Abstract
Production of fumaric acid from alkali-pretreated corncob (APC) at high solids loading was investigated using a combination of separated hydrolysis and fermentation (SHF) and fed-batch simultaneous saccharification and fermentation (SSF) by Rhizopus oryzae. Four different fermentation modes were tested to maximize fumaric acid concentration at high solids loading. The highest concentration of 41.32 g/L fumaric acid was obtained from 20 % (w/v) APC at 38 °C in the combined SHF and fed-batch SSF process, compared with 19.13 g/L fumaric acid in batch SSF alone. The results indicated that a combination of SHF and fed-batch SSF significantly improved production of fumaric acid from lignocellulose by R. oryzae than that achieved with batch SSF at high solids loading.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fumaric acid, a four-carbon unsaturated dicarboxylic acid, serves as an important organic compound and bulk chemical reagent. On account of its non-toxic and non-hygroscopic properties, it is not only used widely in the food and beverage industry but also in etherification and polymer chemistry [1]. Traditionally, fumaric acid has been synthesized using a chemical process [2]. However, this mode of production is energy intensive and environmentally unfriendly, and the development of a biotechnological route for fumaric acid production has captured the attention of researchers.
Lignocellulosic materials (LCMs), derived from agricultural and forestry residues, are a cheap and renewable feedstock from which can be obtained bioenergy and bio-based chemicals such as fumaric acid. The structure of LCMs is complex, which is a potential barrier to their further utilization. LCMs have three structural components: cellulose, a polysaccharide made up of glucose units, with a high degree of crystallinity and polymerization; hemicellulose, made up of various sugar units that can be substituted; and lignin, a polymer of phenolic nature [3]. LCMs are in many ways an ideal raw material for the production of bio-based chemicals using microorganisms, which has many advantages over traditional chemical synthesis-based methods. Feedstocks are widely available in most geographical locations, which can improve the local economy, and fermentation is less energy intensive and generates less toxic pollutants and is therefore more environmentally friendly [4].
Rhizopus species are saprophytic fungi that grow on simple nutrients through fermentation, and Rhizopus oryzae is among the most widely used strains for fumaric acid production [5], which can be produced using lignocellulose-based carbohydrates as a carbon source. A two-stage corn straw utilization strategy was reported to convert concentrated glucose (80 g/L) into 27.79 g/L fumaric acid with a yield of 0.35 g/g glucose [6]. R. oryzae ATCC 20344 can use dairy manure hydrolysate (lignocellulosic materials) supplemented with glucose to produce 31 g/L fumaric acid with a yield of 31 % [7]. In this process, enzymatic hydrolysis of pretreated lignocellulosic material was performed before fermentation by R. oryzae in what was described as separated hydrolysis and fermentation (SHF). However, SHF may meet with several problems, such as end-product inhibition of enzymatic hydrolysis, slow hydrolysis rate, and a low yield and product concentration. A simultaneous saccharification and fermentation (SSF) process could afford enzymatic hydrolysis of lignocellulose and fermentation of released sugars in the same vessel and hence overcome the problems associated with SHF.
Our previous study showed that R. oryzae could produce fumaric acid from alkali-pretreated corncob (APC) in a SSF process [8]. Under optimal conditions, SSF by R. oryzae produced 13.78 g/L fumaric acid from 5 % (w/v) APC, while direct fermentation only yielded 6.21 g/L using an equivalent amount of glucose as the carbon source [8]. Unfortunately, the low level of solids loading resulted in a low concentration of fumaric acid, and a high solids loading is desirable for both technical and economic reasons [9, 10]. Increasing solids loading could not only improve product concentration and reduce production costs but also reduce equipment size, energy consumption, and the burden of downstream processing [3]. An economically feasible process using lignocellulose as feedstock requires a solids concentration above 15 % (on dry basis) [3, 11]. However, high solids loading can lead to the potentially serious problem of high viscosity and lower transfer efficiency. Indeed, this has proved to be the main limiting factor for SSF by R. oryzae at high solids loading [11–14]. Fed-batch SSF may overcome mass transfer limitation and allow higher solids loading, and when applied in the production of ethanol from pretreated corncob, solids loading could be increased from 19 % (w/v) to 25 % (w/v), yielding 84.7 g/L ethanol [15].
In the present study, we investigated production of fumaric acid from alkali-pretreated corncob by R. oryzae CICC 40351 at high solids loading. Fed-batch SSF was compared with batch SSF, and the effect of increasing dry mass (DM) up to a solids loading of 20 % (w/v) was assessed.
Materials and Methods
Materials
Strains
The R. oryzae CICC 40351 strain was purchased from the China Center of Industrial Culture Collection (CICC, Beijing, China).
Raw Materials
Alkali-pretreated corncob (APC) was a gift from Jiangsu Kangwei Biologic Co., Ltd. (Dongtai, Jiangsu, China).
Enzymes
Cellulase and β-glucosidase were purchased from Sigma Aldrich (USA) and Novozymes (Denmark), respectively.
Media
Agar Slant
The composition of the slant medium was as follows: 10.0 g/L glucose, 3.0 g/L yeast extract, 3.0 g/L malt extract, 5.0 g/L peptone, and 20.0 g/L agar. The inoculated slant was cultured in a constant temperature incubator at 30 °C for 1 week, and then stored at 4 °C.
Seed Medium
The seed medium was composed of 40 g/L glucose, 4.4 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.6 g/L KH2PO4, 0.0176 g/L ZnSO4·7H2O, and 0.000498 g/L FeSO4·7H2O. Spores were washed from the slant with sterile water and the spore density was adjusted to 107 spores/mL.
Fermentation Medium
SSF medium contained APC, 0.71 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.6 g/L KH2PO4, 0.01 g/L ZnSO4·7H2O, 0.0004 g/L FeSO4·7H2O, and 30 g/L CaCO3 in 0.05 mol/L NaAc-Ac buffer (pH 4.8).
SHF medium contained enzymatic hydrolysate of APC, 0.71 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.6 g/L KH2PO4, 0.01 g/L ZnSO4·7H2O, 0.0004 g/L FeSO4·7H2O, and 30 g/L CaCO3.
Methods
Pretreatment
APC was derived from alkali-pretreatment of corncobs following digestion in 7 % (w/v) sodium hydroxide at 85–90 °C for 1 h. After filtration, the liquid fraction containing hemicellulose was used for production of xylooligosaccharides, while the solid fraction was soaked in water at a solid-liquid ratio of 1:10 (w/v) and then neutralized by 72 % (w/w) sulfuric acid to pH 4.8–5.0. APC was obtained by filtration to remove the liquid fraction and stored in plastic bags at 4 °C.
Enzymatic Hydrolysis
Enzymatic hydrolysis of APC was conducted in an incubator at 50 °C with shaking at 150 rpm. The dosage of cellulase and β-glucosidase was 25 FPIU/g and 20 IU/g, respectively. The glucose yield was calculated as follows:
Seed Culture
The seed suspension was transferred to seed medium and cultured for 24 h at 35 °C with shaking at 200 rpm.
Simultaneous Saccharification and Fermentation
SSF using free enzymes (cellulase and β-glucosidase) was carried out at 38 °C and 220 rpm in fermentation medium (50 mL) with a 10 % (v/v) inoculum. The conversion yield was calculated as follows:
Fed-Batch Simultaneous Saccharification and Fermentation
Fed-batch simultaneous saccharification and fermentation (Fed-batch SSF) experiments were carried out with different initial solids loading. Fresh substrate was added every 12 h, giving a final solids loading of 20 % (w/v). The fermentation conditions, enzyme loadings, and nutrient concentrations were the same as in the batch SSF.
Separated Hydrolysis and Fermentation
Enzymatic hydrolysis of APC was conducted with a cellulase cocktail at 50 °C and 150 rpm. The supernatant from the enzymatic hydrolysate was obtained by centrifugation at 2742×g for 10 min. The supernatant and other fermentation medium components together formed the separated hydrolysis and fermentation (SHF) medium. Fermentation of SHF medium was carried out at 38 °C and 220 rpm.
Analytical Methods
Fumaric acid, ethanol, and glucose were determined by high-performance liquid chromatography (HPLC) using a Bio-Rad Aminex HPX-87H column and a refractive index detector. The mobile phase was 5 mmol/L H2SO4, and the flow rate was maintained at 0.6 ml/min.
Due to the low solubility of fumaric acid at room temperature [16], the final culture broth was treated with 25 % (w/w) NaOH in order to convert fumaric acid into soluble sodium fumarate. Excess NaOH was neutralized with 36 % (w/w) H2SO4 before samples were taken.
Analysis of the chemical composition of APC was carried out according to the National Renewable Energy Laboratory standard methods for the determination of structural carbohydrates and lignin in biomass [17]. Filter paper activity and β-glucosidase activity were determined according to standard International Union of Pure and Applied Chemistry (IUPAC) procedures [18]. One FPIU was defined as the amount of enzyme that releases 1 μmol of glucose equivalents from Whatman No.1 filter paper per min. One unit of β-glucosidase was defined as the amount of enzyme that converts 1 μmol of cellobiose to 2 μmol of glucose per min.
All data presented in this study are the averages of two experiments. All of experiments were carried in 250-mL flasks containing 50-mL medium.
Results and Discussion
Components and Enzymatic Hydrolysis of Alkali-Pretreated Corncob
Corncob material was treated with sodium hydroxide to generate APC, and the composition was compared with raw material (Table 1). Corncob contains (%, dry weight basis) 36.01 % cellulose, 36.84 % hemicellulose, 17.43 % lignin (including acid-soluble lignin and acid-insoluble lignin), and 3.43 % ash. After alkali pretreatment, APC contains 66.95 % cellulose, 21.87 % hemicellulose, 5.92 % lignin (including acid-soluble lignin and acid-insoluble lignin), and 1.20 % ash. The percentage of cellulose (66.95 %) after pretreatment increased in relation to raw material (36.01 %) due to solubilization of xylan. Alkali pretreatment simultaneously increased cellulose and decreased hemicellulose and lignin, as shown previously [19, 20]. This treatment disrupted the crystalline structure of cellulose to make it more accessible for enzymes to attack APC [21].
High fumaric acid concentration relies on a high cellulose concentration in SSF [15]. In order to improve the cellulose concentration, a simple and easy method involves increasing the solids loading for enzymatic hydrolysis. As shown in Fig. 1, higher solids loading resulted in a higher glucose concentration after enzymatic hydrolysis. A 72-h enzymatic hydrolysis of (20 % w/v) APC released 135.20 g/L glucose, which was 3.47 times higher than that obtained from 5 % (w/v) APC. However, the glucose yield was nearly 100 % at 5 % (w/v) APC compared with 90.87 % at 20 % (w/v) APC. Increasing the APC concentration therefore decreased the glucose yield. The hydrolytic performance of cellulase at high solids loading was likely limited due to a decline in cellulase-binding capacity [11] or mass transfer limitation [3].
Comparison of Simultaneous Saccharification and Fermentation and Separated Hydrolysis and Fermentation at 5 % (w/v) Solids Loading
SSF was tested for production of fumaric acid from lignocellulose by R. oryzae. Firstly, we tried to produce fumaric acid from 5 % (w/v) APC in SSF mode, and compared this with SHF at a 5 % (w/v) substrate concentration.
Figure 2a, b shows a comparison of SSF and SHF at the same solids loading. In SHF, the hydrolysate containing 39.16 g/L glucose was used as a sole carbon source, which resulted from enzymatic hydrolysis of 5 % (w/v) APC. The glucose concentration remained relatively constant during the first 12 h of fermentation in SHF, presumably due to the complex composition in the lignocellulosic hydrolysate that prompted R. oryzae to need a 12-h adaption period [22]. Glucose was exhausted at 60 h in both SSF and SHF, but the fumaric acid concentration was 12.9 % higher in SSF. Indeed, the SSF process secreted up to 14.72 g/L fumaric acid from lignocellulosic material in a one-pot process. When APC was used directly as a sole carbon source for fumaric acid production, a significantly higher yield (0.44 g/g) was achieved using SSF.
Production of Fumaric Acid by Batch Simultaneous Saccharification and Fermentation at Different Solids Loading
Achieving a higher concentration of fumaric acid is dependent on the cellulose content in the SSF system. Table 2 shows production of fumaric acid by batch SSF at 5–15 % (w/v) solids loading. When the substrate concentration is 5 % (w/v), we achieve the highest fumaric acid concentration (14.12 g/L) with a yield of 0.28 g/g substrate. However, a higher DM did not result in a higher fumaric acid concentration. The concentrations of fumaric acid were 8.83 g/L at 10 % (w/v) APC and 10.36 g/L at 15 % (w/v) APC, respectively. Ethanol, a major byproduct, reached a final concentration of 10.85 g/L in batch SSF with 15 % (w/v) APC. Furthermore, >26 g/L residual glucose remained in the SSF medium. A higher substrate concentration is known to decrease fermentation efficiency and allow accumulation of glucose in the SSF medium [23]. High solids loading limited mass transfer to an extent, especially oxygen mass transfer, which generates anaerobic-like conditions. As a consequence, R. oryzae fermentation switched from aerobic-based to anaerobic-based metabolism, and anaerobic-like fermentation increased ethanol and decreased fumaric acid. Zhang et al. [15] reported that a high solids loading of lignocellulosic material during SSF resulted in high-viscosity slurry in the reactor, and the actual product yield was far less than the theoretical yield [15]. We also carried out batch SSF with 20 % (w/v) APC, but unfortunately, no fermentation products were detected (data not shown). Therefore, a very high viscosity fermentation suspension limited oxygen mass transfer at a solids loading of 20 % (w/v), which generated anaerobic-like conditions and diminished fumaric acid production.
To overcome the high solids loading conundrum associated with SSF, a fed-batch mode with prehydrolysis was explored. Intermittent addition of fresh substrate in a fed-batch SSF system could avoid the problem of high viscosity, improve mass transfer, and increase the final product concentration. Intermittent addition of fresh pretreated corncob during the first 24 h of fed-batch SSF achieved a final solids loading of 25 % (w/v) based on an initial solids loading of 19 % (w/v) and resulted in a final ethanol concentration of 84.7 g/L with an ethanol yield of 79.6 % [15].
Production of Fumaric Acid by Fed-Batch Simultaneous Saccharification and Fermentation at High Solids Loading
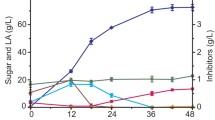
In order to increase the final solids loading to 20 % (w/v) and avoid mass transfer limitation, enzymatic prehydrolysis and a fed-batch approach were applied to the SSF process [24–26]. The operating conditions of different SSF modes and the accompanying results are shown in Table 3 and Fig. 3. In the first three SSF modes (a, 20 % SSF; b, 5 % SSF + 5 % SSF + 5 % SSF + 5 % SSF; c, 10 % SSF + 10 % SSF), a 24-h enzymatic prehydrolysis was performed, followed by batch or fed-batch SSF for production of fumaric acid. The purpose of enzymatic prehydrolysis was not only to increase the initial glucose concentration in the hydrolysate but also to decrease the initial viscosity of the fermentation broth. As a result, diffusion and mixing could be improved significantly [27].
Batch SSF (mode a) with 20 % (w/v) APC was compared with fed-batch SSF b (5 % SSF + 5 % SSF + 5 % SSF + 5 % SSF), and fumaric acid increased from 19.13 to 33.32 g/L (Fig. 3a, b). A high solids loading resulted in high viscosity [15], but the fed-batch SSF mode alleviated the mass transfer limitation and improved production of fumaric acid from 20 % (w/v) APC. The four-stage mode b (5 % SSF + 5 % SSF + 5 % SSF + 5 % SSF) was next compared with the two-stage mode c (10 % SSF + 10 % SSF). As shown in Table 3, there was little difference in fumaric acid concentration between modes b and c (Fig. 3b, c). As a result, adding different amounts of fresh APC every 12 h had no influence on the final fumaric acid concentration. In SSF mode d, the final fumaric acid concentration increased up to 41.32 g/L with a yield of 0.21 g/g substrate, and there was no ethanol byproduct in the SSF medium. SSF mode d involved three stages: 48 h enzymatic hydrolysis with 10 % (w/v) APC and fermentation by R. oryzae for 36 h; fed-batch SSF of 5 % (w/v) APC for 12 h; fed-batch SSF of 5 % (w/v) APC until the end of the SSF process. In this mode, the first stage provided sufficient glucose and improved glucose consumption, and subsequent stages improved mass transfer compared with one large addition at the beginning of the process.
In this study, a combination of SHF and fed-batch SSF at 20 % (w/v) APC produced the highest concentration of fumaric acid (41.32 g/L). This combined mode reduced the viscosity of the fermentation broth and improved glucose consumption. To our knowledge, this is the first report on combining SHF and fed-batch SSF for production of fumaric acid from lignocellulose by R. oryzae at high solids loading.
Although the production of fumaric acid by R. oryzae have been increased by using a combination of SHF and fed-batch SSF at a relatively high solids loading, the fermentative production of fumaric acid is still facing some problems such as lower fumaric acid concentration (< 50 g/L) and lower conversion yield obtained in the present work. Increased solids loading led to higher potential product concentration, reducing equipment’s size, energy consumption, and the burden of the downstream processing [3]. From the perspective of economics, higher fumaric acid concentration would be beneficial for the large-scale production of fumaric acid and cut down the production cost. Further study would focus on improving the concentration of fumaric acid by diverse fermentative modes at higher solids loading.
Conclusions
Four different fermentation modes were assessed for production of fumaric acid by R. oryzae at a high solids loading of lignocellulosic materials. The combination of SHF and fed-batch SSF yielded the highest fumaric acid concentration (41.32 g/L) from 20 % (w/v) APC at 38 °C, compared with 19.13 g/L fumaric acid in a batch SSF process. Consequently, it can be concluded that production of fumaric acid from LCMs with high solids loading was significantly improved by combining SHF and fed-batch SSF processes that reduced viscosity and improved glucose utilization.
References
Carta, F. S., Soccol, C. R., Ramos, L. P., & Fontana, J. D. (1999). Production of fumaric acid by fermentation of enzymatic hydrolysates derived from cassava bagasse. Bioresource Technology, 68, 23–28.
Roa Engel, C. A., Straathof, A. J. J., Zijlmans, T. W., van Gulik, W. M., & van der Wielen, L. A. M. (2008). Fumaric acid production by fermentation. Applied Microbiology and Biotechnology, 78, 379–389.
Romaní, A., Garrote, G., & Parajó, J. C. (2012). Bioethanol production from autohydrolyzed Eucalyptus globulus by simultaneous saccharification and fermentation operating at high solids loading. Fuel, 94, 305–312.
Zhang, Y. H. P. (2009). A sweet out-of-the-box solution to the hydrogen economy: is the sugar powered car science fiction? Energy and Environmental Science, 2, 272–282.
Huang, L., Wei, P., Zang, R., Xu, Z., & Cen, P. (2010). High-throughput screening of high-yield colonies of Rhizopus oryzae for enhanced production of fumaric acid. Annals of. Microbiology, 60, 287–292.
Xu, Q., Li, S., Fu, Y. Q., Tai, C., & Huang, H. (2010). Two-stage utilization of corn straw by Rhizopus oryzae for fumaric acid production. Bioresource Technology, 101, 6262–6264.
Liao, W., Liu, Y., Frear, C., & Chen, S. L. (2008). Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material—dairy manure—using a pelletized filamentous fungus Rhizopus oryzae ATCC 20344. Bioresource Technology, 99, 5859–5866.
Gu, X. M., Zhou, J., Chen, Y., & Li, X. (2015). Simultaneous saccharification and fermentation of alkali-pretreated corncob to fumaric acid by Rhizopus oryzae. Chinese Journal of Bioprocess Engineering, 6(13), 13–17.
Manzanares, P., Negro, M. J., Oliva, J. M., Sáez, F., Ballesteros, I., & Ballesteros, M. (2011). Different process configurations for bioethanol production from pretreated olive pruning biomass. Journal of Chemical Technology and Biotechnology, 86, 881–887.
Wang, W., Kang, L., Wei, H., Arora, R., & Lee, Y. Y. (2011). Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Applied Biochemistry and Biotechnology, 164, 1139–1149.
Jorgensen, H., Vibe-Pedersen, J., Larsen, J., & Felby, C. (2007). Liquefaction of lignocellulose at high-solids concentrations. Biotechnology and Bioengineering, 96, 862–870.
Georgieva, T. I., Hou, X. R., Hilstrom, T., & Ahring, B. K. (2008). Enzymatic hydrolysis and ethanol fermentation of high dry matter wet-exploded wheat straw at low enzyme loading. Applied Biochemistry and Biotechnology, 148, 35–44.
Rudolf, A., Alkasrawi, M., Zacchi, G., & Liden, G. (2005). A comparison between batch and fed-batch simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme and Microbial Technology, 37, 195–204.
Varga, E., Klinke, H. B., Reczey, K., & Thomsen, A. B. (2004). High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol. Biotechnology and Bioengineering, 88, 567–574.
Zhang, M. J., Wang, F., & Su, R. (2010). Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresource Technology, 101(13), 4959–4964.
Zhang, K. (2012). Fumaric acid fermentation by Rhizopus oryzae with integrated separation technologies. PhD Thesis, the Ohio State University, Columbus, USA.
Online: Determination of structural carbohydrates and lignin in biomass 2012. Available through: http://www.nrel.gov/biomass/analytical_procedures.html.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268.
Qin, W., Chen, Y., Zhao, H., Wang, R., & Xiao, D. (2010). Optimization of pretreatment conditions for corn cob with alkali liquor. Transactions of the Chinese Society of Agricultural Engineering, 26(4), 248–253.
Kim, S., & Holtzapple, M. T. (2006). Delignification kinetics of corn Stover in lime pretreatment. Bioresource Technology, 97, 778–785.
Chen, H. Z., Han, Y. J., & Xu, J. (2008). Simultaneous saccharification and fermentation of steam exploded wheat straw pretreated with alkaline peroxide. Process Biochemistry, 43, 1462–1466.
Pietrzak, W., & Kawa-Rygielska, J. (2015). Simultaneous saccharification and ethanol fermentation of waste wheat–rye bread at very high solids loading: effect of enzymatic liquefaction conditions. Fuel, 147, 236–242.
Stenberg, K., Bollók, M., & Réczey, K. (2000). Effect of substrate and cellulase concentration on simultaneous saccharification and fermentation of steam-pretreated softwood for ethanol production. Biotechnology and Bioengineering, 68(2), 204–210.
Jin, M. J., Gunawan, C., Balan, V., & Dale, B. E. (2012). Consolidated bioprocessing (CBP) of AFEX™-pretreated corn stover for ethanol production using clostridium phytofermentans at a high solids loading. Biotechnology and Bioengineering, 109, 1929–1936.
Jin, M. J., Lau, M. W., Balan, V., & Dale, B. E. (2010). Two-step SSCF to convert AFEX-treated switchgrass to ethanol using commercial enzymes and Saccharomyces cerevisiae 424A (LNH-ST. Bioresource Technology, 101, 8171–8178.
Hoyer, K., Galbe, M., & Zacchi, G. (2008). Production of fuel ethanol from softwood by simultaneous saccharification and fermentation at high dry matter content. Journal of Chemical Technology and Biotechnology, 84, 570–577.
Liu, Z. H., Qin, L., & Zhu, J. Q. (2014). Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnology for biofuels, 7(1), 167–182.
Acknowledgments
The authors would like to acknowledge financial support from the Major Program of the Natural Science Foundation of Jiangsu Higher Education (14KJA220003), the Natural Science Foundation of Jiangsu Province (grant no. BK20131426), the Key Research and Development Program of Jiangsu Province (BF2015007), and the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD).
At the same time, I am also grateful to M.S. Gu for her valuable suggestions and help on my thesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Zhou, J., Ouyang, S. et al. Fumaric Acid Production from Alkali-Pretreated Corncob by Fed-Batch Simultaneous Saccharification and Fermentation Combined with Separated Hydrolysis and Fermentation at High Solids Loading. Appl Biochem Biotechnol 181, 573–583 (2017). https://doi.org/10.1007/s12010-016-2232-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2232-3