Abstract
In this paper, the biomass and lutein productivity of the lutein-rich new strain Scenedesmus almeriensis is modelled versus irradiance and temperature. The results demonstrate that S. almeriensis is a mesophile microorganism with an optimal growth temperature of 35°C, and capable of withstanding up to 48°C, which caused culture death. This strain is also tolerant to high irradiances, showing no signs of photoinhibition even at the maximum irradiance essayed of 1625 μE m−2 s−1 accumulating up to 0.55% dry weight (d.wt.) of lutein. The optimal conditions that maximise the biomass productivity also favour the lutein productivity, lutein being a primary metabolite. Maximal biomass and lutein productivities of 0.87 g l−1 day−1 and 4.77 mg l−1 day−1, respectively, were measured. The analysis of light availability inside the cultures, quantified as average irradiance, demonstrates that the cultures were mainly photo-limited, although photosaturation also took place at high external irradiances. The effect of temperature was also investigated finding that the specific maximal growth rate is modified by the temperature according to the Arrhenius equation. The influence of both light availability and temperature was included in an overall growth model, which showed, as a result, capable of fitting the whole set of experimental data. An overall lutein accumulation rate model was also proposed and used in a regression analysis. Simulations performed using the proposed models show that under outdoor conditions a biomass productivity of 0.95 g l−1 day−1 can be expected, with a lutein productivity up to 5.31 mg l−1 day−1. These models may be useful to assist the design and operation optimisation of outdoor cultures of this strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lutein is a xantophile recommended for the prevention of some types of cancer (Demming-Adams and Adams 2002), cardiovascular diseases (Dweyer et al. 2001) and retinal degeneration (Ziegler et al. 1996; VERIS 1997). Lutein is also a food dye allowed by EU and reported as E 161 b. Sales of lutein as food additive in the US amount to about $150 millions per year. Although lutein is present in fruits and vegetables, the estimated daily uptake of 1.5 mg day−1 (Johnson-Down et al. 2002) is far from the recommended 6 mg day−1 (Silva 2004), so that the intake of lutein supplements, particularly in certain segments of population, may be advisable . The current commercial source of pure lutein is marigold (Tagetes erecta L.). However, the low lutein content of marigold flowers, 0.03% dry weight (d.wt.), makes the consideration of other lutein sources interesting. Several microalgae have been proposed as potential producers of lutein, as Muriellopsis sp. (Del Campo et al. 2001, 2007), Chlorella zofingensis (Del Campo et al. 2000), or Chlorella protothecoides (Shi et al. 2000). Recently, a new microalgae strain with a high content in lutein, Scenedesmus almeriensis, has been also proposed as lutein source (Sánchez et al. 2007).
The production of any microalga at commercial scale requires the use of large photobioreactors outdoors. In these systems, the main factors in culture management are the light availability and temperature because any mineral nutrient limitation can be easily overcome. In outdoor conditions the irradiance and temperature change during the day and over the annual cycle, so that is of great interest to know the behaviour of the strain versus irradiance and temperature variations. Many studies on the influence of environmental variables on biomass productivity have been carried out (Guterman et al. 1990; Molina et al. 1994; Acién et al. 1998; Del Río et al. 2008), but few take into account the simultaneous influence of temperature and irradiance, and none considers the effect of these variables on the product yield.
In the present paper, the influence of irradiance and temperature on the biomass productivity, fluorescence of chlorophylls, lutein content and lutein productivity of the lutein-rich new strain Scenedesmus almeriensis is analyzed. This strain was isolated within a farmer’s greenhouse, under high temperature and irradiance conditions and its characteristics have been recently reported (Sánchez et al. 2007). The objective of this work is to model the influence of irradiance and temperature on the growth rate and lutein accumulation rate, to obtain overall models that will allow determining the optimal conditions to achieve the maximum biomass and lutein productivity. The results reported here will allow the design and development of improved systems for the efficient production of lutein-rich cells of Scenedesmus almeriensis at industrial scale.
Materials and methods
Microalga and culture conditions
The microalga Scenedesmus almeriensis was isolated from fresh water in a greenhouse located in Almería, Spain. This strain was identified as new by the “Experimental Phycology and Culture Collection of Algae—SAG” and deposited in the Culture Collection of Algae and Protozoa of the Centre for Hydrology and Ecology, Ambleside, UK, code CCAP 276/24.
The cultures were grown photoautotrophically in four bubble column photobioreactors (2.0 l capacity, 0.06 m diameter, 0.50 m height) jacketed for temperature control. Air was bubbled at 0.5 vol vol−1 min−1. Thermostatted water was circulated through the jacket to keep the temperature set point. The pH was controlled by on-demand injection of carbon dioxide into the air stream entering the culture. The photobioreactors were under artificial illumination simulating a solar cycle using six Phillips PL-32 W/840/4p white-light lamps. The irradiance on the reactor surface was controlled by an automated system to provide a maximum irradiance ranging from 625 to 1625 μE m−2 s−1 at noon. The culture medium used was Mann and Myer’s (1968). The culture medium was prepared by adding salt to tap water and then sterilised by passing through 0.02-μm Whatman filters before entering the photobioreactors.
The experiments were performed in continuous mode. For this, the fresh medium was continuously fed to the reactor during the light period at the dilution rate selected, while the harvest stream was withdrawn by means of a valve located at the top of the photobioreactor. The steady states attained were maintained for at least 5 days to ensure steady state conditions in the biochemical profile of the biomass. At least three samples were measured each day on the last 3 days of every steady state; therefore, the reported values are the means of at least nine experimental measurements.
Analytical procedures
Biomass concentration was determined by dry weight measurements. For this, 10-ml aliquots of the cell suspension were filtered through a 1.2-μm Whatman GF/C filter paper, washed with distilled water, and the filters containing the algae were dried in an oven at 80°C for 24 h. The physiological status of the cells was measured by quantifying the fluorescence of chlorophylls, F v/F m, using a fluorimeter (9917 Sensorial PEA; Hansatech Instruments, UK). For the measurement, a 2-ml sample taken from the bioreactor was immediately placed in a vial (PEA capped vial, 980234) and held in the dark for 5 min. After this period, the fluorimeter illuminated the sample with a saturating red light flash of 0.3-s duration to provide the following values: F o, the minimum value of fluorescence attributed to chlorophyll a; F m, the maximum fluorescence value reached; F v, the variable fluorescence value; and the ratio F v/F m.
Carotenoid content was determined by high-performance liquid chromatography (HPLC) as described by García-Malea et al. (2006). For this, 2-ml aliquots of cell suspension were centrifuged at 2,000×g for 5 min. The pellet containing the cells was ground in a mortar with alumina, and the pigments extracted with 4 ml of pure acetone. The acetone extract was centrifuged at 2,000×g for 5 min to remove alumina. The pellet was then resuspended with 2 ml acetone and centrifuged once more. This procedure was repeated until the supernatant was colourless. Acetone extracts were evaporated under nitrogen gas and the residue re-dissolved in 1 ml of pure ethyl ether, after which, 1 ml of KOH in MeOH (4% w/v) was added. The stirred mixture was allowed to react for 15 min at 0°C in darkness under nitrogen gas. To stop the reaction and to remove excess alkali, 2 ml of 10% NaCl was added and the mixture was stirred. For the separation of phases, the mixture was centrifuged at 2000×g for 2 min. The aqueous phase was removed and discarded. Ether phase was washed twice with 2 ml of 10% NaCl. Ether was then evaporated under nitrogen gas and the remaining pigment re-dissolved in 1 ml of pure acetone and centrifuged to discard particulate material. Pigments were analysed by the HPLC chromatographic method of Del Campo et al. (2000), using a Shimadzu SPD-M10AV HPLC. Separation was performed on a XTerra MSC18 5-μm column (4.6 × 150 mm). The eluents used were: (A) water–ion pair reagent-methanol (1:1:8, v/v) and (B) acetone-methanol (1:1, v/v). The ion-pair reagent was a solution of tetrabutylammonium (0.05 m) and ammonium acetate (1 M) in water. The pigments were eluted at a rate of 1 ml min−1, and detected by measuring absorbance at 360 to 700 nm. Standards of astaxanthin, cantaxanthin, β-carotene and lutein were provided by Sigma Chemical Co., St. Louis, MO, USA. Violaxanthin was from DHI.
Irradiance and numerical methods
Measurements of photosynthetically active irradiance were carried out using a 4π quantum scalar irradiance sensor QSL-100 (Biospherical Instrument, San Diego, CA). The external irradiance, I o, was measured in the centre of the photobireactor filled with cell-free medium. The average irradiance inside the culture was calculated as a function of the external irradiance I o, the light path p, the biomass concentration C b, and the extinction coefficient of the biomass K a, as proposed by Molina et al. (1994),
For each experiment the value of external irradiance, I o, was the maximum external irradiance measured during the simulated solar cycle, the light path was constant (0.06 m), and the value used for the extinction coefficient was of 0.08 m2 g−1. C b was the steady state biomass concentration. The values presented are means of at least nine measurements done during 3 days of steady state.
Statistical analysis of data was carried out with the software Statgraphics version 7.0. Nonlinear regressions and correlations between parameters were performed using the same software.
Results
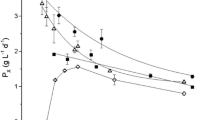
To determine the influence of irradiance on the growth rate and lutein productivity of S. almeriensis, continuous cultures with different dilution rate and external irradiance were carried out. The external irradiance was varied simulating the daylight solar cycle with different maximal irradiances of 650 to 1625 μE m−2 s−1. The dilution rate was modified from 0.01 to 0.07 h−1. The results show that the steady state biomass concentration (C b) increased with the external irradiance, due to a higher light availability, and decreased with increasing dilution rates (Fig. 1a) as can be expected in a continuous culture in which the specific growth rate (μ) must match dilution rate (D) in steady state. Therefore, an increased D requests a higher light availability, which is attained by a decrease in the biomass concentration. It is also observed that the influence of the external irradiance in the steady state biomass concentration is attenuated at high dilution rates, which is justified because at high D there is necessarily a high light availability and the microalgal cells are closer to light saturation. The experimental data of C b ranged from 0.3 to 3.4 g l−1. Regarding the biomass productivity, a maximum value of 0.56 g l−1 day−1 was obtained at D = 0.04 h−1. This same dilution rate maximised the biomass productivity for every irradiance tested (Fig. 1b). On the other hand, the increase of external irradiance enhanced the biomass productivity at every dilution rate, which is the typical behaviour of photo-limited cultures, although the existence of photosaturation and specially photoinhibition should not be overlooked. To determine the state of the culture cells, the fluorescence of chlorophylls was measured (Fig. 1c). The results showed that the fluorescence of chlorophylls remained constant even at the maximal values of dilution rate and irradiance tested, which result in the highest exposures to light, and this allows to discard the existence of the photoinhibition phenomena.
The lutein content of the biomass and the lutein productivity were also determined for every steady state reached at the different external irradiances and dilution rates essayed. In the same fashion as the steady state biomass concentration, the lutein content increased with the irradiance and decreased at increasing dilution rates (Fig. 2a). The experimental data of lutein content ranged from 0.31 to 0.58% d.wt.. The lutein productivity also increased with the irradiance at every dilution rate and the highest lutein productivity at every irradiance was obtained at D = 0.04 h−1 with a maximum of 2.65 mg l−1 day−1 (Fig. 2b). This behaviour is analogous to the previously observed for the biomass productivity, which suggests that lutein can be considered a primary metabolite as its accumulation is growth-linked. Therefore, it can be expected that the optimal conditions for biomass productivity will also be optimal for lutein productivity.
The influence of the temperature on the growth rate and lutein productivity of S. almeriensis was analysed in continuous cultures carried out at D = 0.03 h−1 modifying the external irradiance and temperature. The external irradiance simulated the daylight solar cycle, as in the previous experiments, with different maximal irradiances of 650 to 1625 μE m−2 s−1, whereas the temperature was modified from 10 to 45°C. It is important to note that the thermal death of the culture took place at 48°C, indicating the tolerance of this strain to high temperature. The steady state biomass concentration increased with temperature attaining maximum values between 30 and 40°C, and then decreasing sharply (Fig. 3a) regardless of the irradiance. No interaction between the external irradiance and the temperature was observed in the statistical analysis the higher the irradiance the higher the steady state biomass concentration regardless the temperature of the experiment. The biomass productivity shows a similar behaviour (Fig. 3b). A maximal biomass productivity of 0.87 g l−1 day−1 was measured at 35°C and 1,625 μE m−2 s−1. Measurements of fluorescence of chlorophylls showed that the cultures were not stressed below 40°C as fluorescence values of 0.7 were measured for every irradiance (Fig. 3c). However, strong damage took place at 45°C as shown by a decrease of fluorescence of chlorophylls below 0.4 (Fig. 3c). Thus, it can be concluded that growth was temperature limited at low temperature, below 30°C, optimal at medium temperatures, from 30 to 40°C, and temperature damage was observed above 40°C. This is the typical growth versus temperature relationship for mesophiles organisms.
Regarding the influence of temperature on lutein, the results showed that the lutein content of the biomass increased with temperature at every irradiance (Fig. 4a). Only at the highest temperature of 45°C, with the culture intensely stresses due to thermal stress, the lutein content of the biomass decreased compared to the value measured at 35°C. For these experiments, the lutein content of the biomass ranged from 0.29 to 0.55% d.wt.. Due to the simultaneous increase in biomass productivity and lutein content with temperature, the lutein productivity greatly improved at increasing temperatures, with a maximal lutein productivity of 4.77 mg l−1 day−1 (Fig. 4b) measured at 35°C and 1625 μE m−2 s−1, the maximal irradiance. Only at the highest temperature of 45°C did the lutein productivity decrease due to the reduction in the biomass productivity.
Discussion
The optimal conditions for the production of lutein using the new strain S. almeriensis have been discussed in a recent paper (Sánchez et al. 2007). As reported in this work, a maximum lutein content of 0.54% d.wt. and productivity of 3.8 mg l−1 day−1 were obtained at 33°C and 1625 μE m−2 s−1. In the present paper a maximum lutein content of 0.55% d.wt. in S. almeriensis biomass is also reported, but the lutein productivity is found to increase up to 4.77 mg l−1 day−1 due to an increase in biomass productivity up to 0.87 g l−1 day−1. The lutein content of S. almeriensis is much higher than the 0.03% d.wt. lutein content in Marigold flowers, currently the commercial source (Piccaglia et al. 1998), therefore this microalga can be regarded as an interesting alternative for the production of this substance. However, some other microalgae have also been considered as potential sources for the production of lutein.
Del Campo et al. (2001) referenced a maximum lutein content of 0.6% d.wt. in Muriellopsis sp. cultivated in tubular photobioreactors in summer. Chlorella zofingensis also had high lutein content although only during the first stages of growth (Del Campo et al. 2000). Shi et al. (2000) referenced a maximum lutein content of 0.42% d.wt. in Chlorella protothecoides, in cultures performed under heterotrophic conditions. A comparison among the different data of lutein productivity shows that the values obtained in the present work under optimal conditions are of the same order than the ones obtained under heterotrophic conditions using Chlorella protothecoides, of 8.0 mg l−1 day−1 (Shi et al. 2000), or the 7.0 mg l−1 day−1 obtained by Del Campo et al. (2001) in a 50-l tubular photobioreactor using Muriellopsis sp. Moreover, S. almeriensis has been referenced to give lutein productivities of 8.5 mg l−1 day−1 in a 3000-l outdoor tubular photobioreactor (Molina et al. 2005). Therefore, S. almeriensis can be considered a source of lutein for commercial purposes, especially considering that the consumption of only 0.75 g of S. almeriensis can supply the daily dose of lutein recommended (Krinsky et al. 2003).
To use a microalga as a source of any product, it is necessary to know the influence of culture conditions on the biomass and product generation rate of the strain. Thus, it is very helpful to model the response of the strain to the environmental variables such as irradiance and temperature. These models are an essential tool for the scale up, especially if outdoor photobioreactors are going to be used, as well as for optimizing its operation. In the present paper, continuous cultures of S. almeriensis were performed at a laboratory scale simulating outdoor conditions, at different dilution rates, irradiances and temperatures, to allow modelling of the behaviour of this strain. The average irradiance inside the cultures has been used in this work as an indicator of light availability.
The results showed that the average irradiance inside the cultures increased with increasing external irradiances, although the changes were stronger at high dilution rates and less pronounced at low D (Fig. 5a), which indicates that the cultures were mainly photo-limited in this last situation. On the contrary, at high dilution rates the cultures were photo-saturated. This is evinced because at a constant dilution rate, increasing external irradiances leads to higher steady state biomass concentration, but the increase was not enough to reduce the average irradiance, and therefore the light availability, to the values corresponding to photo-limitation. This behaviour has been previously referenced in outdoor cultures of microalgae in photo-inhibiting conditions, where the average irradiance was found to increase hyperbolically with the external irradiance (Molina et al. 1994; Acién et al. 1998). The data here reported demonstrate that S. almeriensis was not photo-inhibited, in spite of the daily variation of external irradiance and high external irradiance essayed, verifying the high tolerance of genera Scenedesmus to high irradiances (Masojidek et al. 1999).
Temperature had a similar effect on the irradiance–growth rate relationship. In experiments carried out at a constant dilution rate, higher external irradiances led to increased average irradiance inside the culture, although the slope was different for each temperature. At favourable temperatures, ranging from 20° to 40°C, the average irradiance was kept at minimum values because any increase in external irradiance was compensated by an enhanced biomass concentration that increased the culture optical thickness and kept constant the average irradiance (Fig. 5b). However, at unfavourable temperatures, 10°C and 45°C, the average irradiance at a constant dilution rate is higher than the measured at optimal temperatures, and it increases when the external irradiance increases. Thus, an inadequate temperature reduces the efficiency of cells to use light, a higher average irradiance being necessary to maintain the same growth rate.
To establish a relationship between the growth rate and average irradiance a hyperbolic function has been proposed (Molina et al. 1994) (Eq. 2). However, this is only valid for photo-limited cultures. Under photosaturation or photoinhibition conditions this relationship falls short and must be modified if the influence of external irradiance in the parameters of the model is to be taken into account (Acién et al. 1998). The data here reported demonstrate that when the external irradiance increased, the efficiency of the cells to use light diminished, which appears in the model as an increase in the irradiance constant, I k. Accepting a hyperbolic variation of this parameter with the external irradiance (Eq. 3), the following growth model is obtained (Eq. 4).
Fitting the experimental data by nonlinear regression to the proposed Eq. (4), the parameters obtained are μ max = 0.075 h−1, n = 2.02, I k,max = 225 μE m−2 s−1, I’ k = 1436 μE m−2 s−1, r 2 = 0.9712. This equation reproduces well the experimental data for all the range of culture conditions essayed (Fig. 6), giving values of the characteristics parameters that agree with the previously referenced. The maximal growth rate obtained here, 0.075 h−1, is similar to the value of 0.063 h−1 reported for a discontinuous culture of the same strain (Sánchez et al. 2007), and similar to the reported for other strains such as Haematococcus pluvialis of 0.11 h−1 (García-Malea et al. 2006), I. galbana of 0.056 h−1 (Molina et al. 1994) or P. tricornutum of 0.063 h−1 (Acién et al. 1998). The irradiance constant ranged from to 90 to 150 μE m−2 s−1, which compares to values reported for strains such as H. pluvialis of 99 μE m−2 s−1 (García-Malea et al. 2006), I. galbana of 130 μE m−2 s−1 (Molina et al. 1994) or Phaeodactylum tricornutum of 116 μE m−2 s−1 (Acién et al. 1998). Thus, it can be concluded that the regression results obtained for proposed model are coherent and it could be considered for scale-up purposes if the effect of the temperature were included.
Variation of specific growth rate with the average irradiance inside the culture and external irradiance. Points correspond to experimental data whereas lines correspond to simulated data using Eq. (3)
To accomplish this, the proposed Eq. 4 has been used to fit separately each set of experimental values of growth rate versus external and average irradiance carried out a different temperature (Fig. 5b). If the values of n = 2.02, I k,max = 225 μE m−2 s−1 and I’ k = 1436 μE m−2 s−1 are considered constants, a value of μ max is obtained for each temperature series by nonlinear regression (Fig. 7). The results obtained from this procedure show that the specific maximum growth rate increased with the temperature up to 35°C, and then decreased sharply. As reaction kinetic constants typically fit to the Arrhenius equation, the following correlation between growth rate and temperature is proposed (Roels 1983):
where A 1and A 2 are characteristics constants related with the formation of activated compounds driving either favourable (A 1, the positive term) or detrimental processes (A 2, the negative term), while E a1 and E a2 are the corresponding activation energies for each the positive and negative part of the correlation.
The positive term of Eq. 5 allows consideration of the enhancement of growth rate with temperature up to 35°C, while the second one takes into account any negative effect of temperature observed above 35°C in the growth rate including the thermal death. By fitting the experimental data (Fig. 7) to the proposed equation (Eq. 5) the value of characteristics parameters are obtained as A 1 = 3.20 × 105 h−1, E a1 = 3.75 × 104 J mol−1, A 2 = 1.44 × 1012 h−1, E a1 = 7.85 × 104 J mol−1, r 2 = 0.9897. These values agree with the referenced for other microorganisms such as 5.8 × 104 J mol−1 for Aspergillus nidulans or 6.8 × 104 J mol−1 for E. coli (Pirt 1975), pointing out the sensitivity of S. almeriensis to temperature, higher than these other microorganisms. By combining the two proposed equations (Eqs. 4 and 5) the following overall growth model considering the influence of irradiance and temperature on the growth of S. almeriensis was obtained,
The whole experimental data set can now be fitted to the proposed overall growth model by nonlinear regression, allowing to determine the final characteristics parameters as A 1 = 3.20 × 105 h−1, E a1 = 3.75 × 104 J mol−1, A 2 = 1.44 × 1012 h−1, E a1 = 7.85 × 104 J mol−1, n = 2.28, I k,max = 202 μE m−2 s−1 and I’ k = 726 μE m−2 s−1, r 2 = 0.8685. The model fits the experimental data and allows to simulate the behaviour of the system. It also allows determination of the optimal operational conditions and maximum productivity attainable. In this sense, a simulation showed that the maximal productivity of 0.95 g l−1 day−1 can be obtained at 35°C and the maximum irradiance considered of 1900 μE m−2 s−1 (Fig. 8). This irradiance can be reached during summer time under outdoor conditions, but in wintertime the maximum irradiance at outdoor conditions is lower, i.e. 1000 μE m−2 s−1. In these conditions the optimal temperature is also 35°C, but the biomass productivity decreases to 0.62 g l−1 day−1.
The lutein productivity has also been modelled by calculating the specific lutein accumulation rate, q p, as the ratio of lutein productivity to biomass concentration on steady state (Eq. 7). The variation of specific lutein accumulation rate with the dilution rate for each external irradiance essayed showed that lutein is a primary metabolite because its accumulation increases linearly with the dilution rate (Eq. 8), although with different slopes depending on the irradiance (Fig. 9). The results show that the higher the external irradiance the higher the increase of the specific accumulation rate with the dilution rate. Accepting a linear relationship between these variables (Eq. 9), the following model taking into account the influence of external irradiance and growth rate on the specific accumulation rate of lutein in S. almeriensis is obtained (Eq. 10).
The values of the characteristics parameters can be calculated, again, by a nonlinear regression of experimental data to Eq. 10. The values obtained for the parameters are a = 24.70 mg g−1, b = 0.01 mg g−1 (μE m−2 s−1)−1, r 2 = 0.9858. Although this type of model is usual in bioprocess technology (Pirt 1975), as far as we know, there are no similar models for microalgal products, other than the proposed for the accumulation of astaxanthin by H. pluvialis (Del Río et al. 2005, 2007). In H. pluvialis the astaxanthin is a secondary metabolite with a product yield value Y p/x of 0.80 mg g−1. For S. almeriensis this value of product yield is much higher, ranging from 29.9 to 38.9 mg g−1, confirming the role of lutein as a primary metabolite in S. almeriensis.
Regarding the influence of temperature on the lutein productivity, it was observed that at every temperature the specific accumulation rate of lutein increased linearly with the external irradiance. A linear regression analysis shows that the slope of this variation is constant, but the origin ordinate changes with temperature (Fig. 10). Assuming a linear variation of the origin ordinate with the temperature the following overall lutein accumulation rate model is obtained (Eq. 11).
Fitting all the experimental data to the proposed equation (Eq. 11) by nonlinear regression, the following values of characteristics parameters are obtained, aa = 16.37 mg g−1, ab = 2.30 mg g−1 °C−1, b = 0.019 mg g−1 (μE m−2 s−1)−1, r 2 = 0.9028. The model fits adequately the experimental data and it could be used for scale-up purposes and to determine the optimal conditions maximizing lutein productivity. Simulation shows how the optimal conditions for the production of lutein are the same as those obtained for the production of biomass, confirming the role of lutein as a primary metabolite (Fig. 11). The optimal conditions are 35°C temperature and external irradiance of 1900 μE m−2 s−1, the maximum lutein productivity being 5.31 mg l−1 day−1. If the external irradiance diminishes to 1000 μE m−2 s−1, as in wintertime outdoors, the lutein productivity falls to 3.02 mg l−1 day−1.
Summarising, the behaviour of S. almeriensis versus external irradiance and temperature has been modelled. The results demonstrate that S. almeriensis is a mesophile microorganism with an optimal temperature of 35°C, tolerant to high external irradiances, showing no photoinhibition at irradiances up to 1625 μE m−2 s−1. S. almeriensis accumulates up to 0.55% d.wt. of lutein; this pigment is a primary metabolite in this strain. Thus, the conditions that maximise biomass productivity are the same that for lutein productivity. Maximal productivities of 0.87 g l−1 day−1 of biomass and 4.77 mg l−1 day−1 of lutein were measured. However, these values can be increased up to 0.95 g l−1 d−1 of biomass and 5.31 mg l−1 day−1 of lutein, at outdoor conditions. Thus, S. almeriensis is adequate for outdoor culture and could be a promising source of lutein.
References
Acién FG, García F, Sánchez JA, Fernández JM, Molina E (1998) Modelling of biomass productivity in tubular photobioreactors for microalgal cultures: effects of dilution rate, tube diameter and solar irradiance. Biotechnol. Bioeng 58:605–616
Del Campo JA, Moreno J, Rodríguez H, Vargas MA, Rivas J, Guerrero MG (2000) Carotenoid content of chlorophycean microalgae: factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76:51–59
Del Campo JA, Rodriguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 85:289–295
Del Campo JA, García-González M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina E, Guerrero MG (2005) Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol Bioeng 91(7):808–815
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina E, Guerrero MG (2008) Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol Bioeng 100(2):397–402 DOI https://doi.org/10.1002/bit.21770
Demming-Adams B, Adams WW III (2002) Antioxidants in photosynthesis nutrition. Science 298:2149–2153
Dweyer JH, Navab M, Dwyer KM, Hassan K, Sun P, Shircore A, Hama-Levy S, Hough G, Wang X, Drake T, Merz NB, Fogelman AM (2001) Oxygenated carotenoid lutein and the progression of early atherosclerosis. The Los Angeles atherosclerosis study. Circulation 103:2922–2927
García-Malea MC, Acién FG, Fernández JM, Cerón MC, Molina E (2006) Continuous production of green cells of Haematococcus pluvialis: modelling of the irradiance effect. Enzyme Microb Technol 38:981–989
Guterman H, Vonshak A, Ben-Yaakov S (1990) A macromodel for outdoor algal mass production. Biotechnol Bioeng 35:809–819
Johnson-Down L, Saudny H, Gray-Donald K (2002) Food habits of Canadians: lutein and lycopene intake in the Canadian population. J Am Diet Assoc 102(7):988–991
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann Rev Nutr 23:171–201
Mann JE, Myers J (1968) On pigments, growth and photosynthesis of Phaeodactylum tricornutum. J Phycol 4:349–355
Masojidek J, Torzillo G, Koblizek M, Kopecky J, Bernardini P, Sacchi A, Komenda J (1999) Photoadaptation of two members of the Chlorophyta Scenedesmus and Chlorella in laboratory and outdoor cultures: changes in chlorophyll fluorescence quenching and the xanthophylls cycle. Planta 209(1):126–135
Molina E, García F, Sánchez JA, Fernández JM, Acién FG, Contreras A (1994) A mathematical model of microalgal growth in light limited chemostat culture. J Chem Technol Biotechnol 61:167–173
Molina E, Fernández JM, Acién FG, Sánchez JF, García J, Magán JJ, Pérez J (2005) Production of lutein from the microalga Scenedesmus almeriensis in an industrial size photobioreactor: case study. Oral presentation at the 10th Internacional Conference on Applied Phycology, Kunming, China
Piccaglia R, Marotti M, Grandi S (1998) Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind Crops Prod 8(1):45–51
Pirt SJ (1975) Principles of microbe and cell cultivation. Blackwell Scientific Publications, London
Roels JA (1983) Energetics and kinetics in biotechnology. Elsevier, New York
Sánchez JF, Fernández JM, Acién FG, Pérez J, Molina E (2007) Influence of culture conditions in the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem 43(4):398–405
Shi XM, Zhang ZH, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb Technol 27:312–318
Silva S (2004) Luteína, alimento para tu vista. Food Ingredients 80–81
VERIS (Vitamin E Research and Information Service) (1997) Efficacy of carotenoids. VERIS Research Summary, August Vitamin E Research and Information Service, LaGrange, Illinois
Ziegler RG, Colavito EA, Hartge P, McAdams MJ, Schoenberg JB, Mason TJ, Fraumeni JF (1996) Importance of a-carotene, b-carotene and other phytochemicals in the etiology of lung cancer. J Natl Cancer Inst 88:612–615
Acknowledgements
This research was supported by Ministerio de Educación y Ciencia (CTQ2005-00335/PPQ), Junta de Andalucía, Plan Andaluz de Investigación (CVI 131 &173), and Fundación CAJAMAR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, J.F., Fernández-Sevilla, J.M., Acién, F.G. et al. Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79, 719–729 (2008). https://doi.org/10.1007/s00253-008-1494-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1494-2