethanol (Warnicke et al. 2007).
Metabolic engineering of ethanol-producing microorganisms
To construct an efficient organism that can be used in large operations, important traits such as broad substrate utilization range simultaneously hydrolyzing the cellulose, high osmo-tolerance, high ethanol yields and productivity even at high temperatures, high ethanol tolerance, increased tolerance to inhibitors, and minimal nutrient supplementation are required (Zaldivar et al. 2001). An enormous amount of work has been done to search for suitable ethanologens from lignocelluloses, and efforts are underway to create a suitable microorganism that can be used on a larger scale in biorefineries. Hahn-Hagerdal et al. (2007) and Nevoigt (2008) elegantly reviewed the developments of recombinant yeast strains for simultaneous conversion of both pentose and hexose sugars from lignocellulose hydrolysates into ethanol.
The first xylose-fermenting S. cerevisiae strain was developed through the insertion and expression of xylose-metabolizing genes from P. stipitis (Kotter and Ciriacy 1993). Later, xylose-fermenting strains of S. cerevisiae were constructed by introducing the genes encoding xylose isomerase from the bacterium Thermus thermophilus (Walfridsson et al. 1996) and the anaerobic fungus Piromyces sp. (Kuyper et al. 2005), respectively. Ethanol production using lignocellulosic feedstock from recombinant and wild-type microorganisms is summarized in Table 3. Katahira et al. (2006) constructed a recombinant yeast strain that could ferment xylose and cellooligosaccharides by integrating genes for the intercellular expressions of xylose reductase and xylitol dehydrogenase from P. stipitis and xylulokinase from S. cerevisiae, as well as a gene for displaying β-glucosidase from Aspergillus acleatus on the cell surface. This strain produced 30 g/l ethanol from acid hydrolysate of wood chips (73 g/l total sugars).
Sanchez et al. (2010) developed a recombinant S. cerevisiae strain showing improved arabinose and xylose utilization by adopting evolutionary engineering. Jin et al. (2005) explored an inverse metabolic engineering approach to identify gene targets for improved xylose assimilation in recombinant S. cerevisiae expressing XYL1 and XYL2 from P. stipitis. The resulting recombinant strain exhibited a 100% increase in the growth rate and a 70% increase in ethanol production (0.033 versus 0.019 g ethanol/g cells·h) on xylose compared to the parental strain. Another industrially favorable microorganism, the recombinant S. cerevisiae D 452-2 strain, was developed for ethanol production from xylose expressing protein engineered NADH-preferring xylose reductase from P. stipitis NBRC 1687 (Watanabe et al. 2007).
Endo et al. (2008) identified the genes required for tolerance to vanillin in S. cerevisiae. Seventy-six deletion mutants were identified as vanillin-sensitive mutants and classified under the functional categories for chromatin re-modeling and vesicle transport, suggesting that these functions are important for vanillin tolerance. This study provided a biotechnological basis for molecular engineering as well as for screening of more robust yeast strains that may be useful in bioethanol fermentation.
The production of desirable compounds from microbes can often require a complete reprogramming of their innate metabolism. The evolution of such complex traits requires simultaneous modification of the expression levels of many genes, which may not be achievable by sequential multigene modifications. It could be helpful in the development of high ethanol-tolerant microbial strains. Alper et al. (2006) called this cellular engineering approach “global transcription machinery engineering”; it includes the alteration of key proteins regulating the global transcriptome and generates, through them, a new type of diversity at the transcriptional level. Following this, they observed 69%, 41%, and 15% improvement in volumetric and specific ethanol productivities and ethanol yield from an S. cerevisiae mutant compared with the wild species. Genome shuffling is a classical genetic engineering approach that uses iterative cycles of genome recombination and selection to combine the useful alleles of many parental strains into single cells showing the desired phenotype. P. stipitis was developed by genome shuffling several times for improved tolerance to hardwood spent sulfite liquor, resulting in improved ethanol production (Bajwa et al. 2010). The genome sequences of Z. mobilis ZM4 and P. stipitis revealed insights into the metabolic pathways responsible for pentose conversion into ethanol (Seo et al. 2005; Jeffries 2006).
Current status of genetic engineering in bioenergy crops
Genetic engineering of crops in order to increase structural carbohydrate content and reduce lignin levels is a promising path that may result in reduced pretreatment severity, facilitate the hydrolysis process, and help recover the maximum amount of sugars. In addition to this, cellulose and hemicellulose degradation enzymes are also being expressed in the cell wall, which decreases the overall cellulase enzyme load during saccharification of biomass (Sticklen 2008). Torney et al. (2007) reviewed the genetic engineering approaches to improve bioethanol production from maize. These approaches were intended to increase stress tolerance, photosynthesis rate, grain yield, and production of biomass conversion enzymes in planta (Table 4). These approaches could also be incorporated for the improvement of weedy crops in terms of increased biomass weight, cell wall composition, and biomass conversion assisted by enzyme expression in planta.
The lignin biosynthesis pathway has been a major area of research in plant biotechnology (Harris et al. 2009). It may be helpful to reduce the lignin content by increasing the amount of cellulose for improved digestion and pulping efficiency (Reddy et al. 2005). Chen and Dixon (2007) studied the downregulation of lignin biosynthetic genes in alfalfa, which revealed an increment in fermentable sugars for improved ethanol production; they advocated the downregulation of lignin-synthetic genes in other energy crops such as switch grass, Miscanthus, and poplar. Li et al. (2008b) constructed transgenic poplar plants using antisense technology, resulting in a 40% decrease in lignin and a 14% increase in cellulose content. Wei et al. (2001) reviewed the methods developed for altering lignin-biosynthetic genes in forest tree species. In another prospect for the genetic engineering of biofuel crops, Vega-Sanchez and Ronald (2010) suggested that the complete elucidation of lipid metabolism may facilitate the fatty acid biosynthetic pathways in cell wall synthesis. This could help in the development of the next generation of biofuel crops by increasing fatty acid contents and optimizing the hydrolysis of plant cell walls to release fermentable sugars for eventual conversion into bioethanol.
A genome sequence study on Populus trichocarpa (poplar), a potential bioenergy crop, reveals the potential of applying genomics to the challenge of optimizing energy crops. The shown traits will be used to maximize the biomass yield per unit land area (Tuskan et al. 2006). When using metagenomics, namely genetic material recovered directly from environmental samples, there is no need to cultivate cells. At the same time, the impetus to exploit “omics” approaches to capture new biotechnologies for plant cell wall deconstruction and the production of second-generation biofuels has reached new heights (Morrison et al. 2009).
Economic analysis of bioethanol production and commercialization
A steady state progress has been made in the bioconversion of lignocellulosic biomass for ethanol production. Despite the achievements made in the laboratory, the successful commercialization of ethanol remains a challenging task for commercialization (Wyman 2007; Himmel and Beyer 2009). The key issues relates to the cost and regular supply of feedstock and the balance between judicious usage of lignocellulosics, the economics of ethanol production and the environment (Banerjee et al. 2010). The economics of ethanol production using different raw materials are compared in Table 5. It is revealed that the cost of cellulosic ethanol is not competitive with grain-based ethanol as yet (Table 5).
Cellulosic ethanol commercialization is the process of building an industry out of methods of turning cellulose-containing organic matter into fuel. Companies such as Iogen Corporation, Mascoma Corporation, Lignol Energy Corporation, and Abengoa Bioenergy etc. are building refineries that can process biomass to turn into ethanol. Companies viz. Genencore Inc, Diversa Corporation, Novozymes Inc, and Dyadic Corporation are engaged producing enzymes that could enable a cellulosic ethanol in future. In recent years, the growth of commercial plants for bioethanol across the USA has mushroomed, including 26 new plants under construction in 2008 alone (Banerjee et al. 2010; Chandel et al. 2010a). The induction of cheap and surplus lignocellulosics having least economic and food/feed value (weedy materials, switch grass, Miscanthus, groundnut shell, sugarcane leaves, Brassica compestris stalks, cotton stalks, coffee spent, municipal solid waste, etc.) should be more explored.
The process integration, improved microbial traits for simultaneous production of cellulases and ethanol from mixed sugars, and improvements in the distillation process to get water-free ethanol will lead to a new manufacturing paradigm (Banerjee et al. 2010).
The implementation of bioethanol would generate more employment opportunities and income in rural areas and would reduce greenhouse gas emissions, which makes it worthwhile for the government to encourage biofuels by providing tax benefits (Himmel and Beyer 2009). It is recommended that appropriate policy objectives be imposed to foster bioethanol commercialization. These policy objectives could include the correction of certain tax anomalies, exemption from excise duty and sales tax, deregulation of feedstock and its pricing, and simplification of licensing for bioethanol production (Wyman 2007; Chandel et al. 2010a).
Future perspectives and challenges
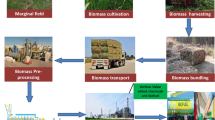
Currently, the ability to produce biofuel is largely dependent upon lignocellulosic materials. Weedy materials may be the next-generation choice for biofuel, as they do not impose additional growth requirements for sustainability. It is advised to select C4 grasses such as sugarcane, switch grass, and Miscanthus, which have marginal requirements for growth. The increased demand for ethanol can be met by focused exploration of cheap lignocellulosic feedstock; pretreatment; elimination of detoxification steps (removal of fermentation inhibitors); a cost-effective, highly thermostable, synergistically acting enzyme mixture; development of robust fermentation microorganisms; and process integration to minimize process energy demand, including cost-efficient use of lignin (Fig. 2).
The development of efficient microorganisms can follow three paths: (1) making P. stipitis, C. shehatae, and recombinant E. coli more resistant to inhibitors; (2) genetic engineering of microorganisms (i.e., S. cerevisiae or Z. mobilis) for xylose fermentation and insertion of a laccase gene to eliminate the detoxification step for pentose hydrolysates; and (3) metagenomics of natural genes to produce an efficient fermentation process. In addition to optimize ethanol yields, a variety of microorganisms can be developed with the ability to utilize cellulosic and hemicellulosic sugars and tolerate high alcohol content and fermentation inhibitors. A more efficient distillation procedure for fermented broth must also be developed to economize the overall process. Developing a cheap process for ethanol recovery from lignocellulose hydrolysate fermented broth is one of the biofuel industry’s biggest challenges.
To create a sustainable generation of biofuels, using modern genetic engineering tools to produce tailor-made perennial plants and trees with increased amounts of biomass is an unavoidable necessity (Somerville et al. 2010; Harris et al. 2009). However, despite the promise of modern genetic engineering techniques, concerns about the environmental impact of genetically engineered plants cannot be ignored. In the USA, the FDA, USDA, and EPA are responsible for ensuring the safety of crops through regulations (Ragauskas et al. 2006). Several agencies in other countries monitor GE crops and frame guidelines for the safe application of recombinant genes in agro-industries (Singh 2010).
Conclusion
Lignocellulosic biomass is gaining popularity as a source of fermentable sugars for liquid fuel production. To use wood and/or weedy substrates as energy crops for commercial production, significant improvements will be required in the growth of feedstock. Recent advances in functional genomics and plant biotechnology have helped identify the genes and transcription factors that control wood formation and cellulase composition in fungi and bacteria. These advancements include potential approaches to develop ethnologic traits for fermenting pentose and hexose sugars and withstanding fermentative inhibitors, which may provide significant opportunities to genetically optimize tree crops as a cheap feedstock with high cellulase titers and high ethanol production.
References
Ackerson MD, Clausen EC, Gaddy JL (1991) Production of ethanol from MSW via concentrated acid hydrolysis of the lignocellulosic fraction. In: Klass DL (ed) Energy from biomass wastes Vol.XV. Institute of Gas Technology, Chicago, IL, pp 725–743
Agbogbo FK, Wenger KS (2007) Production of ethanol from corn stover hemicelluloses hydrolysate using Pichia stipitis. J Ind Microb Biotechnol 34:723–727
Alam MZ, Mamun AA, Qudsieh IY, Muyibi SA, Salleh HM, Omar NM (2009) Solid state bioconversion of oil palm empty fruit bunches for cellulase enzyme production using a rotary drum bioreactor. Biochem Eng J 46(1):61–64
Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G (2006) Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314:1565–1568
Alriksson B, Rose SH, van Zyl WH, Sjöde A, Nilvebrant NO, Jönsson LJ (2009) Cellulase Production from spent lignocellulose hydrolysates by recombinant Aspergillus niger. Appl Environ Microbiol 75:2366–2374
Arifeen N, Wang R, Kookos I, Webb C, Koutinas AA (2009) Optimization and cost estimation of novel wheat bio-refining for continuous production of fermentation feedstock. Biotechnol Prog 23:872–880
Bajwa PK, Pinel D, Martin VJJ, Trevors JT, Lee H (2010) Strain improvement of the pentose-fermenting yeast Pichia stipitis by genome shuffling. J Microbiol Methods 81:179–186
Bak JS, Kim MD, Choi IG, Kim KH (2010) Biological pretreatment of rice straw by fermenting with Dichomitus squalens. N Biotechnol 30:424–434
Banerjee S, Mudliar S, Sen R, Giri B, Satpude D, Chakrabarti T, Pandey RA (2010) Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels Bioprod Bioref 4:77–93
Barbosa MF, Beck MJ, Fein JE, Potts D, Ingram LO (1992) Efficient fermentation of Pinus sp. acid hydrolysates by an ethanologenic strain of Escherichia coli. Appl Environ Microbiol 58:1382–1384
Betts WB, Dart RK, Ball AS, Pedlar SL (1991) Biosynthesis and structure of lignocellulose. In: Betts WB (ed) Biodegradation: natural and synthetic materials. Springer, Berlin, Germany, pp 139–155
Biswas GCG, Ransom C, Sticklen M (2006) Expression of biologically active Acidothermus cellulolyticus endoglucanase in transgenic maize plants. Plant Sci 171:617–623
Brulc JM, Antonopoulos DA, Miller ME, Wilson MK, Yannarell AC, Dinsdale EA, Edwards RE, Frank ED, Emerson JB, Wacklin P, Coutinho PM, Henrissat B, Nelson KE, White BA (2009) Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases. Proc Natl Acad Sci 106:1948–1953
Camassola M, Dillon AJP (2008) Biological pretreatment of sugar cane bagasse for the production of cellulases and xylanases by Penicillium echinulatum. Indus Crops Prod 29:642–647
Chandel AK, Chan EC, Rudravaram R, Narasu ML, Rao LV, Ravindra P (2007a) Economics and environmental impact of bioethanol production technologies: an appraisal. Biotechnol Mol Biol Rev 2:14–32
Chandel AK, Kapoor RK, Singh AK, Kuhad RC (2007b) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Biores Technol 98:1947–1950
Chandel AK, Narasu ML, Rudravaram R, Ravindra P, Narasu ML, Rao LV (2009a) Bioconversion of de-oiled rice bran (DORB) hemicellulosic hydrolysate into ethanol by Pichia stipitis NCIM3499 under optimized conditions. Int J Food Eng 2:1–12
Chandel AK, Narasu ML, Chandrasekhar G, Manikeyam A, Rao LV (2009b) Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Biores Technol 100:2404–2410
Chandel AK, Singh OV, Chandrasekhar G, Rao LV, Narasu ML (2010a) Key-drivers influencing the commercialization of ethanol based biorefineries. J Comm Biotechnol 16:239–257
Chandel AK, Singh OV, Rao LV (2010b) Biotechnological applications of hemicellulosic derived sugars: state-of-the-art. In: Singh OV, Harvey SP (eds) Sustainable biotechnology: renewable resources and new perspectives. Springer, Netherland, pp 63–81
Chandel AK, Singh OV, Chandrasekhar G, Rao LV, Narasu ML (2010c) Bioconversion of novel substrate, Saccharum spontaneum, a weedy material into ethanol by Pichia stipitis NCIM3498. Biores Technol. doi:10.1016/j.biotech.2010.08.016
Chandra M, Kalra A, Sangwan NS, Gaurav SS, Darokar MP, Sangwan RS (2009a) Development of a mutant of Trichoderma citrinoviride for enhanced production of cellulases. Biores Technol 100:1659–1662
Chandra RP, Ewanick SM, Chung PA, Au-Yeung K, Del Rio L, Mabee W, Saddler JN (2009b) Comparison of methods to assess the enzyme accessibility and hydrolysis of pretreated lignocellulosic substrates. Biotechnol Lett 31:1217–1222
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761
Desai SG, Guerinot ML, Lynd LR (2004) Cloning of the l-lactate dehydrogenase gene and elimination of lactic acid production via gene knockout in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl Microbiol Biotechnol 65:600–605
Dien BS, Iten LB, Skory CD (2005) Converting herbaceous energy crops to bioethanol; a review with emphasis on pretreatment processes. In: Hou CT (ed) Handbook of industrial biocatalysis, Chapter 23. Taylor and Francis, Boca Raton, FL, pp 1–11
Dogaris I, Vakontios G, Kalogeris E, Mamma D, Kekos D (2009) Induction of cellulases and hemicellulases from Neurospora crassa under solid-state cultivation for bioconversion of sorghum bagasse into ethanol. Ind Crops Prod 29:404–411
Endo A, Nakamura T, Ando A, Tokuyasu K, Shima J (2008) Genome-wide screening of the genes required for tolerance to vanillin, which is a potential inhibitor of bioethanol fermentation in Saccharomyces cerevisiae. Biotechnol Biofuels 1:3
Everitt JH, Lonard RL, Little CR (2007) Weeds in South Texas and Northern Mexico. Texas Tech University Press, Lubbock
Farrell A, Plevin R, Turner B, Jones A, O'Hare M, Kammen D (2006) Ethanol can contribute to energy and environmental goals. Science 311:506–508
Gupta R, Sharma KK, Kuhad RC (2009) Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, a woody substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis NCIM 3498. Biores Technol 100:1214–1220
Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953
Harris D, Stork J, Debolt H (2009) Genetic modification in cellulose-synthase reduces crystallinity and improves biochemical conversion to fermentable sugar. Glob Change Biol Bioener 1:51–61
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Nat Acad Sci 103:11206–11210
Himmel ME, Beyer EA (2009) Lignocellulose conversion to biofuels: current challenges, global perspectives. Curr Opin Biotechnol 20:316–317
Hinman ND, Schell DJ, Riley CJ, Bergeron PW, Walter PJ (1992) Preliminary estimate of the cost of ethanol production for SSF technology. Appl Biochem Biotechnol 34(35):639–649
Huber GW, Dale BE (2009) Grassoline at the pump. Sci Am Ind 4:40–45
Jeffries TW (2006) Engineering yeasts for xylose metabolism. Curr Opin Biotechnol 17:320–326
Jin YS, Alper H, Yang YT, Stephanopoulos G (2005) Improvement of xylose uptake and ethanol production in recombinant Saccaromyces cerevisiae through an inverse metabolic engineering approach. Appl Environ Microbiol 71:8249–8256
Jobling S (2004) Improving starch for food and industrial applications. Curr Opin Plant Biol 7:210–218
Kadam KL, Camobreco VJ, Glazebrook BE, Forrest LH, Jacobson WA, Simeroth DC, Blackburn WJ, Nehoda KC (1999) Environmental life cycle implications of fuel oxygenate production from California biomass. National Renewable Energy Laboratory: Golden, Colorado. NREL Report no. NREL/TP-580-25688
Katahira SA, Mizuike FH, Kondo A (2006) Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide- assimilating yeast strain. Appl Microbiol Biotechnol 72:1136–1143
Kim S, Dale EB (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 26:361–375
Kotter P, Ciriacy M (1993) Xylose fermentation by Sacharomyces cerevisiae. Appl Microbiol Biotechnol 38:776–783
Kuhad RC, Mehta G, Gupta R, Sharma KK (2010) Fed batch enzymatic saccharification of newspaper cellulosics improves the sugar content in the hydrolysates and eventually the ethanol fermentation by Saccharomyces cerevisiae. Biomass Bioenergy 34:1189–1194
Kumar R, Singh S, Singh OV (2008) Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35:377–391
Kumar A, Singh LK, Ghosh S (2009a) Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipitis. Biores Technol 100:3293–3297
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009b) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Kurup SC, Snishamol C, Prabhu NG (2005) Cellulase production by native bacteria using water hyacinth as substrate under solid state fermentation. Malay J Micorbiol 1:25–29
Kuyper M, Hartog MMP, Toirkens MJ, Almering MJH, Winkler AA, Van Dijken JP, Pronk JT (2005) Metabolic engineering of a xyloseisomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res 5:399–409
Lark N, Xia Y, Qin CG, Gong CS, Tsao GT (1997) Production of ethanol from recycled paper sludge using cellulase and yeast, Kluveromyces marxianus. Biomass Bioenergy 12:135–143
Larsson S, Cassland P, Jönsson LJ (2001) Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl Environ Microbiol 67:1163–1170
Lawford HG, Rousseau JD (1991) Fuel ethanol production from hard wood hemicelluloses hydrolysate by genetically engineered Escherichia coli carrying genes from Zymomonas mobilis. Biotechnol Lett 13:191–196
Lefebvre S, Lawson T, Fryer M, Zakhleniuk OV, Lloyd JC, Raines CA (2005) Increased sedoheptulose-1, 7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138:451–460
Lemus R, Lai R (2005) Bioenergy crops and carbon sequestration. Crit Rev Plant Sci 24:1–21
Li W, Zhang WW, Yang MM, Chen YL (2008a) Cloning of the thermostable cellulase gene from newly isolated Bacillus subtilis and its expression in Escherichia coli. Mol Biotechnol 40:195–201
Li X, Weng JK, Chapple C (2008b) Improvement of biomass through lignin modification. Plant J 54:569–581
Liming X, Xueliang S (2004) High-yield cellulase production by Trichoderma reesei ZU-02 on corn cob residue. Biores Technol 91:259–262
Lu XM, Yin WB, Hu ZM (2006) Chloroplast transformation. Methods Mol Biol 318:285–303
Lynd LR, van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583
Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, Danchin EG, Grigoriev IV, Harris P, Jackson M, Kubicek CP, Han CS, Ho I, Larrondo LF, de Leon AL, Magnuson JK, Merino S, Misra M, Nelson B, Putnam N, Robbertse B, Salamov AA, Schmoll M, Terry A, Thayer N, Westerholm-Parvinen A, Schoch CL, Yao J, Barabote R, Nelson MA, Detter C, Bruce D, Kuske CR, Xie G, Richardson P, Rokhsar DS, Lucas SM, Rubin EM, Dunn-Coleman N, Ward M, Brettin TS (2008) Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotechnol 26:553–560
Moiser N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Biores Technol 96:673–686
Morrison M, Pope PB, Denman SE, McSweeney CS (2009) Plant biomass degradation by gut microbiomes: more of the same or something new? Curr Opin Biotechnol 20:358–363
Neureiter M, Danner H, Thomasser C, Saidi B, Braun R (2002) Dilute-acid hydrolysis of sugarcane bagasse at varying conditions. Appl Biochem Biotechnol 98:49–58
Nevoigt E (2008) Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mole Biol Rev 72:379–412
Ng TL, Eheart JW, Cai X, Miguez F (2010) Modeling Miscanthus in the soil and water assessment tool (SWAT) to simulate its water quality effects as a bioenergy crop. Environ Sci Technol 44:7138–7144
Nigam JN (2001) Ethanol production from wheat straw hemicelluloses hydrolysate by Pichia stipitis. J Biotechnol 87:17–27
Olofsson K, Palmqvist B, Lidén G (2010) Improving simultaneous saccharification and co-fermentation of pretreated wheat straw using both enzyme and substrate feeding. Biotechnol Biofuels 3:17
Olsson L, Hahn-Hagerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol 18:312–331
Olsson L, Hahn-Hagerdal B, Zacchi G (1995) Kinetics of ethanol production by recombinant Escheichia coli K011. Biotechnol Bioeng 45:356–365
Parawira W, Tekere M (2010) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol. doi:10.3109/07388551003757816
Parrish DJ, Fike JH (2009) Selecting, establishing, and managing switch grass (Panicum virgatum) for biofuels. Methods Mol Biol 581:27–40
Pasha C, Valli N, Rao LV (2007) Lantana camara for fuel ethanol production using thermotolerant yeast. Lett Appl Microbiol 44:666–672
Perlack RD, Wright A, Turhollow R, Stokes GB, Erbach D (2005) Biomass as feedstock for a bioenergy and bio-products industry: the technical feasibility of a billion-ton annual supply. Washington, USDA. DOE/GO-102005-2135, ORNL/TM- 2005/66
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Reddy MSS, Chen F, Shadle G, Jackson L, Aljoe H, Dixon RA (2005) Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc Natl Acad Sci 102:16573–16578
Rubin EM (2008) Genomics of cellulosic biomass. Nature 454:841–845
Sanchez RG, Karhumaa K, Fonseca C, Nogué VS, Almeida JRM, Larsson CU, Bengtsson O, Bettiga M, Hahn-Hägerdal B, Gorwa-Grauslund MF (2010) Improved xylose and arabinose utilization by an industrial recombinant Saccharomyces cerevisiae strain using evolutionary engineering. Biotechnol Biofuels 3:13
Sarkar P, Bosneaga E, Auer M (2009) Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J Exp Bot 60:3615–3635
Scordia D, Cosentino SL, Jeffries TW (2010) Second generation bioethanol production from Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hack. Biores Technol 101:5358–5365
Seo JS, Chong HY, Park HS, Yoon KO, Jung C, Kim JJ, Hong JH, Kim H, Kim JH, Kil JI, Park CJ, Oh HM, Lee JS, Jin SJ, Um HW, Lee HJ, Oh SJ, Kim JY, Kang HL, Lee SY, Lee KJ, Kang HS (2005) The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat Biotechnol 23:63–68
Shou H, Bordallo P, Wang K (2004) Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. J Exp Bot 55:1013–1019
Singh OV (2010) Regulation and safety assessment of genetically engineered food. Stu Ethics Law Technol. doi:10.2202/1941-6008.1100
Somerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for lignocellulosic biofuels. Science 329:790–792
Sørensen A, Teller PJ, Hilstrøm T, Ahring BK (2008) Hydrolysis of Miscanthus for bioethanol production using dilute acid presoaking combined with wet explosion pre-treatment and enzymatic hydrolysis. Biores Technol 99:6602–6607
Sreenath HK, Koegel RG, Moldes AB, Jeffries TW, Straub RJ (2001) Ethanol production from alfalfa fiber fractions by saccharification and fermentation. Proc Biochem 36:1199–1204
Sticklen M (2008) Plant genetic engineering for biofuel production: towards affordable cellulosic ethanol. Nat Rev Genet 9:433–443
Sukumaran RK, Singhania RR, Mathew GM, Pandey A (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Ren Ener 34:421–424
Suryawati L, Wilkins MR, Bellmer DD, Huhnke RL, Maness NO, Banat IM (2009) Effect of hydrothermolysis process conditions on pretreated switchgrass composition and ethanol yield by SSF with Kluyveromyces marxianus IMB4. Proc Biochem 44:540–545
Szijártó N, Faigl Z, Réczey K, Mézes M, Bersényi A (2004) Cellulase fermentation on a novel substrate (waste cardboard) and subsequent utilization of home-produced cellulase and commercial amylase in a rabbit feeding trial. Indus Crops Prod 20:49–57
Tabka MG, Herpoël-Gimbert I, Monod F, Asther M, Sigoillot JC (2006) Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enz Microb Technol 39:897–902
Taherzadeh MJ, Niklasson C, Lidén G (1999) Conversion of dilute-acid hydrolyzates of spruce and birch to ethanol by fed-batch fermentation. Biores Technol 69:59–66
Tilman D, Hill J, Lehman C (2007) Carbon-negative biofuels from low-input high-diversity grass and biomass. Science 314:1598–1600
Torney F, Moeller L, Scarpa A, Wang K (2007) Genetic engineering approaches to improve bioethanol production from maize. Curr Opin Biotechnol 18:193–199
Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, Schein J, Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A, Brunner A, Busov V, Campbell M, Carlson J, Chalot M, Chapman J, Chen GL, Cooper D, Coutinho PM, Couturier J, Covert S, Cronk Q, Cunningham R, Davis J, Degroeve S, Déjardin A, Depamphilis C, Detter J, Dirks B, Dubchak I, Duplessis S, Ehlting J, Ellis B, Gendler K, Goodstein D, Gribskov M, Grimwood J, Groover A, Gunter L, Hamberger B, Heinze B, Helariutta Y, Henrissat B, Holligan D, Holt R, Huang W, Islam-Faridi N, Jones S, Jones-Rhoades M, Jorgensen R, Joshi C, Kangasjärvi J, Karlsson J, Kelleher C, Kirkpatrick R, Kirst M, Kohler A, Kalluri U, Larimer F, Leebens-Mack J, Leplé JC, Locascio P, Lou Y, Lucas S, Martin F, Montanini B, Napoli C, Nelson DR, Nelson C, Nieminen K, Nilsson O, Pereda V, Peter G, Philippe R, Pilate G, Poliakov A, Razumovskaya J, Richardson P, Rinaldi C, Ritland K, Rouzé P, Ryaboy D, Schmutz J, Schrader J, Segerman B, Shin H, Siddiqui A, Sterky F, Terry A, Tsai CJ, Uberbacher E, Unneberg P, Vahala J, Wall K, Wessler S, Yang G, Yin T, Douglas C, Marra M, Sandberg G, Van de Peer Y, Rokhsar D (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604
Varshney VK, Gupta PK, Naithani S, Khullar R, Bhatt A, Soni PL (2006) Carboxy methylation of alpha-cellulose isolated from Lantana camara with respect to degree of substitution and rheological behavior. Carbohydr Polym 63:40–45
Vega-Sanchez ME, Ronald PC (2010) Genetic and biotechnological approaches for biofuel crop improvement. Curr Opin Biotechnol 21:218–224
von Sivers MV, Zacchi G, Olsson L, Hahn-Hägerdal B (1994) Cost analysis of ethanol production from willow using recombinant Escherichia coli. Biotechnol Prog 10:555–560
Walfridsson M, Bao X, Anderlund M, Lilius G, Bulow L, Hahn-Hagerdal B (1996) Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xyl A gene, which expresses an active xylose (glucose) isomerase. Appl Environ Microbiol 12:4648–4651
Wang Z, Chen X, Wang J, Liu T, Liu Y, Zhao L, Wang G (2007) Increasing maize seed weight by enhancing the cytoplasmic ADPglucose pyrophosphorylase activity in transgenic maize plants. Plant Cell Tissue Organ Cult 88:83–92
Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernández M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR (2007) Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 450:560–565
Watanabe S, Saleh AA, Pack SP, Annaluru N, Kodaki T, Makino K (2007) Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein-engineered NADH-preferring xylose reductase from Pichia stipitis. Microbiol 153:3044–3054
Wayman M, Parekh SR (1990) Biotechnology of biomass conversion; Fuels and chemicals from renewable resources. Open University Press, Milton, Keynes
Wei T, Ogbon J, McCoy A (2001) Genetic engineering and lignin biosynthetic regulation in forest tree species. J Forestry Res 12:75–83
Wilson DB (2009) Cellulases and biofuels. Curr Opin Biotechnol 20:295–299
Wingren A, Galbe M, Zacchi G (2003) Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog 9:1109–1117
Wiselogel A, Tyson S, Johnson D (1996) Biomass feedstock resources and composition. In: Wyman CE (ed) Handbook on bioethanol: production and utilization. Taylor & Francis, Washington, DC, pp 105–118
Wooley R, Ruth M, Glassner D, Sheehan J (1999) Process design and costing of bioethanol technology: a tool for determining the status and direction of research and development. Biotechnol Prog 15:794–803
Wyman CE (2007) What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol 25:153–157
Yang B, Wyman CE (2006) BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnol Bioeng 94:611–617
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34
Zhao X, Zhang L, Liu D (2007) Comparative study on chemical pretreatment methods for improving enzymatic digestibility of Crofton weed stem. Biores Technol 99:3729–3736
Zhao X, Zhang L, Liu D (2010) Pretreatment of Siam weed stem by several chemical methods for increasing the enzymatic digestibility. Biotechnol J 5:493–504
Zhu Y, Lee YY, Elander RT (2007) Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and co-fermentation. Appl Biochem Biotechnol 137–140:721–738
Zhu JY, Zhu W, O’ Bryan PJ, Dien BS, Tian S, Gleisner R, Pan XJ (2010) Ethanol production from SPORL-pretreated lodgepole pine: preliminary evaluation of mass balance and process energy efficiency. Appl Microbiol Biotechnol 86:1355–1365
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandel, A.K., Singh, O.V. Weedy lignocellulosic feedstock and microbial metabolic engineering: advancing the generation of ‘Biofuel’. Appl Microbiol Biotechnol 89, 1289–1303 (2011). https://doi.org/10.1007/s00253-010-3057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-3057-6