Abstract
Dwindling reserves of fossil fuel and petroleum derivatives, rising oil prices, concern about environmental impact, and supply insecurity demand environmentally sustainable energy sources. Production of fuels and chemicals through microbial fermentation of plant material that uses renewable feedstock is a desirable alternative to petrochemicals. Lignocellulose represents the most widespread and abundant source of carbon in nature and is the only source that could provide a sufficient amount of feedstock to satisfy the world’s energy and chemical needs in a renewable manner. Typically, most of the agricultural lignocellulosic biomass is comprised of about 10–25% lignin, 20–30% hemicellulose, and 40–50% cellulose. The processing and utilization of this substrate are complex, differing in many aspects from crop-based ethanol production. Sustainable and economically viable manufacturing of bioethanol from lignocellulose raw material is dependent on the availability of a robust ethanol-producing microorganism, able to ferment all sugars present in the feedstock. Thus, an obvious target in the field of metabolic engineering has been the tailoring of such a microorganism, combining advantageous traits from different microorganisms with classical procedures such as random mutagenesis. Nowadays research is being directed to develop metabolically and genetically engineered Saccharomyces strains and other ethanol-fermenting microbes that has the potential to utilize wide range of substrates including pentose and hexose sugars, ability for direct and efficient ethanol production from cellulosic materials, and ability to tolerate ethanol stress. Although it is still in its infancy, metabolic engineering and synthetic biology offer great potential to overcome the challenges associated with lignocellulose bioconversion.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
Rapid depletion of bioenergetics resources and the environmental compliance concerning the greenhouse gases has attracted the awareness in nonconventional fuel from natural resources. Production of fuels and chemicals through microbial fermentation of plant material that uses renewable feedstock is a desirable alternative to petrochemicals. Mature technologies for ethanol production are crop based, utilizing substrates such as sugarcane juice and corn starch. But there are huge concerns regarding the increasing diversion of starch- or sucrose-rich crop materials and land from food to biofuel production. This has shifted attention to the use of lignocellulose-derived bioethanol as a biofuel (Morales et al. 2015). For the past few years, the biomass-based ethanol has caught the attention of global industry. Agro-industrial biomass comprised on lignocellulosic waste is an inexpensive, renewable, and abundant and provides a unique natural resource for large-scale and cost-effective bioenergy collection. Recently lignocellulosic biomasses have gained increasing research interests and special importance because of their renewable nature (Chen 2014). To expand the range of natural bioresources, the rapidly developing tools of genetic engineering can lower the conversion costs and also enhance target yield of the product of interest.
Bioethanol can be used as fuel with significant characteristics like high octane number, low cetane number, and high heat of vaporization. Its main drawbacks are the corrosiveness, low flame luminosity, lower vapor pressure, miscibility with water, and toxicity to ecosystems. One crucial problem with bioethanol fuel is the availability of raw materials. The supply of feedstocks for bioethanol production can vary season to season and depends on geographic locations. Lignocellulosic biomass, such as forest-based woody materials, agricultural residues, and municipal waste, is prominent feedstock for bioethanol because of its high availability and low cost (Dominguez et al. 2015). In addition, the supply and the attentive use of microbes render the bioethanol production process highly peculiar.
Lignocellulosic (cellulosic) biomass-derived ethanol is often termed as “second generation” or “2G” as the “first generation” or “1G” ethanol is derived from sugarcane, corn, wheat, and other starchy feedstocks (Jordan et al. 2012), the most promising near-/long-term fuel candidate. In addition, cellulosic biomass-derived ethanol may serve as a precursor to other fuels and chemicals that are currently derived from unsustainable sources and/or are proposed to be derived from cellulosic biomass. Bioethanol production will be probably the most successful biofuel because it has plenty of usable forms (heat, power, electricity, or vehicle fuel). The benefits estimated from mandated use of cellulosic biofuels include nation’s energy security through domestic production of transportation fuel and environmental improvement through greenhouse gas mitigation and other particulate emissions associated with fossil fuel combustion. Additional benefits include creating new markets for agricultural products, keeping productive farmland in use, and improving trade balances.
16.2 Physicochemical Characteristics of Lignocellulosic Biomass
Biomass refers to renewable organic materials, including agricultural products and agricultural wastes, wood and its wastes, animal wastes, urban wastes, aquatic plants, and so on. Lignocellulosic complex is regarded as the most abundant biopolymer in the earth constantly generated through photosynthesis and as one of the potential raw materials for ethanol production (da Silva 2016). About 50% of the world biomass is considered as the lignocellulosic biomass, and its total annual production is estimated to be approximately 10–50 billion ton (Mood et al. 2013). Generally, lignocellulosic biomass for bioethanol (as fuel) production can be differentiated into the following six groups: crop residues (sugarcane bagasse, sweet sorghum bagasse, pulp, wheat straw, rice straw, rice hulls, and barley straw), softwood (pine and spruce), hardwood (aspen and poplar), cellulose wastes material such as newsprint and waste office paper, herbaceous biomass material (timothy grass, alfalfa hay, switch grass, coastal Bermuda grass), and municipal solid wastes (Singh et al. 2012).
Lignocellulose, the principal component of the plant cell walls, is mainly composed of cellulose (40–60% of the total dry weight), hemicellulose (20–40%), and lignin (10–25%) (Dionisi et al. 2015), together with small amounts of other components, like acetyl groups, minerals, and phenolic substituents. Depending on the type of lignocellulosic biomass, these polymers are organized in complex non uniform three-dimensional structures to different degrees and varying relative composition. Lignocellulose has evolved to resist degradation, and this robustness or recalcitrance of lignocellulose stems from the crystallinity of cellulose, hydrophobicity of lignin, and encapsulation of cellulose by the lignin-hemicellulose matrix (Agbor et al. 2011). Its composition depends not only on the type of plant but also on the selected part of the plant (Brown 1999) and on growth conditions (Wiselogel and Johnsson 1996). This material differs from products with high sugar and starch content (McMillan 1997).
The major component of lignocellulosic biomass is cellulose, being the most abundant and easily available carbohydrate polymer all around the earth which is a major polysaccharide constituent of plant cell wall, composed of repeating (1,4)-D-glucopyranose units, which are attached by β-1,4 linkages with an average molecular weight of around 100,000 (Himmel et al. 2007). Naturally cellulose molecules exist as bundles which aggregated together in the form of microfibrils order, i.e., crystalline and amorphous regions (Taherzadeh and Karimi 2008).
The second most abundant polymer after cellulose is hemicellulose which is heterogeneously branched in nature. The backbone of the hemicellulose polymer is built up by sugar monomers like xylans, mannans, and glucans, with xylans and mannans being the most common (Ladisch and Lee 2005); in this case xylanases are the enzymes involved in its degradation. Hemicelluloses differ in composition too; hardwood hemicelluloses contain mostly xylans, whereas softwood hemicelluloses contain mostly glucomannans. The heteropolymers of hemicellulose are composed of different 5- and 6-carbon monosaccharide units: pentoses (xylose, arabinose), hexoses (mannose, glucose, galactose), and acetylated sugars. Hemicelluloses are embedded in the plant cell walls to form a complex network of bonds that provide structural strength by linking cellulose fibers into microfibrils and cross-linking with lignin (Schellar and Ulvskov 2010). Cellulose and hemicellulose bind tightly with noncovalent attractions to the surface of each cellulose microfibril. Hemicellulose degrades quickly due to its amorphous nature (Hamelinck et al. 2005). Among other important aspects of the structure and composition of hemicellulose are the lack of crystalline structure, mainly due to the highly branched structure, and the presence of acetyl groups connected to the polymer chain.
Lignin is generally the most complex and smallest fraction of the biomass. Lignin is a three-dimensional polymer of phenylpropanoid units. The oxidative coupling of three different phenylpropane building blocks, monolignols: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, forms the structure of lignin. The corresponding phenylpropanoid monomeric units in the lignin polymer are identified as p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units, respectively. Lignin acts like a glue by filling the gap between and around the cellulose and hemicellulose complexion with the polymers. It is present in almost all kind of cellulosic plant biomass and acts as a protective sheet against cellulosic and hemicellulosic components of the biomass materials. It adds compressive strength to the plant tissue and the individual fibers, stiffness to the cell wall, and resistance against insects and pathogens (Rubin 2008).
16.3 Cellulosic Biomass to Ethanol
In 2014 the global production of bioethanol reached 24.5 billion gallon, up from 23.4 billion gal in 2013 which shows the international bioethanol market is at a very dynamic stage. More than half (60%) of global bioethanol production is based on sugarcane conversion, and the rest (40%) comes from other crops (Dufey 2006). The United States (corn) and Brazil (sugarcane) are the global producers as they produce 70% of the global bioethanol production.
Thus most ethanol produced to date as biofuel is generated from edible crops. However, this “first generation” approach led to the “food versus fuel” conflict and dilemma leading to search for alternative biomass sources for the “next generation biofuels” mostly based on cellulose (Valentine et al. 2012). Plant cell walls are the most abundant renewable resource on our planet with 150-170×109 tons produced annually (Pauly and Keegstra 2008). The major components of plant cell walls are cellulose, hemicellulose, and lignin that comprise around 90% of its dry biomass (Gibson 2012). Lignocellulose offers several benefits over sugar and starch as a substrate for bioethanol production (Morales et al. 2015). Lignocellulosic biomass, which covers a large category of agricultural biomass, has minimized the potential conflict between land use for food production and production of energy feedstock. Thus, the production of ethanol from the cell walls of non-crop plants or nonedible parts of plants is considered a sustainable solution for biofuel production. This is despite the current difficulties related to the costs, high energy inputs, and harsh conditions required to process the complex cell wall polymers into fermentable sugars. The raw material is cheaper compared to conventional agricultural feedstock and can be produced along with lower involved fertilizers, pesticides, and energy. Biofuels from lignocellulosic biomass have low emissions of greenhouse gas and reduce environmental impacts, including climate change. Biofuels might also provide employment in rural areas. A large number of studies for developing large-scale ethanol production from lignocellulosic biomass have been carried out worldwide. The complex composition of lignocellulosic materials is a key factor affecting the efficiency of bioethanol production during the conversion processes (Dixon 2013), and this is directly related to the composition of lignocellulosic material. Cellulose and hemicellulose are the two main polymers of the biomass that break down into fermentable sugars, which are further converted into ethanol, but the breakdown of lignocellulosic biomass is a complicated and energy-consuming process (Kaur et al. 2013).
Low-carbon biofuels from commercial-scale cellulosic ethanol have become a reality in recent times. Numerous cellulosic ethanol refineries have now come online worldwide with several more in the pipeline (European Biofuels Technology Platform 2015). To date, the largest cellulosic ethanol industrial-scale refinery is the Beta Renewables/Novozymes-funded plant situated at Crescentino in Northwestern Italy which commenced operations in October 2013. The facility is entirely self-sufficient, using the lignin and biogas by-products to power the plant which generates 75 million liters annually of cellulosic ethanol, enough fuel for more than 50,000 cars. In the United States, there are over 200 corn-based ethanol plants in operation (Gnansounou 2010). Many of these bioethanol plants are evolving to become cellulosic ethanol production facilities utilizing cheaper agricultural residues and nonfood substrates. Encouraging yields ranging from 68 to 83 gallons per tonne of biomass have recently been reported by several bioenergy groups such as Abengoa Bioenergy, Iogen Energy, and Poet, LLC from their respective pilot cellulosic ethanol plants (Guo et al. 2015). With the inevitable upsurge in oil price back to pre-2014 levels an unavoidable reality allied with advancements in the relevant technology, industrial-scale lignocellulosic bioethanol will continue to spread worldwide in the near future.
16.4 Processes for Bioethanol Production
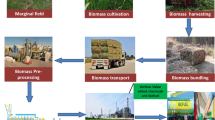
One of the most important goals of lignocellulosic biomass refining is to fractionate lignocellulose into its three major components: cellulose, hemicelluloses, and lignin. Single-step treatment methods, like pyrolysis, are not efficient. Although they render lower costs, deconstruction of the lignocellulosic biomass takes place since these methods generally rely on high temperatures. It is highly inconvenient and difficult to separate the targeted chemicals and fuels via single-step methods because the produced bio-oil consists of a mixture of hundreds of compounds. For downstream and efficient separations, additional costs and various pretreatment methods are required. Application of the pretreatment methods changes the natural-binding characteristics of lignocellulosic materials by modifying the supramolecular structure of cellulose-hemicellulose-lignin matrix. Therefore, this robust and complex structure in order to be converted into bioalcohols requires a multistep process, including pretreatment, enzymatic hydrolysis, and fermentation that increases the cost of biofuels production significantly (Kumagai et al. 2014).
Pretreatment is a processing step to make lignocellulosic biomass more amenable to biological conversion at high yields that otherwise suffers from low yields and high processing costs (Wyman et al. 2013). Pretreatment methods are divided into different categories such as mechanical, chemical, physicochemical, and biological methods or various combinations of these (Barakat et al. 2013). Various pretreatment options were reported to fractionate, solubilize, hydrolyze, and separate cellulose, hemicellulose, and lignin components. Some of them include milling, irradiation, microwave, steam explosion, ammonia fiber explosion (AFEX), supercritical CO2 and its explosion, SO2, alkaline hydrolysis, liquid hot-water pretreatment, organosolv processes, wet oxidation, ozonolysis, dilute- and concentrated-acid hydrolyses, and biological pretreatments (Saha 2005). A few new promising pretreatments that have recently been developed include cosolvent-enhanced lignocellulosic fractionation (CELF) (Nguyen et al. 2015a, b), cosolvent-based lignocellulosic fractionation (COSLIF) (Zhang et al. 2007), extractive ammonia (EA) pretreatment (Chundawat et al. 2013), γ-valerolactone (GVL) pretreatment (Shuai et al. 2016; Wu et al. 2016), pretreatment applying ionic liquid(s) (Konda et al. 2014), sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL) (Zhu et al. 2009), and switchable butadiene sulfone pretreatment (de Frias and Feng 2013). The common goal of these methods is to reduce the biomass in size and open its physical structure. Each of these methods has been reported to have distinct advantages and disadvantages.
The performance of the hydrolysis is highly associated to the pretreatment process (Girio et al. 2010). During this reaction, cellulose and hemicellulose are hydrolyzed into simple and soluble compound available for further conversion (fermentation) to ethanol (Chandel et al. 2007). There are two different methods of hydrolysis processes that involve either acidic or enzymatic reactions. Acidic reactions require high quantity of acid which makes its usage less attractive (Hamelinck et al. 2005). Also, when acids are used in the hydrolysis, the phenomenon of chemical dehydration occurs on monosaccharide resulting in the appearance of other compounds like aldehydes. These limitations led to the researchers’ interest to focus on enzymatic hydrolysis. Compelling pretreatment is fundamental to an efficient enzymatic hydrolysis. Eggeman and Elander (2005) have demonstrated that Trichoderma reesei is a very efficient fungus to produce industrial grade cellulolytic enzymes. The use of metallic compounds like Ca2+ and Mg2+ could intensify the enzymatic hydrolysis (Liu et al. 2010).
High cost of cellulase and other accessory enzymes required for biological conversion of pretreated lignocellulosic biomass into sugars is another major impediment in the commercialization of lignocellulosic biomass to fuels and chemicals (Culbertson et al. 2013). In addition to enzymes, low accessibility to (hemi) cellulose, their strong inhibition by components generated during pretreatment (e.g., phenols) (Kim et al. 2011), and enzymatic saccharification (Kumar and Wyman 2014) are one of the main reasons for high loading of enzymes required for commercially viable sugar yields. In addition, enzymes’ unproductive binding to lignin (Li et al. 2013) and pseudo-lignin (Kumar and Wyman 2013) also lowers the amount of enzymes available and affects their effectiveness. Although cellulase end-product inhibition by glucose can be alleviated in a process configuration called simultaneous saccharification and fermentation (SSF), and inhibition by cellobiose and hemicellulose oligomers can be alleviated by supplementing cellulase with accessory enzymes, low reaction rates at fermentation temperatures (32–37 °C) (Elia et al. 2008) and inhibition by ethanol still pose a challenge to high yields and titers at low enzyme loadings (Podkaminer et al. 2011). The discovery of novel non-hydrolytic enzymes like polysaccharide monooxygenases (LPMOs) appears to be highly promising in reducing cellulase and ultimately overall processing costs (Agger et al. 2014). LMPOs have certain limitations like they require electron donor (Muller et al. 2015) and make cellulase cocktails less stable (Scott et al. 2016); in addition to loss of some of the carbohydrates and requirement of different process configurations, the aldonic acids resulting from polysaccharides oxidation by LPMOs can be inhibitory to enzymes as well as microbes (Cannella et al. 2012). Thus, it is still to be seen whether these new non-hydrolytic enzymes would be advantageous in the long run.
Fermentation is the following step and requires the presence of microorganisms to degrade sugars into alcohols and other end products. Typically S. cerevisiae converts the sugars into ethanol under anaerobic conditions at a temperature of 30 °C. In this pathway other by-products are also generated in the form of CO2 and N-based compounds. S. cerevisiae is known as the most studied microorganism for the fermentation of lignocellulosic hydrolyzates that ferment the glucose contained in hydrolyzate while unable to ferment pentose sugar such as xylose. S. cerevisiae is a prevalent microorganism and provides a high yield of ethanol (12.0–17.0% w/v; 90% of the theoretical yield) from sugars (Kumar et al. 2009).
Fermentation of biomass hydrolyzates involves processes done in separate units (hydrolysis and fermentation). This system is known as separate hydrolysis and fermentation (SHF). However, on the other hand, simultaneous saccharification and fermentation (SSF) is a process which is performed in a single unit.
16.4.1 Separate Hydrolysis and Fermentation (SHF)
The SHF is the traditional method for bioethanol production in which hydrolysis and fermentation are performed in separate units. In this process, a fraction contains the cellulose in an accessible form after pretreatment is subjected to hydrolysis. After completion of hydrolysis, the obtained cellulose hydrolyzate is converted into ethanol by fermentation. The interesting feature of SHF is that every step could be conducted at its optimal conditions so that the probability of product recovery is more (Sharma et al. 2011). The most important parameters to be taken into consideration for saccharification step are availability of cellulose (for glucose conversion), reaction time, temperature, pH, optimal enzyme unit, and substrate loading (Oberoi et al. 2012). Several studies have reported the weakness of S. cerevisiae to ferment only hexose sugars and the interest for versatile-acting microorganisms increased. To date, extensive research has been conducted to develop microorganisms which enable to (i) ferment pentose and hexose sugars synchronously available from the hemicellulose fraction and (ii) endure under inhibitory conditions.
16.4.2 Simultaneous Saccharification and Fermentation (SSF)
The SSF process is more attractive than the SHF due to high ethanol yield and less energy consumption. In this process, cellulases (for hydrolysis) along with microorganisms (for fermentation) are added in the same process unit allowing glucose formation and immediate consumption of glucose by microbial cells, resulting into ethanol production. Therefore, the inhibitory effect of sugars on cellulases is neutralized. However, the use of more diluted media makes the final product of low concentration. Moreover, this process proceeds at nonoptimal parameters of hydrolysis, and, at the same time, higher enzyme dosages are required, which enhance substrate conversion rate as well as the cost of the process. Alkasrawi et al. (2003) reported that the addition of nonionic surfactants, such as Tween-20 and Tween-80, to the steam-exploded wood in a batch of SSF by using S. cerevisiae gave 8% increment in ethanol yield, 50% reduction in cellulases dosage (from 44 FPU/g to 22 FPU/g of cellulose), and decrease in the time period required for reaching maximum ethanol concentration. It is felt that the surfactant prevents the unuseful adsorption of cellulases to lignin.
16.5 Consolidated Bioprocessing (CB)
Three main steps in lignocellulosic biomass conversion – enzymes production, biological hydrolysis of biomass to sugars and oligomers, and fermentative metabolites (e.g., ethanol) production – can be combined into a single bioprocessing system “direct microbial conversion (DMC)” (Demain et al. 2005) or lately known as “consolidated bioprocessing (CBP)” (Li et al. 2014).
A novel development, the consolidated bioprocessing (CBP) proceeds by producing all required enzymes and ethanol using a single type of microorganisms in a single reactor. CBP is considered as the ultimate evolution of biomass-to-bioethanol conversion technology, since it implies neither capital nor operating costs (Lynd et al. 2008) for dedicated enzyme production together with a reduced consumption of substrate for enzyme production.
Increasing evidence suggests that CBP may be feasible (Okamoto et al. 2014). Ever since the concept of CBP was proposed in 1996, CBP research has focused on the development of new and even more effective CBP microorganisms, which has been a key challenge (Lynd et al. 2005). Bacteria and yeast have been the primary candidates for CBP research, and some progress has been made in this regard (den Haan et al. 2015). There are several cellulolytic/non-cellulolytic and thermophilic/mesophilic candidate microorganisms for CBP including bacteria, e.g., Clostridium thermocellum (Shao et al. 2011), Thermoanaerobacterium saccharolyticum (Shaw et al. 2008), Clostridium phytofermentans (Jin et al. 2012), and Caldicellulosiruptor bescii (Chung et al. 2014), and yeasts, e.g., S. cerevisiae and thermotolerant K. marxianus (Yamada et al. 2013). Thermophiles have an added advantage of higher hydrolysis rates and less probability of contaminations at fermentation temperatures of >60 °C than mesophiles that usually operate at temperatures <50 °C (Olson et al. 2012). Caldicellulosiruptor bescii has recently been engineered to produce ethanol at high metabolic yield; however, the productive yields are too low for commercial application yet (Chung et al. 2014). In addition to thermophilic and other bacteria, research is also underway in modifying yeasts to convert them into CBP organisms (Yamada et al. 2013). However, most of these genetically engineered strains still need some supplementation of exogenous enzymes for high ethanol yields.
Fungi have not been widely proposed as CBP microorganisms, but there are a few recent reports of researchers developing strains of the fungi Fusarium oxysporum and Trichoderma reesei with enhanced CBP potential (Huang et al. 2014).
16.6 Genetic Engineering for the Fermentation of Lignocellulose into Ethanol
The utilization of lignocellulose as a raw material for a fermentation process imposes many demands on the potential microorganism, which therefore must display many of the features, viz., broad substrate utilization range, high ethanol yields and productivity, minimal by-product formation, high ethanol tolerance, etc.
The ability to utilize all sugars present in lignocellulose substrate is a prerequisite for the efficient production of ethanol from the raw material. Given the high ethanol on glucose (and sucrose) as well as the high ethanol tolerance of S. cerevisiae and Z. mobilis, an obvious approach was to expand their substrate utilization range, so that all monosaccharides in lignocellulosic materials are utilized. The preferred microorganism in crop-based processes, Saccharomyces cerevisiae, is unable to ferment pentoses and is therefore of limited use for lignocellulose substrates with a high content of pentoses, unless the necessary pathways are inserted and expressed. The same restriction applies to the ethanologen bacterium Zymomonas mobilis. Thus, the efficient utilization of xylose in hemicellulose in addition to glucose in cellulose by a recombinant xylose-fermenting S. cerevisiae strain would offer an opportunity to reduce the production cost of bioethanol significantly (Oh et al. 2013). To date, numerous studies regarding the metabolic engineering of S. cerevisiae for xylose utilization have been reported, and many reviews have already addressed the current advancement in metabolic engineering of xylose-fermenting strains and factors which affect xylose metabolism in yeasts (Xin et al. 2014).
With ethanol being a low value-added product, the overall yield in the conversion of sugars to ethanol is crucial. Utilizing crop sugars as substrates for ethanol production, yields of 90–95% of the theoretical can be obtained using S. cerevisiae or Z. mobilis, and yields in this range are also required for an economically feasible process based on lignocellulose as raw material. However, of equal importance to the yield is a high productivity, since the depreciation of capital investments also contributes significantly to the cost of ethanol production (Zaldivar et al. 2001). E. coli and several enteric bacteria naturally possess a broad substrate utilization range, converting hexoses (glucose, mannose, galactose, fructose), pentoses (xylose and arabinose), and uronic acids (galacturonic acid, glucuronic acid) to the central metabolite, pyruvate. This compound is further converted to a near-equal mix of ethanol, lactate, acetate, and formate (H2O plus CO2). Normally, fermentations are carried out at pH 7.0 and at temperatures between 30 and 35 °C. The main strategy to increase ethanol production in E. coli and make it suitable for lignocellulose processes was to redirect the carbon flux toward ethanol production, which was achieved in three main steps. The insertion of pdc and adhB genes from Z. mobilis, encoding for highly active ethanologenic enzymes; enabled E. coli to produce ethanol and CO2 from hexoses and pentoses at high efficiency; Control of a single promoter creating the PET (production of ethanol) operon (Ingram et al. 1987). The PET operon was subsequently introduced into several bacterial hosts. The direction of carbon flux toward ethanol formation was favored by the expression of high levels of heterologous pdc and adhB as well as by the fact that the original PDC from Z. mobilis has an affinity toward pyruvate higher than other homologous enzymes competing for pyruvate in E. coli, e.g., lactate dehydrogenase.
A well-known by-product in yeast fermentation is glycerol. During the formation of biomass, there is a net conversion of the cytosolic cofactor NAD+ to NADH. Since the respiratory chain is nonfunctional under anaerobic conditions, the only route to reconvert the cofactor to NAD+ is through glycerol formation. Thus, glyceraldehyde-3-P is converted to dihydroxyacetone-P to glycerol-3-P and further to glycerol. There are two genes, GPD1 and GPD2, encoding glyceraldehyde-3-phosphate dehydrogenase, the enzyme that regenerates NAD+ from NADH while converting dihydroxyacetone-P to glycerol-3-P, but Gpd2 is the most important for glycerol formation (Nissen et al. 2000). To overcome the various inhibitory substances, metabolic, genetic, evolutionary, and gene disruption strategies were used. For example, a GPD2 mutant of S. cerevisiae, grown under anaerobic conditions, had a 40% reduction in glycerol levels (relative to the amount of substrate consumed) and 8% higher ethanol yield than the unmodified strain. Succinate is another by-product generated in ethanol production. In order to eliminate succinate formation in ethanologenic E. coli KO4 and consequently increase the ethanol yield, the fumarate reductase gene (frd) was deleted generating E. coli strain KO11. In this strain, the channeling of a small fraction of phosphoenolpyruvate toward the formation of succinate was avoided (Ohta et al. 1991).
For low-value products such as ethanol, a product concentration as high as possible is essential for the process economy. What normally occurs is that, as the ethanol concentration in the broth increases, most microorganisms begin to experience some impairment of membrane integrity. According to Dombek and Ingram (1986), the 24 h response to ethanol stress correlates with the type of lipids in the cellular membrane. In fact, the two well-known ethanologens, S. cerevisiae and Z. mobilis, display peculiar membrane structures. Thus, the membrane of S. cerevisiae is rich in sterols, whereas the membrane of Z. mobilis is exceptionally rich in the fatty acid cis vaccenic acid, as well as in compounds known as hopanoids (analogous to sterols). S. cerevisiae tolerates up to 21% (w/v) ethanol (Walker 1998), whereas Z. mobilis tolerates up to 12% (w/v) ethanol (Rogers et al. 1996). Besides the cell membrane composition, factors such as the activity of plasma membrane ATPase and superoxide dismutase and the capacity of a strain to produce trehalose contribute to the ethanol tolerance trait in yeasts (Jeffries and Jin 2000). Some candidate proteins involved in the expression of stress-related genes like the zinc finger protein (MacPherson et al. 2006) and alcohol sensitive ring/PHD finger 1 protein (Asr1p) (Betz et al. 2004) also play a role in ethanol tolerance in Saccharomyces cerevisiae. Lastly, the global transcription machinery engineering (gTME) technology can reprogram gene transcription and then improve glucose/ethanol tolerance of yeast cells.
16.7 Future Prospects
With industrial development growing rapidly and increasing demand for energy, there is an urgent need for environmentally friendly energy sources. Bioethanol is considered as an important renewable fuel to replace fossil fuels. Lignocellulosic bioethanol is a potential pathway for the global producers which provide renewable fuels. Lignocellulosic biomass represents as a promising candidate for ethanol production, including their output, input energy ratio, and their huge availability in both tropical and temperate regions.
In recent years, using metabolic engineering along with random mutagenesis techniques, advancement in terms of the enhancement of microorganism capabilities by adding/modifying traits such as tolerance to ethanol and inhibitors, hydrolysis of cellulose/hemicellulose, thermotolerance, reduced need nutrient supplementation, and improvement of sugar transport is underway. Further, improving pretreatment method and identifying metabolic pathways through genetic engineering for pentose fermentation, genomic sequencing, environmental genomics, and/or metagenomic technologies may assist to make bioethanol production more economical, practical, and commercially feasible.
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29:675–685
Agger JW, Isaksen T, Várnai A, Vidal-Melgosa S, Willats WGT, Ludwig R, Horn SJ, Eijsink VGH, Westereng B (2014) Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc Natl Acad Sci U S A 111(17):6287–6292
Alkasrawi M, Eriksson T, Borjesson J, Wingren A, Galbe M, Tjerneld F, Zacchi G (2003) The effect of tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzym Microb Technol 33:71–78
Barakat A, de Vries H, Rouau X (2013) Dry fractionation process as an important step in current and future lignocellulose biorefineries. Bioresour Technol 134:362–373
Betz C, Schlenstedt G, Bailer SM (2004) Asrp, a novel yeast ring/PHD finger protein, signals alcohol stress to the nucleus. J Biol Chem 279:28174–28181
Brown RM Jr (1999) Cellulose structure and biosynthesis. Pure Appl Chem 71(5):767–775
Cannella D, Hsieh CW, Felby C, Jorgensen H (2012) Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol Biofuels 5(1):261
Chandel AK, Chan E, Rudravaram R, Narasu ML, Rao LV, Ravindra P (2007) Economics and environmental impact of bioethanol production technologies: an appraisal. Biotechnol Mol Biol Rev 2:14–32
Chundawat SPS, Bals B, Campbell T, Sousa L, Gao D, Jin M, Eranki P, Garlock R, Teymouri F, Balan V, Dale BE (2013) Primer on ammonia fiber expansion pretreatment. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, New York, pp 169–200
Chen H (2014) Biotechnology of lignocellulose: theory and practice. Springer, Dordrecht/Heidelberg/New York
Chung D, Cha M, Guss AM, Westpheling J (2014) Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc Natl Acad Sci U S A 111(24):8931–8936
Culbertson A, Jin M, da Costa SL, Dale BE, Balan V (2013) In-house cellulase production from AFEXTM pretreated corn stover using Trichoderma reesei RUT C-30. RSC Adv 3(48):25960–25969
da Silva (2016) Adding value to agro-industrial wastes. Ind Chem 2:2
de Frias JA, Feng H (2013) Switchable butadiene sulfone pretreatment of Miscanthus in the presence of water. Green Chem 15(4):1067–1078
Demain AL, Newcomb M, Wu JHD (2005) Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev 69(1):124–154
den Haan R, van Rensburg E, Rose SH, van Gorgens JF, van Zyl WH (2015) Progress and challenges in the engineering of non-cellulolytic microorganisms for consolidated bioprocessing. Curr Opin Biotechnol 33:32–38
Dionisi D, Anderson JA, Aulenta F, McCue A, Paton G (2015) The potential of microbial processes for lignocellulosic biomass conversion to ethanol: a review. J Chem Technol Biotechnol 90:366–383
Dixon RA (2013) Microbiology: break down the walls. Nature 493:36–37
Dombek KM, Ingram LO (1986) Determination of the intracellular concentration of ethanol in Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol 51:197–200
Dominguez-Bocanegra AR, Torres-Munoz JA, Lopez RA (2015) Production of bioethanol from agro-industrial wastes. Fuel 149:85–89
Dufey A (2006) Biofuels production, trade and sustainable development: emerging issues, Environmental economics programme, sustainable markets discussion paper no. 2. International Institute for Environment and Development (IIED), London
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatments technologies. Bioresour Technol 96:2019–2025
Elia TP, Jose MO, Mercedes B, Lisbeth O (2008) Comparison of SHF and SSF processes from steam-exploded wheat straw for ethanol production by xylose-fermenting and robust glucose-fermenting Saccharomyces cerevisiae strains. Biotechnol Bioeng 100(6):1122–1131
European Biofuels Technology Platform (2015) Newsletter 21, January 2015
Gibson LG (2012) The hierarchical structure and mechanics of plant materials. J Royal Soc Interface 9:2749–2766
Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Lukasic R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101:4775–4800
Gnansounou E (2010) Production and use of lignocellulosic bioethanol in Europe: current situation and perspectives. Bioresour Technol 101:4842–4850
Guo M, Song W, Buhain J (2015) Bioenergy and biofuels: history, status, and perspective. Renew Sust Energy Rev 42:712–725
Hamelinck CN, Van Hooijdonk G, Faaij APC (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 28:384–410
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuel production. Science 315:804–807
Huang J, Chen D, Wei Y, Wang Q, Li Z, Chen Y, Huang R (2014) Direct ethanol production from lignocellulosic sugars and sugarcane bagasse by a recombinant Trichoderma reesei strain HJ48. Sci World J 2014:798683
Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF (1987) Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 53:2420–2425
Jeffries TW, Jin Y-S (2000) Ethanol and thermotolerance in the bioconversion of xylose by yeasts. Adv Appl Microbiol 47:222–268
Jin M, Gunawan C, Balan V, Dale BE (2012) Consolidated bioprocessing (CBP) of AFEX™-pretreated corn stover for ethanol production using Clostridium phytofermentans at a high solids loading. Biotechnol Bioeng 109(8):1929–1936
Jordan DB, Bowman MJ, Braker JD, Dien BS, Hector RE, Lee CC, Mertens JA, Wagschal K (2012) Plant cell walls to ethanol. Biochem J 442(2):241–252
Kaur B, Sharma M, Soni R, Oberoi HS, Chadha BS (2013) Proteome-based profiling of hypercellulase-producing strains developed through interspecific protoplast fusion between Aspergillus nidulans and Aspergillus tubingensis. Appl Biochem Biotechnol 163:577–591
Kim Y, Ximenes E, Mosier NS, Ladisch MR (2011) Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym Microb Technol 48(4–5):408–415
Konda N, Shi J, Singh S, Blanch H, Simmons B, Klein-Marcuschamer D (2014) Understanding cost drivers and economic potential of two variants of ionic liquid pretreatment for cellulosic biofuel production. Biotechnol Biofuels 7(1):86
Kumagai A, Kawamura S, Lee SH, Endo T, Rodriguez M Jr, Mielenz JR (2014) Simultaneous saccharification and fermentation and a consolidated bioprocessing for Hinoki cypress and Eucalyptus after fibrillation by steam and subsequent wet-disk milling. Bioresour Technol 162:89–95
Kumar R, Wyman CE (2013) Physical and chemical features of pretreated biomass that influence macro-/micro-accessibility and biological processing. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, New York, pp 281–310
Kumar R, Wyman CE (2014) Strong cellulase inhibition by mannan polysaccharides in cellulose conversion to sugars. Biotechnol Bioeng 111(7):1341–1353
Kumar S, Singh SP, Mishra IM, Adhikari DK (2009) Recent advances in production of bioethanol from lignocellulosic biomass. Chem Eng Technol 32:517–526
Ladisch MR, Lee YY (2005) Coordinated development of leading biomass pretreatment technologies. Bioresour Technol 96:1959–1966
Li H, Pu Y, Kumar R, Ragauskas AJ, Wyman CE (2013) Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol Bioeng 111(3):485–492
Li J, Lin J, Zhou P, Wu K, Liu H, Xiong C, Gong Y, Xiao W, Liu Z (2014) One-pot simultaneous saccharification and fermentation: a preliminary study of a novel configuration for cellulosic ethanol production. Bioresour Technol 161:171–178
Liu H, Zhu JY, Fu S (2010) Effects of lignin-metal complexation on enzymatic hydrolysis of cellulose. J Agric Food Chem 58:7233–7238
Lynd LR, Van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583
Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE (2008) How biotech can transform biofuels. Nat Biotechnol 26(2):169–172
MacPherson S, Larochelle M, Turcotte B (2006) A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev 70:583–604
McMillan JD (1997) Bioethanol production: status and prospects. Renew Energy 10(2–3):295–302
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Gholam N, Gholami MH, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sust Energ Rev 27:77–93
Morales M, Quintero J, Conejeros R, Aroca G (2015) Life cycle assessment of lignocellulosic bioethanol: environmental impacts and energy balance. Renew Sust Energy Rev 42:1349–1361
Muller G, Várnai A, Johansen KS, Eijsink VGH, Horn SJ (2015) Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol Biofuels 8(1):1–9
Nguyen TY, Cai CM, Kumar R, Wyman CE (2015a) Co-solvent pretreatment reduces costly enzyme requirements for high sugar and ethanol yields from lignocellulosic biomass. Chem Sus Chem 8(10):1716–1725
Nguyen TY, Cai CMZ, Osman O, Kumar R, Wyman CE (2015b) CELF pretreatment of corn stover boosts ethanol titers and yields from high solids SSF with low enzyme loadings. Green Chem. https://doi.org/10.1039/C5GC01977
Nissen TL, Hamann CW, Kielland-Brandt MC, Nielsen J, Villadsen J (2000) Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 16:463–474
Oberoi HS, Babbar N, Dhaliwal SS, Kaur U, Chadha BS, Bhargav VK (2012) Ethanol production from alkali-treated rice straw via simultaneous saccharification and fermentation using newly isolated thermotolerant Pichia kudariavzavii. Indian J Biotechnol 39:557–566
Oh EJ, Ha SJ, Kim SR, Lee WH, Galazka JM, Cate JHD, Jin YS (2013) Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae. Metab Eng 15:226–234
Ohta K, Beall DS, Mejia JP, Shanmugam KT, Ingram LO (1991) Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase II. Appl Environ Microbiol 57:893–900
Okamoto K, Uchii A, Kanawaku R, Yanase H (2014) Bioconversion of xylose, hexoses and biomass to ethanol by a new isolate of the white rot basidiomycete Trametes versicolor. Springerplus 3:121
Olson DG, McBride JE, Joe Shaw A, Lynd L (2012) Recent progress in consolidated bioprocessing. Curr Opin Biotechnol 23(3):396–405
Pauly M, Keegstra K (2008) Cell wall carbohydrates and their modification as a resource for biofuels. Plant J 54(4):559–568
Podkaminer KK, Shao X, Hogsett DA, Lynd LR (2011) Enzyme inactivation by ethanol and development of a kinetic model for thermophilic simultaneous saccharification and fermentation at 50°C with Thermoanaerobacterium saccharolyticum ALK2. Biotechnol Bioeng 108(6):1268–1278
Rogers PL, Lee KJ, Tribe DE (1996) Kinetics of alcohol production by Zymomonas mobilis at high sugar concentrations. Biotechnol Lett 1(4):165–170
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454:841–845
Saha BC (2005) Enzymes as biocatalysts for conversion of lignocellulosic biomass to fermentable sugars. In: Hou CT (ed) Handbook of industrial biocatalysis. CRC Press LLC, West Palm Beach
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Scott BR, Huang HZ, Frickman J, Halvorsen R, Johansen KS (2016) Catalase improves saccharification of lignocellulose by reducing lytic polysaccharide monooxygenase-associated enzyme inactivation. Biotechnol Lett 38(3):425–434
Shao X, Jin M, Guseva A, Liu C, Balan V, Hogsett D, Dale BE, Lynd L (2011) Conversion for Avicel and AFEX pretreated corn stover by Clostridium thermocellum and simultaneous saccharification and fermentation: insights into microbial conversion of pretreated cellulosic biomass. Bioresour Technol 102(17):8040–8045
Sharma M, Soni R, Nazir A, Oberoi HS, Chadha BS (2011) Evaluation of glycosyl hydrolyses in the secretome of Aspergillus fumigatus and saccharification of alkali-treated rice straw. Appl Biochem Biotechnol 163(5):577–591
Shaw AJ, Podkaminer KK, Desai SG, Bardsley JS, Rogers SR, Thorne PG, Hogsett DA, Lynd LR (2008) Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. Proc Natl Acad Sci 105(37):13769–13774
Shuai L, Questell-Santiago YM, Luterbacher JS (2016) A mild biomass pretreatment using [gamma]-valerolactone for concentrated sugar production. Green Chem 18:937–943
Singh LK, Majumder CB, Ghosh S (2012) Bioconversion of hemicellulosic fraction of perennial kans grass (Saccharum spontaneum) biomass to ethanol by Pichia stipitis: a kinetic study. Int J Green Energy 9:5
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Valentine J, Clifton-Brown J, Hastings A, Robson P, Allison G, Smith P (2012) Food vs. fuel: the use of land for lignocellulosic ‘next generation’ energy crops that minimize competition with primary food production. GCB Bioenergy 4:1–19
Walker GM (1998) Yeast physiology and biotechnology. Wiley, London
Wiselogel JT, Johnsson D (1996) Biomass feed- stock resources and composition. In: Wyman CE (ed) Handbook on bioethanol: production and utilization. Taylor and Francis, Washington, DC
Wu M, Yan ZY, Zhang XM, Xu F, Sun RC (2016) Integration of mild acid hydrolysis in γ-valerolactone/water system for enhancement of enzymatic saccharification from cotton stalk. Bioresour Technol 200:23–28
Wyman CE, Dale BE, Balan V, Elander RT, Holtzapple MT, Ramirez RS, Ladisch MR, Mosier NS, Lee YY, Gupta R, Thomas SR, Hames BR, Warner R, Kumar R (2013) Comparative performance of leading pretreatment technologies for biological conversion of corn stover, poplar wood, and switchgrass to sugars. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, London, pp 239–259
Xin FX, Wu YR, He JZ (2014) Simultaneous fermentation of glucose and xylose to butanol by Clostridium sp. strain BOH3. Appl Environ Microbiol 80:4771–4778
Yamada R, Hasunuma T, Kondo A (2013) Endowing non-cellulolytic microorganisms with cellulolytic activity aiming for consolidated bioprocessing. Biotechnol Adv 31(6):754–763
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34
Zhang YHP, Ding SY, Mielenz JR, Cui JB, Elander RT, Laser M, Himmel ME, McMillan JR, Lynd LR (2007) Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol Bioeng 97(2):214–223
Zhu JY, Pan XJ, Wang GS, Gleisner R (2009) Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour Technol 100(8):2411–2418
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer (India) Pvt. Ltd.
About this chapter
Cite this chapter
Maheshwari, N.V. (2018). Agro-industrial Lignocellulosic Waste: An Alternative to Unravel the Future Bioenergy. In: Kumar, A., Ogita, S., Yau, YY. (eds) Biofuels: Greenhouse Gas Mitigation and Global Warming. Springer, New Delhi. https://doi.org/10.1007/978-81-322-3763-1_16
Download citation
DOI: https://doi.org/10.1007/978-81-322-3763-1_16
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-3761-7
Online ISBN: 978-81-322-3763-1
eBook Packages: EnergyEnergy (R0)