Abstract
The objective of this research was to investigate the kinetics of lipid production by Yarrowia lipolytica SKY7 in the crude glycerol-supplemented media with and without the control of pH. Lipid and citric acid production were improved with the pH control condition. There was no significant difference observed in the biomass concentration with or without the pH control. In the pH-controlled experiments, the biomass and lipid concentration reached 18 and 7.78 g/L, (45.5% w/w), respectively, with lipid yield (Yp/s) of 0.179 g/g at 60 h of fermentation. The lipid production was directly correlated with growth and the process was defined as growth associated. After 60 h of fermentation, the lipid degradation was noticed in the pH-controlled reactor whereas it occurred after 84 h in the pH-uncontrolled reactor. Apart from lipid, citric acid was produced as the major extracellular product in both fermentations but the much lower concentration in uncontrolled pH. Based on the experimental results, it is evident that controlling the pH will enhance the lipid production by 15% compared to pH-uncontrolled fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever growing energy demand and rapid industrialization leads to the generation of a vast amount of waste materials in various fields throughout the world. This necessitates the search for alternative waste disposal methods. One of the most important ways of waste treatment and disposal is the use of their carbon and other nutrients as a substrate to grow various microbes to generate value-added products. In the present scenario, energy (in terms of oil, electricity, heat, etc.) has become a vital human need. A rapid depletion of energy resources such as fossil fuels and their adverse impact on environment leads the exploration of fuel alternatives [1, 7]. The revolution in green-energy technologies resulted in the advancement of research and demonstration of biofuels (biodiesel, bioethanol, biohydrogen, etc.) from renewable alternative sources. One such example is biodiesel from yellow grease, used cooking oil, etc. [31]. The production of each ton of biodiesel generates 100 kg of glycerol as a by-product. On the other hand, glycerol is generated along with wastewater from ethanol- and soap-producing industries [9]. Thus, the growth of biorefineries may lead to the generation of a large quantity of biologically transformable waste materials. Among these wastes, glycerol has gained much attention of the research community due to a rapid growth of the biodiesel industry. Moreover, surplus availability of glycerol, which has substantially decreased the market price of glycerol ($0.04–$0.33/kg of crude glycerol) [1, 6].
Microbes have the advantage to store different energy-reserve materials such as lipids and polymers, in specialized storage structures. In the case of oleaginous microbes, the excess carbon is converted to lipid and is stored in specialized organelles called lipid bodies, which are embedded in phospholipid monolayer. The lipids are mainly accumulated as triacylglycerides (TAG) or steryl esters [4]. In microbes, TAG mainly consists of C:16 and C:18 or longer chain length fatty acid molecules and as similar to vegetable oils [2, 24]. This property of microbial oils makes it a suitable source for biodiesel production. In spite of ample amount of research reports available for microbial oil production using different substrates, the fermentation process challenges with reference to physicochemical aspects are yet to be satisfied.
Several oleaginous microorganisms have been identified and reported to accumulate lipids more than 70% of the cell dry weight. For example, Lipomyces starkeyi 64.9% [19], Rhodotorula gluttinis were reported to accumulate lipid up to 72% of cell dry weight when glucose was used as a carbon source [2], Trichosporon porosum 34.1% [16], 65% by Cryptococcus curvatus on cheese whey [32], 49% on crude glycerol [11], 72.5% by Mortirella isabellina on commercial glucose medium [8], and so on. Various isolates of Y. lipolytica were reported to accumulate 18.9% w/w lipid on crude glycerol, 43% w/w on biodiesel derived glycerol, 40% w/w industrial lipid plus glycerol, 30.1% w/w industrial waste, 31% on industrial by-products as a carbon source, and 58% in whey medium. When molecular tools based on Y. lipolytica were developed, the understanding about the lipid accumulation process was well addressed, which enhanced the knowledge about the biosynthesis of storage lipids [2, 3]. Compared to the other lipid-accumulating strains, Y. lipolytica has gained more attention due to the presence of multigene families to assimilate wide array of carbon sources such as hydrophobic as well as hydrophilic substrates. The ability of Y. lipolytica to produce different value-added metabolites such as storage lipids, aroma compounds (γ-decalactone), single cell protein [14], and sugar alcohols [21] has made it very popular. Apart from the above mentioned properties, Y. lipolytica has high tolerance towards glycerol which is reported to be the best substrate for lipid and citric acid production by this organism [27, 30]. Therefore, the present study is dedicated to investigating the ability of Y. lipolytica strain SKY7 (isolated from nitrogen-deficient soil samples collected from the region of Quebec, Canada) to produce lipid from crude glycerol. To enhance the biomass and lipid accumulation, physicochemical parameters, such as pH, DO (dissolved oxygen) mixing rate, were important. However, most of the existing literature on lipid production deals with the chemical composition of the medium and failed to explain the physicochemical parameters (pH) associated with biomass and lipid production. The influence of pH on lipid production has a great importance since the lipid accumulation is favored by a slightly acidic pH (5 to 6.5). Similarly, most of the fungal strains grow well in an optimum pH range of 6.0 and 7.0 [29]. In unbuffered media, the pH falls rapidly during the growth of microbes resulting in growth retardation. In fact, the growth retardation is a result of poor nutrient assimilation by the microbes due to the change in pH, which alters the cell membrane permeability [17]. Moreover, there are studies showing a significant reduction (above 40%) lipid content at low pH. In the present study, we investigated the influence of pH control during fermentation on biomass and lipid production since the pH affects the biomass production and thereby reduces the overall lipid yield. In addition, if fermentation could be conducted without pH control with less effect on lipid production, it could lead to a decrease in the cost of operation. The lipid production was studied in two identical 15-L fermenters to evaluate the influence of pH on biomass and lipid production with and without control of pH. The data obtained were analyzed to study the kinetics of biomass and lipid production with and without pH control.
Materials and Methods
Organism and Pre-inoculum Preparation
Yarrowia lipolytica isolate SKY7 (gene bank accession KF908256.1) was used in the present study. The culture was maintained in YPD (yeast extract, peptone, and dextrose) agar slants and revived at every 30 days interval. The crude glycerol sample was obtained from a biodiesel-producing industry in Quebec, Canada. Pre-inoculum was prepared in the crude glycerol-enriched media with the following composition used: 20 g/L crude glycerol, 1 g/L (NH4)2SO4, 1 g/L Na2HPO4, 2.7 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, and 0.3 g/L yeast extract. The medium was sterilized in an autoclave at 121 °C for 15 min and a loop full of pure culture was aseptically inoculated. The flasks were incubated at 28 °C in an agitator operated at 180 rpm (revolutions per minute) for 36 h.
Fermentation Conditions
To evaluate the influence of pH on biomass and lipid accumulation, fermentation was simultaneously performed (to ensure the experimental variations, the experiments were conducted twice and the results were presented with standard deviations) in two identical 15-L bioreactors (Biogenie, Quebec, Canada) with a working volume of 10 L. The bioreactors were equipped with programmable logic control system, which includes dissolved oxygen (DO) probe, antifoam, mixing and aeration, temperature, and pH control system. The above parameters were maintained using the program ifix 3.5, Intellution, USA. The pH meter (Mettler Toledo, USA) was calibrated using buffers pH 4 and 7.0 (VWR, Canada). The DO probe was calibrated to zero using sodium sulfate solution and to 100% saturation by purging the air in distilled water. All fermentation experiments were conducted with 100 g/L crude glycerol and C/N ratio of 101. The other medium components are 1 g/L (NH4)2SO4, 1 g/L Na2HPO4, 2.7 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, and 1.5 g/L yeast extract. The medium was sterilized at 121 °C for 30 min. After sterilization, the medium was cooled to 28 °C and aseptically inoculated with 5% v/v 36-h grown pure culture of Y. lipolytica SKY7. The samples were collected aseptically at each 6 h interval. The samples were analyzed for biomass, lipid, citric acid, and residual glycerol.
Analytical Methods
Quantification of Biomass, Lipid, and Residual Glycerol
Fermented broth (25 mL) was used for biomass and lipid quantification. The collected samples were centrifuged at 4000 rpm for 10 min and the supernatant was collected in a clean tube. The biomass was washed twice with distilled water and the cell pellets were collected by centrifugation. Each sample (pellet) for biomass quantification was transferred to a clean pre-weighed aluminum pan and it was kept at 105 °C until it reaches a constant weight. Chloroform- and methanol-based conventional methods of lipid extraction were employed for lipid quantification [13]. Wet sample was mixed with 15 mL of 2:1 chloroform: methanol mixture and zirconium beads (0.7 mm) were added to the mixture and the cells were disrupted by bead beater for 3 min (BioSpec Products, Bartlesville, OK, USA). The samples after bead beating were filtered through Whatman filter paper with a pore size of 0.45 μm. The solids, thus obtained, were re-suspended in 1:1 chloroform-methanol mixture and the procedure was repeated. The resulting filtrates were pooled in a pre-weighed glass tube and the solvent was evaporated under low nitrogen pressure. The residual samples were further dried at 60 °C in a hot air oven until the sample reaches a constant weight.
Glycerol in the centrifuged supernatant was estimated based on a chemical method. Pure glycerol (Sigma-Aldrich, Canada) was used as the calibration standard [5]. Total dissolved nitrogen was analyzed by the Shimadzu VCPH automated analyzer. Citric acid estimation was carried out according to the method described by Marier et al. 1958 [20]. Appropriately diluted samples were mixed with pyridine and acetic anhydride. The reaction mixture was incubated in a water bath preset at 60 °C. After 5 min of incubation, the reaction mixture was cooled to room temperature and the OD (optical density) was measured at 510 nm (Varian Cary 50, USA). Citric acid (Fisher, Canada) was used as the calibration standard.
Determination of Yield
The lipid and biomass production rates were determined by the following equations. The specific growth rate (μ) was calculated according to Eq. 1.
where X is the biomass concentration (g/L) at time t (h).
The biomass yield (Yx/s g/g) was calculated according to Eq. 2, where X is the biomass concentration (g/L) and S is the substrate consumed to generate the biomass at time t (h).
The lipid yield (Y P/S g/g) was calculated based on Eq. 3, where P is the lipid concentration (g/L) and S is the substrate consumed to generate the biomass at time t (h):
The lipid content (Y P/X g/g) was calculated from Eq. 4:
The specific growth rate (μ), specific lipid production rate (Ʋ), and lipid productivity were calculated based on the model described by Giridhar et al. 2000 [15].
Characterization of Lipid
Lipid sample of 25 mg was trans-esterified using acidified methanol. Decahexanoic acid was used as the internal standard. The trans-esterified lipid fraction was extracted using hexane and the samples were further characterized by GC (Agilent 7890B) equipped with flame ionization detector. Column length was 60 m (Agilent J&W); the carrier gas was helium at a flow rate of 1.18 mL/min with the oven temperature 230 °C. Trans-esterified sample (1 μL) was injected with an automated sample injector and the sample analysis was performed with Agilent GC chem station software. A 37 component FAME mixture from Supelco was used as the calibration standard at different concentrations.
Results and Discussion
Effect of Control of pH on Biomass and Lipid Production
In the present study, the crude glycerol solution used consists of glycerol concentration 78% (w/v), methanol (1.28% w/v), soap (2.4% w/v), catalyst 0.12% (w/v), and water content of 2.48% (w/v). To calculate the C/N ratio, the carbon present in glycerol was taken into account whereas the carbon in methanol and soap was not included since the amount was negligible.
Microbial assimilation of carbon source depends on the pH of the medium. The pH influences the surface properties of the cell membrane and thereby affects the carbon assimilation process. Several studies showed that a pH range of 6 to 6.5 is suitable for lipid production [26]. In the present investigation, initial pH of both fermenters was adjusted to 6.5. In one reactor, the pH was not controlled and in the other, pH was controlled by adding 4 N NaOH. Due to a rapid decline in pH in the uncontrolled reactor, the cells transformed into pseudohyphae. In the case of uncontrolled fermentation, the secretion of organic acids (mainly citric acid) into the medium resulted in significant drop in pH (6.5 to 3.98) (Fig. 1). The production of citric acid by oleaginous microorganisms has been reported by several authors [26, 28]. From 60 h onward, the pH of the uncontrolled fermentation started to increase. After 108 h of fermentation, in the pH-uncontrolled reactor, the cells started to aggregate and pH of the medium increased to 4.3. This increase in pH could be attributed to maintaining the ionic balance in cellular metabolism; microbes tend to pump protons to intracellular space, which may lead to a slight increase in pH [10, 34].
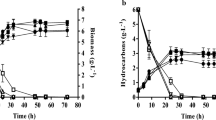
The variation in concentration of biomass, lipid, and glycerol (g/L) with respect to fermentation time is presented in Fig. 2. A short lag phase at the beginning of the fermentation was observed in both the pH-controlled and the uncontrolled reactors. During the lag period (0–6 h), a biomass increase of 1.25 and 0.24 g/L was observed in the pH-uncontrolled and the controlled reactors, respectively. This short lag period was brought by the sudden change in the medium composition, i.e., from a low concentration of glycerol (20 g/L) during inoculum production to high concentration of glycerol (100 g/L) in the fermenter medium. A substantial growth was achieved from 6 to 18 h of fermentation in both reactors. The increase in biomass concentration was 9.3 g/L in the pH-controlled and 10.14 g/L in the pH-uncontrolled reactors during this time. The biomass growth was decreased from 18 to 40 h. After 40 h, the increase in biomass was almost two times higher in the pH-controlled reactor (3.52 g/L pH-controlled, 1.62 g/L pH-uncontrolled). At 60 h, the biomass concentrations in the pH-controlled and the pH-uncontrolled reactor were 18 and 19.54 g/L, respectively. The biomass concentration was almost stable from 80 h onwards in both reactors. Growth reduction in both reactors could be because of the depletion of nutrients. On the other hand, a decrease in growth in the pH-uncontrolled reactor was additionally contributed by low pH (4 to 4.5) of the medium. At the end of fermentation (120 h), the biomass concentration was approximately the same in both reactors (20.58 g/L pH-controlled and 20.48 g/L pH-uncontrolled).
A monotonous decrease of glycerol concentration was observed in both reactors until 80 h of fermentation (Fig. 2). After 80 h, glycerol consumption and increase in biomass and lipid concentration were very low. At this point, the consumed glycerol in the case of the pH-controlled reactor was mostly converted into citric acid (Table 1) or other fermentation co-products and in the case of the pH-uncontrolled reactor, it may be used mainly for cell maintenance and some other co-product formation (polyalcohols, glycogens, etc.) [33].
The lipid concentration (Fig. 2) was found to increase until 60 h in the case of the pH-controlled and until 78 h in the case of the pH-uncontrolled reactor. However, a significantly low increase in lipid concentration (0.9 g/L) was observed in the pH-uncontrolled fermentation from 60 to 78 h. From 6 to 18 h (the growth phase) of fermentation, the lipids produced in the pH-controlled and the uncontrolled reactors were 3.46 and 2.8 g/L, respectively. The concentrations of lipid at 48 h in the controlled and the uncontrolled reactor were 2.94 and 1.38 g/L, respectively. From 48 to 60 h, 1.12 g/L lipid was produced in the pH-controlled reactor, whereas no substantial lipid production (0.2 g/L) was noticed in the uncontrolled reactor. In the case of a pH-controlled reactor, the lipid started to decrease after 60 h of fermentation. From 60 to 120 h of fermentation, 1.98 g/L of lipid was degraded, whereas in the pH-uncontrolled reactor, the lipid degradation was detected from 78 h. The maximum lipid accumulation of 7.78 g/L in the pH-controlled reactor was observed at 60 h. The biomass concentration at 60 h was 18 g/L with the lipid content of 45.44% w/w. The pH control resulted in 15% (2 g/L) higher lipid concentration than the pH-uncontrolled experiment (Fig. 2).The maximum lipid concentration 6.68 g/L with biomass concentration 20.6 g/L and lipid content of 33.8% (w/w) was observed at 78 h in the pH-uncontrolled fermentation. A slightly low lipid concentration can be correlated with the pH of the medium. The pH of the fermentation medium affects the cell membrane property and it has been reported that growth rate was higher when the pH was above 4.5 [35].
Y. lipolytica is a well-known industrial scale citric acid producer. The concentration of citric acid was similar in both reactors at 24 h of fermentation. In the case of a pH-controlled reactor, the citric acid concentration was noticed to considerably increase from 24 h and reached 5.71 g/L at 60 h and 11.1 g/L at 120 h (Table 1). In the case of pH-uncontrolled fermentation, rapid decline in pH (Fig. 2) inhibits the citric acid production. The maximum concentration of citric acid obtained was 1.2 g/L at 72 h. On the other hand, the citric acid production (citrate concentration in the cytoplasm) is one of the important factors that control the lipid accumulation. Citrate is known as the acetyl donor for fatty acid biosynthesis and is transported from mitochondria to the cytoplasm as a result of nitrogen depletion in the medium. Further, the intracellular citrate is believed to be converted into fatty acids. Besides, depletion in nitrogen concentration is noticed to increase the extracellular citrate. Similarly, in the pH-controlled experiment, the citric acid concentration was noticed to be 2.35 g/L at 48 h. However, it was noticed to increase to 5.71 g/L at 72 h and these observations well support with Evans and Ratledge et al. (1983) [12]. On the other hand, the optimum pH for citric acid production is reported to be 5.5 to 6.5. Citric acid production by Y. lipolytica in glycerol-based fermentation media under buffered and non-buffered media was evaluated by Papanikolaou et al. (2002) [27]. The results showed improved citric acid production in buffered media which is well agreed with our observation. Therefore, under a pH-controlled condition under nitrogen-limited environment, the citric acid production was significantly improved after 48 h. Lipid biosynthesis requires a certain concentration of citrate in the cytoplasm which activates the intracellular lipid-synthesizing enzymes. Citric acid is the substrate for ATP citrate lyase, which is involved in lipid production metabolism. Citric acid activates the ACC (acetyl-CoA carboxylase), which is involved in the conversion of acetyl-CoA to malonyl-CoA (a step in lipid synthesis) [18]. A constant supply of citrate will generate adequate amounts of acetyl-CoA in the cytoplasm by the enzyme ACL (ATP citrate lyase). Narlin et al. (1983) showed that 2.0 mM citrate concentration resulted in a steady rate of ACC activity [23]. Further, the relationship between the cellular concentration of citrate and carboxylase activity was confirmed by Moss and Lane [22]. Thus, the supply of acetyl-CoA required for lipid synthesis depends on the cellular ACL and the ACC catalytic activity and these enzymes are guarded by cellular citrate concentration. In the case of pH-uncontrolled fermenter, a low citrate concentration in the medium (0.55 g/L at 24 h and 1.25 g/L at 66 h, (Table 1)) reflects that the intracellular citric acid concentration in the cells would be low. The low citric acid concentration reduced the lipid production, which resulted in a little lower lipid concentration in the pH-uncontrolled fermentation. Thus, pH control favors the citric acid production that resulted in slightly higher biomass lipid content and higher medium lipid concentration in a shorter time than the pH-uncontrolled fermentation.
From the above observations, it is clear that the fermentation can be stopped at 60 h. At this instant, 53.9% glycerol (added initially) was assimilated (23.2% w/w was converted to lipid-free biomass, 17.7% w/w to lipid, and 13% w/w to citric acid) under the pH-controlled condition. In the case of the pH-uncontrolled fermentation, 48.38% glycerol was assimilated (32.0% w/w was converted to lipid-free biomass, 13.4% w/w to lipid, and 2.9% w/w to citric acid). The above comparison shows that 8.7 and 4.3% more glycerol was used for biomass production and lipid production, respectively, in the pH-controlled reactor than the uncontrolled pH. Thus, controlling the pH during fermentation can lead to a little higher lipid production.
Variation of Yield Factors, Specific Growth Rate, and Specific Lipid Production Rate During pH-Controlled and Uncontrolled Fermentation
The values of biomass and lipid yields with respect to glycerol consumed in the case of pH-controlled and uncontrolled fermentation are presented in Fig. 3a, b. In both cases, Y X/S (biomass produced/g of glycerol consumed) increased with the fermentation time until 24 h. The maximum biomass yield (Y X/S ) of 0.54 g/g (Y X/S 0.35 g/g of lipid-free biomass) for pH-controlled and 0.51 g/g (Y X/S 0.34 g/g of lipid-free biomass) for pH-uncontrolled fermentation was attained at 24 h (Fig. 3a, b). Thereafter, the Y X/S value decreased with fermentation time in both cases.
The lipid yield (Y P/S , g lipid produced/g of glycerol consumed) increased in the beginning of the fermentation (0 to 18 h) and then decreased until the end of fermentation (Fig. 3a, b). In the case of the pH-controlled fermentation, the Y P/S value reached 0.2 g/g at 18 h whereas it was 0.16 g/g in the pH-uncontrolled reactor. The high value of lipid yield (Y P/S ) in the beginning of the fermentation could be attributed to lipid production as a part of cell wall synthesis. From 24 to 60 h, the Y P/S decreased (from 0.18 to 0.17 g/g) very slowly in the pH-controlled reactor. In the case of the pH-uncontrolled fermentation, Y P/S also decreased slowly, but for a longer period (24 to 78 h, Y P/S 0.15 to 0.13 g/g). Almost constant Y P/S value or a slow decrease up to 60 h of fermentation leads to the conclusion that the fermentation should be terminated at 60 h. After 60 h of fermentation, the Y P/S value declined in both cases. The decline in Y P/S value could be explained by the lipid-free biomass concentration synthesized from 24 to 78 h. The Y X/S value (with respect to lipid-free biomass) was noticed to be higher than the Y P/S value from 24 to 78 h (Y P/S 0.13 g/g and Y X/S lipid-free biomass 0.3 g/g). This indicates that most of the assimilated glycerol were directed towards biomass synthesis. Further, lipid-free biomass yield reached maximum (0.35 g/g) at 24 h in the pH-controlled reactor followed by a decrease and reached 0.21 g/g at 60 h. In the pH-uncontrolled fermentation, the lipid-free biomass yield reached maximum 0.34 g/g at 30 h and then decreased slightly 0.3 g/g at 60 h. This high lipid-free biomass production results in low lipid accumulation.
A different trend of lipid yield with respect to biomass (Y P/X , g lipid produced/g of biomass) in the pH-uncontrolled reactor was perceived (Fig. 3b). The lipid content of the biomass declined from 0.45 to 0.31 g/g within the first 30 h of fermentation and then remained almost constant (from 36 h onward) until the end of fermentation. In the case of the pH-controlled fermentation, after a short decline from 12 to 24 h of fermentation, an increasing trend in the Y P/X value was observed for 24 h (0.34 g/g) to 60 h (0.45 g/g), which reflected high biomass lipid content at 60 h (45.4% w/w). After 60 h, a sharp decline in Y P/X value was evident (Fig. 3a), which indicates the lipid degradation in the pH-controlled fermentation. The lipid degradation could be attributed to β-oxidation of the produced lipid by the organism. In the case of the pH-uncontrolled fermentation, the lipid degradation was noticed after 78 h (about 1 g/L lipid degraded from 78 to 120 h).
The specific growth rates (μ) in both reactors reached a maximum at 12 h of fermentation (Fig. 4a, b). A higher value of μ was observed for the pH-uncontrolled reactor (0.18 h−1) than the pH-controlled reactor (0.14 h−1). This shows that from 6 to 12 h, high growth occurred with or without control of pH. After 12 h of fermentation, the specific growth rate value declined until the end of fermentation in both reactors. The decrease in specific growth rate could be due to the nutrient limitation in the medium. The specific lipid production rate (Ʋ) also followed a similar trend. The value of Ʋ for both fermentations was found to decrease with fermentation time (Fig. 4a, b) and reached near zero at around 60 h. In the case of the pH-uncontrolled fermentation, specific lipid production rate (Ʋ) was very low from 24 to 42 h. In the pH-controlled fermentation, the Ʋ was higher at 12 h (0.061 h−1). The Ʋ value was found to be 0.031 h−1 at 12 h for the pH-uncontrolled reactor, which is almost 50% lower than the pH-controlled fermentation.
On the contrary, lipid production in both reactors occurred throughout the fermentation but decreased with fermentation time. The lipid productivity in case of the pH-controlled fermentation increased from 0.12 to 0.21 g/L/h from 12 to 18 h followed by a slow decline and reached 0.12 g/L/h at 60 h (Fig. 4a). The maximum lipid production rate of 0.25 g/L/h was found at 12 h in the pH-uncontrolled reactor, which declined rapidly and reached to 0.1 g/L/h at 60 h (Fig. 4b). The rapid decrease in lipid productivity with respect to the fermentation time in both cases could be attributed to a decrease in the malic enzyme activity [36]. It was reported that the malic enzyme (intracellular) activity decreases exponentially when nitrogen in the medium is depleted (discussed later). At the end of fermentation (120 h), with or without pH control, the lipid productivity was noted to be the same (0.05 g/L/h). These results clearly established that after 60 h, the lipid produced is not significant.
Relation Between Specific Growth Rate and Specific Lipid Production Rate
The specific growth rates and specific lipid production rates were computed by differentiating the concentration (biomass and lipid) versus time data and the results are presented in Fig. 5, which revealed that the lipid production was proportional to growth. In general, the lipid accumulation has been defined as non-growth associated [29]. The maximum specific growth rate was attained between 6 and 12 h (Fig. 4a, b) in both cases and at this time, a substantial amount of lipid was also synthesized (1.42 with and 3.02 g/L without pH control at 12 h). Thus, the lipid synthesis even occurred during the early stage of fermentation.
These observations suggest that controlling the pH at an optimal level slightly improved the lipid productivity. In the case of the pH-uncontrolled fermentation, lipid concentration 6.12 g/L and biomass concentration 19.54 g/L were attained at 66 h and there was no significant increase afterwards (Fig. 2). On the other hand, 7.78 g/L of lipid with 18 g/L biomass was attained at 60 h of fermentation in the pH-controlled reactor. Meanwhile, in experiments with controlled pH, Y P/S (0.17 at 60 h) stayed significantly high until 60 h compared to the pH-uncontrolled experiment (Y P/S ; 0.14 at 60 h). Further, lipid productivity and specific lipid production tend to near zero in both cases. This suggests that the fermentation should be terminated before 60 h.
Evolution of C/N Ratio on pH-Controlled and Uncontrolled Conditions
The change in carbon flux alters the lipid accumulation in oleaginous microbes [2]. It is advocated that a low concentration of carbon in the medium leads to β-oxidation of stored lipids for re-utilization by the organism. However, in the presence of an abundance of carbon with high C/N molar ratio in the medium, Y. lipolytica generates low biomass and high lipid concentration. Further, a very high C/N ratio represses the metabolism due to nitrogen deficiency, which reduces the lipid accumulation and favors the secondary metabolite formation. In the present study initial, C/N ratio was 113 for the pH-controlled and 115 for the pH-uncontrolled fermentation (Fig. 6). The C/N ratio increased with the fermentation time. The nitrogen content of the fermentation broth reached 0.1 and 0.09 g/L in 24 h in the pH-controlled and the uncontrolled fermentation, respectively, and after that remained approximately unchanged during the rest of the fermentation period (Fig. 6). The glycerol was continuously assimilated by the organism and thus decreasing the carbon concentration in the medium, which resulted in a decrease of the C/N ratio below 300 in both fermentations. At this moment, the lipid production rate and an increase in biomass concentration were very low; therefore, it seems that most of the assimilated glycerol were diverted for cell maintenance purpose. Under the nitrogen-limited condition, the glycerol consumed could also be diverted to the synthesis of secondary metabolites such as organic acids (citric acid, isocitric acid) or polyalcohols (erythritol, mannitol, etc.). Citric acid production was enhanced when the nitrogen content was low or unavailable in the media (from 24 h) in the pH-controlled reactor (Table 1). Synthesis of citric acid should have concomitantly increased the lipid production; however, due to unavailability of nitrogen in the medium, the rate of the lipid production drastically decreased.
This can be further explained by correlating the specific lipid production rate with the C/N ratio. The specific lipid production rates of the pH-controlled and the uncontrolled fermentation were noted to decline with increasing C/N ratio and reached very low at 24 h in both cases (Fig. 4a, b) when the nitrogen content of the medium was very low (0.1 and 0.09 g/L). It is reported that when the organism enters the lipid synthesis mode, it needs a certain amount of nitrogen (as ammonium ion), which could serve as the co-factor for several enzymes in lipid biosynthesis [3, 26]. Thus, the present observation and the previously reported studies clearly suggest that it is necessary to maintain a low concentration of usable nitrogen in the medium after the growth, which will help to increase the lipid content of the cells.
Influence of pH Control on Lipid Composition
The composition of lipid considerably depends on the carbon source, the concentration of carbon and the C/N ratio of the medium. There are several studies, which showed that the lipid profile changed with fermentation time [25]. Table 2 presents the lipid composition of Y. lipolytica (SKY7) for the pH-controlled and the uncontrolled experiments. Oleic acid was found to be the major component in all cases. Y. lipolytica SKY7 isolate produced the following fatty acids in high quantity: myristic (C14:0), palmitic (C16:0), stearic (C18:0), oleic cis-9 (C18:1n9-cis), linoleic (C18:2n6c), and arachidonic (C20:4n6). Other than the above-listed triacylglycerols (TAG), this isolate also produced C15:0, C15:1, palmitoleate (C16:1n7), elaidic (C18:1n9t), linolenate (C18:3n3), arachidic (C20:0), eicosenoate (C20:1n9), and lignoceric (C24:0) in minor quantities (data not shown). Linoleic acid concentration decreased from 27.76% (24 h) to 15.25% w/w (120 h) in the case of pH-controlled fermentation. In the case of the pH-uncontrolled reactor, linoleic acid was decreased from 31.76% (24 h) to 12.88% (120 h). Myristic acid concentration decreased from 24 to 48 h and after that remained almost constant. The other TAGs were noted to increase with fermentation time.
Table 2 Lipid profile of Y. lipolytica in different culture conditions (digits represent % of the total lipid)
Summary and Conclusion
The results revealed that controlling pH at 6.5 produced 7.78 g/L and uncontrolled 6.58 g/L (60 h) of lipid. In both cases, early lipid production was observed and this could be helpful to reduce the fermentation time. The specific lipid production was proportional to the specific growth rate and most of the lipid was produced during 60 h fermentation. Due to the low nitrogen concentration in the medium, the growth ended before 18 h in both fermentations. In conclusion, controlling pH leads to increase in lipid production significantly and further favors citric acid production in late fermentation stage.
References
Ayoub, M., & Abdullah, A. Z. (2012). Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renewable and Sustainable Energy Reviews, 16, 2671–2686.
Beopoulos, A., Cescut, J., Haddouche, R., Uribelarrea, J. L., Molina-Jouve, C., & Nicaud, J. M. (2009). Yarrowia lipolytica as a model for bio-oil production. Progress in Lipid Research, 48, 375–387.
Beopoulos, A., Chardot, T., & Nicaud, J. M. (2009). Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie, 91, 692–696.
Beopoulos, A., Mrozova, Z., Thevenieau, F., Le Dall, M. T., Hapala, I., Papanikolaou, S., Chardot, T., & Nicaud, J. M. (2008). Control of lipid accumulation in the yeast Yarrowia lipolytica. Applied and Environmental Microbiology, 74, 7779–7789.
Bondioli, P., Della, B., & Laura. (2005). An alternative spectrophotometric method for the determination of free glycerol in biodiesel. European Journal of Lipid Science and Technology, 107, 153–157.
Burton, R., & Fan, X. (2009). Recent development of biodiesel feedstocks and the applications of glycerol: a review. The Open Fuels & Energy Science Journal, 2, 100–109.
Campbell, C. J. (2006). The Rimini Protocol an oil depletion protocol: heading off economic chaos and political conflict during the second half of the age of oil. Energy Policy, 34, 1319–1325.
Chatzifragkou, A., Fakas, S., Galiotou-Panayotou, M., Komaitis, M., Aggelis, G., & Papanikolaou, S. (2010). Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. European Journal of Lipid Science and Technology, 112, 1048–1057.
Ciriminna, R., Pina, C. D., Rossi, M., & Pagliaro, M. (2014). Understanding the glycerol market. European Journal of Lipid Science and Technology, 116, 1432–1439.
Cristina, V., Paulo, A., Miguel, C., & Isabel, C. (1998). The H1-ATPase in the plasma membrane of Saccharomyces cerevisiae is activated during growth latency in octanoic acid-supplemented medium accompanying the decrease in intracellular pH and cell viability. Applied and Environmental Microbiology, 64, 779–783.
Cui, Y., Blackburn, J. W., & Liang, Y. (2012). Fermentation optimization for the production of lipid by Cryptococcus curvatus: use of response surface methodology. Biomass and Bioenergy, 47, 410–417.
Evans, C. T., Scragg, A. H., & Ratledge, C. (1983). A comparative study of citrate efflux from mitochondria of oleaginous and non-oleaginous yeasts. European J Biochem, 130, 195–204.
Folch, J., Lees, M., & Slane-Stanley, G. H. (1957). Simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry, 226, 497–509.
Gao, Y., Li, D., & Liu, Y. (2011). Production of single cell protein from soy molasses using Candida tropicalis. Annals of Microbiology, 62, 1165–1172.
Giridhar, R., & Srivastava, A. K. (2000). Model based constant feed fed-batch L-sorbose production process for improvement in L-sorbose productivity. Chemical and Biochemical Engineering Quarterly, 14, 133–140.
Ines, S., Silla, H., Steffen, G., Thomas, R., Katrin, O., Christoph, S., & Anke, N. (2014). Characterization of newly isolated oleaginous yeasts—Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express, 4, 24.
Jarboe, L. R., Royce, L. A., & Liu, P. (2013). Understanding biocatalyst inhibition by carboxylic acids. Frontiers in Microbiology, 4, 272.
Landman, A. D., & Dakshinamurti, K. (1975). Acetyl-Coenzyme A Carboxylase role of the prosthetic group in enzyme polymerization. Biochemical Journal, 145, 545–548.
Lin, J., Shen, H., Tan, H., Zhao, X., Wu, S., Hu, C., & Zhao, Z. K. (2011). Lipid production by Lipomyces starkeyi cells in glucose solution without auxiliary nutrients. Journal of Biotechnology, 152, 184–188.
Marier, J. R., & Boulet, M. (1958). Direct determination of citric acid in milk with an improved pyridine-acetic anhydride method. Journal of Dairy Sciences, 41, 1683–1692.
Mhairi, W., Philippe, H., & Jette, T. (2013). Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. AMB Express, 3, 1–9.
Moss, J., & Lane, M. D. (1972). Acetyl Coenzyme A Carboxylase. The Journal of Biological Chemistry, 247, 4952–4959.
Narlin, B., Beaty, S., & Lane, M. D. (1983). Kinetics of activation of acetyl-coA Carboxylase by citrate relationship to the rate of polymerization of the enzyme. The Journal of Biological Chemistry, 258, 13043–13050.
Papanikolaou, S., & Aggelis, G. (2003). Selective uptake of fatty acids by the yeast Yarrowia lipolytica. European Journal of Lipid Science and Technology, 105, 651–655.
Papanikolaou, S., Chevalot, I., Komaitis, M., & Geor. (2001). Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek, 80, 215–224.
Papanikolaou, S., Chevalot, I., Komaitis, M., Marc, I., & Aggelis, G. (2002). Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Applied Microbiology and Biotechnology, 58, 308–312.
Papanikolaou, S., Muniglia, L., Chevalot, I., Aggelis, G., & Marc, I. (2002). Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. Journal of Applied Microbiology, 92, 737–744.
Ratledge, C. (2004). Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie, 86, 807–815.
Ratledge, C., & Wynn, J. P. (2002). The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Advances in Applied Microbiology, 51, 1–51.
Rywińska, A., Juszczyk, P., Wojtatowicz, M., Robak, M., Lazar, Z., Tomaszewska, L., & Rymowicz, W. (2013). Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass and Bioenergy, 48, 148–166.
Savaliya, M. L., Dhorajiya, B. D., & Dholakiya, B. Z. (2013). Recent advancement in production of liquid biofuels from renewable resources: a review. Research on Chemical Intermediates, 41, 475–509.
Seo, Y. H., Lee, I., Jeon, S. H., & Han, J.-I. (2014). Efficient conversion from cheese whey to lipid using Cryptococcus curvatus. Biochemical Engineering Journal, 90, 149–153.
Tomaszewska, L., Rakicka, M., Rymowicz, W., & Rywinska, A. (2014). A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Research, 14, 966–976.
Ullah, A., Orij, R., Brul, S., & Smits, G. J. (2012). Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Applied and Environmental Microbiology, 78, 8377–8387.
Van Leeuwen, J., Rasmussen, M. L., Sankaran, S., Koza, C. R., Erickson, D. T., Mitra, D., & Jin, B. (2012). Fungal treatment of crop processing wastewaters with value-added co-products. Sustainable Bioenergy and Bioproducts, Green Energy and Technology, 8, 13–44.
Wynn, J. P., bin Abdul Hamidt, A., & Ratledge, C. (1999). The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiology, 145, 1911–1917.
Acknowledgements
The authors would like to acknowledge the Natural Sciences and Engineering Research Council of Canada (grant A4984, strategic grant 412994–11, Canada Research Chair) for financial support. We are grateful to the technical staffs of INRS-ETE for their timely help to analyze the samples on GC-FID.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors have read and agreed with the contents of the manuscript. The authors indicate no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Kuttiraja, M., Dhouha, A. & Tyagi, R.D. Harnessing the Effect of pH on Lipid Production in Batch Cultures of Yarrowia lipolytica SKY7. Appl Biochem Biotechnol 184, 1332–1346 (2018). https://doi.org/10.1007/s12010-017-2617-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2617-y