Abstract
Unsaturated medium-chain-length polyhydroxyalkanoates (MCL-PHA) were produced at a productivity of 0.63–1.09 g PHA l−1 h−1 with final PHA content ranging from 42.6 to 55.8% in single-stage, carbon-limited, fed-batch fermentations of Pseudomonas putida KT2440. A mixture of nonanoic acid (NA) and 10-undecenoic acid (UDA=) was fed exponentially to control growth rate. Varying the specific growth rate (0.14 h−1 vs. 0.23 h−1) at similar substrate feed ratios (NA:UDA= = 5:1) had little effect on the final PHA content and relative composition. However, decreasing the NA:UDA= ratio decreased the final amount of PHA produced from 56% with NA:UDA= = 5.07:1 to only 42% at NA:UDA= = 2.47:1. The molar fraction of all 3-hydroxyalkanoate monomers in the PHA product was relatively constant throughout each fermentation, indicating that the final product was homogeneous rather than a mixture of different copolymers. A linear relationship between unsaturation of the PHA produced and unsaturation of the carbon feed was found, which demonstrates the feasibility of producing unsaturated MCL-PHAs with controlled polymeric composition in a fed-batch process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medium-chain-length poly(3-hydroxyalkanoates) (MCL-PHAs) are intriguing materials because of their biocompatibility, biodegradability, and great diversity. The large variety of possible monomer side chains allows diverse material properties to be attained (Hazer and Steinbüchel 2007) making them candidates for many applications, including drug delivery (Pouton and Akhtar 1996) and tissue engineering (Chen and Wu 2005). MCL-PHAs typically consist solely of repeating units of 3-hydroxyalkanoates (3-OH-alkanoates), but MCL-PHAs containing functional groups in the side chain, often referred to as functionalized PHAs (Hazer and Steinbüchel 2007), can also be synthesized. These functional groups, such as carbon–carbon double (Fritzsche et al. 1990), or triple bonds (Kim et al. 1998), and halogen (Kim et al. 1996), or phenyl groups (Hazer et al. 1996), are usually incorporated by appropriate microorganisms grown on carbon source(s) containing the corresponding functional group(s). To date, however, few such functionalized PHAs have been produced at even gram scale, possibly due to the toxicity of the functional carbon substrates, inefficiency in substrate utilization by the cultures employed, and/or unavailability of such substrates as many of them are not commercially available. Among functionalized PHAs, those containing olefinic groups in the side chains, i.e., unsaturated PHAs, may be the most useful (Hazer and Steinbüchel 2007) due to the subsequent chemical modification that is possible. These include chlorination (Arkin et al. 2000), cross-linking (Dufresne et al. 2001), carboxylation (Kurth et al. 2002) and epoxidation (Park et al. 1998). Since the melting and glass transition temperature vary according to the fraction of unsaturated components (Kim et al. 1995), and since different applications have different requirements, the degree of unsaturation needs to be finely controlled.
Most studies on the synthesis of MCL-PHAs have employed strains of Pseudomonas (Sun et al. 2007b). Edible oils (Ashby and Foglia 1998) and carbohydrates (Sanchez et al. 2003) have been shown to support the synthesis of MCL-PHAs with unsaturated fractions, but the degree of unsaturation could not be controlled using those carbon sources. However, when mixtures of saturated and unsaturated aliphatic acids were used as the carbon sources, there is a clear relationship between the fraction of the unsaturated carbon source in the medium and the unsaturated fraction in the PHA (Kim et al. 1995; Kellerhals et al. 1999; Hartmann et al. 2006). Unfortunately, the highest volumetric productivity of unsaturated PHAs reported was only 0.20 g PHA l−1 h−1 in a closed-loop fed-batch process (Kellerhals et al. 1999), which is much less than that of saturated MCL-PHAs (Sun et al. 2007b). In addition, the degree of unsaturation the PHA produced varied considerably during the fermentation, leading others to conclude that chemostat production is the most suitable method of producing MCL-PHA of defined monomeric composition (Hartmann et al. 2006). However, chemostats are not often used in industrial axenic fermentations due to the probability of contamination.
Some of the difficulty in obtaining homogeneous defined monomeric composition in batch culture is due to the imposition of the nitrogen or phosphorous limitation generally believed to be necessary for efficient production. We have recently reported efficient MCL-PHA production by single-stage, carbon-limited fed-batch fermentations of Pseudomonas putida KT2440 (Sun et al. 2007a). Neither nitrogen nor phosphorus limitation was necessary. In the current study, this fed-batch technique was applied to the production of unsaturated MCL-PHAs and to control their monomeric composition. The effect of growth rate on PHA composition was also investigated.
Materials and methods
Microorganism and growth medium
Pseudomonas putida KT2440 (ATCC 47054) was maintained in lyophiles and on nutrient agar plates at 4 °C. For all fermentations, 9 g l−1 of glucose plus 1 g l−1 of nutrient broth was the inoculum medium. The initial fermentation medium contained about 0.66 g l−1 of a mixture of nonanoic acid (NA, 98%, Spectrum) and 10-undecenoic acid (UDA=, 97%, Sigma Aldrich) in the same molar ratio as in the substrate feed of each fed-batch fermentation. Nitrogen was supplied by using a 14% (w/v) NH4OH solution for pH control. Other medium components are as reported by Sun et al. (2007a). Additional MgSO4·7H2O was fed at a ratio of 0.033 g MgSO4·7H2O g−1 carbon mixture added, assuming a YX/Mg of 240 g g−1 (Sun et al. 2006) and a YX/C of 0.80 g g−1.
Fermentation conditions
Inocula were cultivated in 500-ml shake flasks (150 ml medium) at 30 ± 1 °C and 200 rpm for about 12 h. Fed-batch fermentations were carried out at 28 ± 1 °C with 3.0 l initial working volume in a Minifors 5-l stirred tank bioreactor (Infors HT, Bottmingen, Switzerland). Data acquisition, pH control, dissolved oxygen control, and exit gas CO2 measurement were described previously (Sun et al. 2007a). Substrate feeding using peristaltic pumps was automatically controlled based on the mass of the reservoir containing the substrate mixture. Antifoam 204 (Sigma Aldrich) was added manually when required.
Substrate feeding and control methods
Three carbon-limited fed-batch fermentations, each at a controlled specific growth rate (μ), were carried out at different molar ratios of nonanoic acid to undecenoic acid as follows: (a) NA:UDA= = 4.98:1 (molar ratio) and μ = 0.15 h−1; (b) NA:UDA= = 5.07:1 and μ = 0.25 h−1; (c) NA:UDA= = 2.47:1 and μ = 0.25 h−1. To achieve the desired specific growth rate, substrate was fed continuously to the culture according to the feed Eq. (1):
Where ΔSt (g) is the total substrate required to produce biomass ΔXt (g) at cultivation time t (h), YX/C is the yield of biomass from substrate, assumed to be constant at 0.80 g g−1 based on a previous study (Sun et al. 2007a), X0 (g) is the initial biomass, obtained by measuring the optical density of the inoculum, and μ (h−1) is the desired specific growth rate.
Analytical procedures
Cell dry weight of lyophilized biomass was determined after centrifugation of 5 ml culture broth at 10,000 × g for 15–30 min and washing once with distilled water. The supernatant of the centrifuged broth was saved for analysis of nonanoic acid (NA), undecenoic acid (UDA=), and other major nutrients. Phosphate and ammonium concentration were determined colorimetrically (Sun et al. 2007a). Nonanoic acid and undecenoic acid concentration were determined by GC analysis after methylation (Ramsay et al. 1991), using benzoic acid as the internal standard. The methylation procedure for GC analysis of cellular PHA content and composition was as described by Sun et al. (2007a). A gas chromatograph (CP-3800, Varian Inc.) equipped with flame-ionization-detector was used in all GC analysis. The GC parameters were: injector temperature 250 °C, detector temperature 275 °C, 1-μl injection, split ratio 10. The oven temperature profile was: 90 °C, 0.5 min, 6 °C min−1 to 96 °C, 7 °C min−1 to 131 °C, 20 °C min−1 to 181 °C, 5 min. The MCL-PHA standard for the GC analysis was prepared by acetone extraction of biomass from the final fermentation broth, as described by Jiang et al. (2006). The monomeric composition was confirmed by GC-MS and their molar ratio was determined by proton NMR. The short-chain-length PHA standard was poly(3-hydroxybutyrate(HB)-co-3-hydroxyvalerate(HV)) (81 and 19 mol % of HB and HV, respectively), purchased from Zeneca Bioproducts Inc. The molecular weight of the extracted polymer was analyzed at 40 °C using a Waters 2695 Gel Permeation Chromatograph equipped with four Styragel columns of pore size 100, 500, 103, and 104 Å coupled to a Waters 410 differential refractive index detector, with polystyrene as the standard. Distilled tetrahydrofuran was used as solvent and the mobile phase at 1 ml min−1. All analyses were done in duplicate with the average of the results presented in this paper.
Results
Production of unsaturated MCL-PHAs by exponential feeding of nonanoic acid and 10-undecenoic acid
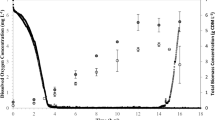
A fed-batch fermentation was conducted by exponentially feeding nonanoic acid (NA) and undecenoic acid (UDA=) at a molar ratio of 4.98:1. The desired specific growth rate (μ) was set at 0.15 h−1 in the feed equation (Eq. 1). As seen in Fig. 1, the overall biomass increased exponentially, and the actual μ was determined to be 0.14 h−1 by curve fitting (dashed line in Fig. 1). Apart from the lag phase, the culture was limited by the carbon feeding rate until the 42 h point of the fermentation. At this time, cell growth became limited by oxygen, causing both nonanoic acid and undecenoic acid to accumulate rapidly in the fermentation broth. The other major nutrients, ammonium and phosphorus, were maintained at concentrations very similar to those of a previous study (Sun et al. 2007a). The inoculum (grown on glucose and nutrient broth) contained negligible PHA, but the PHA content increased to 55.6% of cell dry weight by the end of the fermentation, giving a cumulative unsaturated MCL-PHA productivity of 0.71 g l−1 h−1. The PHA content achieved a plateau at a biomass concentration of 20 g l−1.
Exponential feeding of nonanoic acid (NA) and 10-undecenoic acid (UDA =; molar ratio 4.98:1) with desired μ = 0.15 h−1. The dissolved oxygen (DO) data are shown after 30 h, before which it was kept around 40% air saturation. Curve fitting (dashed line) was done by the nonlinear regression function of Sigmaplot 9.01
Fermentations with a higher desired μ (0.25 h−1) were conducted at different nonanoic acid to undecenoic acid ratios (NA:UDA= = 5.07:1 and 2.47:1; Fig. 2) to determine the effect of growth rate and substrate mixture on the PHA synthesis. The growth curves of the two fermentations were very similar, indicating that the ratio of nonanoic acid and undecenoic acid had little effect on biomass yield. Using a nonanoic acid to undecenoic acid ratio of 5.07:1, the PHA content plateaued at around 55%, very close to that of the μ = 0.14 h−1 fermentation which had a similar substrate ratio (NA:UDA= = 4.98:1), despite the much higher actual specific growth rate (0.23 h−1 vs. 0.14 h−1). In contrast, at NA:UDA= = 2.47:1, the PHA content achieved the plateau at only 42%. The overall PHA productivity of the fermentation where μ = 0.25 h−1 and NA:UDA= = 4.98:1 increased to 1.09 g PHA l−1 h−1 due to the relatively high PHA content achieved in a shortened fermentation time. The final yield of PHA from carbon source consumed during the fermentations was 0.49 g PHA g C−1 and 0.50 g PHA g C−1 for the NA:UDA= = 4.98:1 and NA:UDA= = 5.07:1 fermentations, but only 0.31 g PHA g C−1 for the NA:UDA= = 2.47:1 fermentation, as its final PHA content was only 43%.
Monomeric composition
Throughout the fermentations, MCL-PHAs containing 3-OH-alkanoates with saturated or unsaturated side chains were synthesized. During each fermentation the relative molar fraction of saturated and unsaturated components remained fairly stable (Fig. 3b,c). The molar fractions of all detectable components in the final extracted PHAs are shown in Table 1. Despite the difference in nonanoic acid to undecenoic acid ratio in the feed or the actual cell growth rate, 3-OH-nonanoate was always the most abundant among the saturated components, while 3-OH-nonenoate was the most abundant of the unsaturated components. The molar ratio of the total saturated to unsaturated PHA monomers was also quite constant throughout each fermentation (Fig. 3a). This ratio was always lower than the corresponding ratio of nonanoic acid to undecenoic acid in the feed (Table 1).
a Linear relationship between total molar concentration of saturated components and unsaturated components from three fermentations. b Cumulative monomeric composition of the MCL-PHA synthesized during fermentation with NA:UDA= = 5.07:1 and μ = 0.23 h−1. c Cumulative monomeric composition of the MCL-PHA synthesized during fermentation with NA:UDA= = 2.47:1 and μ = 0.24 h−1. The dash–dot line in b and c divides saturated and unsaturated components. C5 3-OH-valerate, C7 3-OH-heptanoate, C7:1 3-OH-heptenoate, C9 3-OH-nonanoate, C9:1 3-OH-nonenoate, C11 3-OH-undecanoate, C11:1 3-OH-undecenoate
The weight average molecular weight (M w) of the final extracted polymer from these fermentations were all around 115 kDa with a polydispersity of 1.8, similar to that of the saturated MCL-PHAs when nonanoic acid was fed alone.
Discussion
Effect of specific growth rate and substrate composition on PHA synthesis
At very different specific growth rates (0.14 h−1 vs. 0.23 h−1) but with similar substrate composition (NA:UDA= of 4.98:1 vs. 5.07:1), the final achievable PHA content (Table 2) and composition (Table 1) were almost identical. However, increasing the fraction of unsaturated carbon substrate (undecenoic acid in this case) had a great impact on the final achievable PHA content (Table 2). When grown on nonanoate alone, the same organism in the same medium can accumulate as much as 75% of dry weight biomass as PHA (Sun et al. 2007a). Adding UDA= to the feed (NA:UDA= of 5:1) decreased this to 56%. A further increase of UDA= in the feed (NA:UDA= of 2.5:1) resulted in a final PHA content of only 43%. Kim et al. (1995) reported similar observations in batch fermentations.
It is known that the optimal carbon chain length for the synthesis of MCL-PHA in P. putida from aliphatics is eight or nine carbons (Sun et al. 2007b). It is likely that the addition of an eleven-carbon compound such as undecenoate, will not only be incorporated into the polymer chain less rapidly than nonanoate, but will also slow the rate of nonanoate incorporation by competitive substrate inhibition. Consequently, both the final PHA content and the yield of PHA from carbon source decreased when the undecenoic acid in the feed was increased.
There was a linear relationship between the degree of unsaturation in the final PHA product and that in the feed, with slightly higher degree of unsaturation in the PHA than that of the feed substrates, as indicated by the slope of 1.1 of the regression line (Fig. 4). A slightly higher degree of unsaturation in the PHA was also found in the study of Kellerhals et al. (1999). This linear relationship demonstrates that it is possible to accurately predict the degree of unsaturation based on the substrates used, thus allowing production of unsaturated MCL-PHA with controlled compositions. The higher degree of unsaturation in the PHA produced is likely due to enzyme affinities for different substrates in PHA synthesis and β-oxidation, the two dominant metabolic routes for nonanoate and undecanoate.
Relationship between unsaturation of the final MCL-PHA product and unsaturation of the feed substrates. Each data point is from a different fermentation. NA alone data is taken from a previous study (Sun et al. 2007a)
Process design for unsaturated MCL-PHA production
Although a highly promising material, unsaturated MCL-PHAs have not been produced at a scale comparable to that of saturated MCL-PHAs. Ideally, PHA fermentations should be highly productive and produce biomass containing a large amount of product of homogeneous monomeric composition. In a closed-loop fed-batch process, unsaturated MCL-PHAs were produced up to 33% of the dry biomass with a productivity of 0.20 g PHA l−1 h−1 by co-feeding of Na-octanoate and Na-undecenoate (Kellerhals et al. 1999; Table 2). Since feeding of the two substrates was individually controlled and thus added at different rates, the ratio of saturated/unsaturated PHA monomers produced varied throughout the cultivation. Thus, the final product was almost certainly a mixture of copolymers with different degrees of unsaturation. In another study (Hartmann et al. 2006), chemostat cultivation produced unsaturated MCL-PHAs with constant monomeric composition (i.e., a homogeneous final product), while batch processes resulted in some variation in the composition throughout the cultivation. This led Hartmann et al. (2006) to conclude that batch cultivation was less suitable than chemostat for achieving desired monomeric composition. However, the PHA productivity of the chemostat process was very low (0.04–0.07 g PHA l−1 h−1).
By employing carbon-limited exponential feeding in a fed-batch process, unsaturated MCL-PHAs were produced at a much higher productivity (0.63–1.09 g PHA l−1 h−1) and final PHA content (42.6 to 55.8%) than has been previously reported (Table 2). The substrates used (nonanoic and undecenoic acids) are among the most common substrates used to produce unsaturated MCL-PHAs (Sun et al. 2007b), and are miscible, making the feeding process much simpler. The process is completely automatic without requiring expensive instrumentation. In addition, the ratio of saturated to unsaturated monomers of the PHAs produced remained quite constant throughout the fermentations, regardless of the specific growth rate or the substrate composition (Fig. 3). The current process can almost certainly be applied to produce other types of functionalized MCL-PHAs.
References
Arkin AH, Hazer B, Borcakli M (2000) Chlorination of poly(3-hydroxyalkanoates) containing unsaturated side chains. Macromolecules 33:3219–3223
Ashby RD, Foglia TA (1998) Poly(hydroxyalkanoate) biosynthesis from triglyceride substrates. Appl Microbiol Biotechnol 49:431–437
Chen GQ, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Dufresne A, Reche L, Marchessault RH, Lacroix M (2001) Gamma-ray crosslinking of poly (3-hydroxyoctanoate-co-undecenoate). Int J Biol Macromol 29:73–82
Fritzsche K, Lenz RW, Fuller RC (1990) Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol 12:85–91
Hartmann R, Hany R, Pletscher E, Ritter A, Witholt B, Zinn M (2006) Tailor-made olefinic medium-chain-length poly[(R)-3-hydroxyalkanoates] by Pseudomonas putida GPo1: batch versus chemostat production. Biotechnol Bioeng 93:737–746
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74:1–12
Hazer B, Lenz RW, Fuller RC (1996) Bacterial production of poly-3-hydroxyalkanoates containing arylalkyl substituent groups. Polymer 37:5951–5957
Jiang X, Ramsay JA, Ramsay BA (2006) Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J Microbiol Methods 67:212–219
Kellerhals MB, Kessler B, Witholt B (1999) Closed-loop control of bacterial high-cell-density fed-batch cultures: production of mcl-PHAs by Pseudomonas putida KT2442 under single-substrate and cofeeding conditions. Biotechnol Bioeng 65:306–315
Kim YB, Lenz RW, Fuller RC (1995) Poly-3-hydroxyalkanoates containing unsaturated repeating units produced by Pseudomonas oleovorans. J Polym Sci Pol Chem 33:1367–1374
Kim O, Gross RA, Hammar WJ, Newmark RA (1996) Microbial synthesis of poly(beta-hydroxyalkanoates) containing fluorinated side-chain substituents. Macromolecules 29:4572–4581
Kim DY, Kim YB, Rhee YH (1998) Bacterial poly(3-hydroxyalkanoates) bearing carbon–carbon triple bonds. Macromolecules 31:4760–4763
Kurth N, Renard E, Brachet F, Robic D, Guerin P, Bourbouze R (2002) Poly(3-hydroxyoctanoate) containing pendant carboxylic groups for the preparation of nanoparticles aimed at drug transport and release. Polymer 43:1095–1101
Park WH, Lenz RW, Goodwin S (1998) Epoxidation of bacterial polyesters with unsaturated side chains. I. Production and epoxidation of polyesters from 10-undecenoic acid. Macromolecules 31:1480–1486
Pouton CW, Akhtar S (1996) Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Adv Drug Deliv Rev 18:133–162
Ramsay BA, Saracovan I, Ramsay JA, Marchessault RH (1991) Continuous production of long-side-chain poly-beta-hydroxyalkanoates by Pseudomonas oleovorans. Appl Environ Microbiol 57:625–629
Sanchez RJ, Schripsema J, da Silva LF, Taciro MK, Pradella JGC, Gomez JGC (2003) Medium-chain-length polyhydroxyalkanoic acids (PHA(mcl)) produced by Pseudomonas putida IPT 046 from renewable sources. Eur Polym J 39:1385–1394
Sun Z, Ramsay JA, Guay M, Ramsay BA (2006) Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl Microbiol Biotechnol 71:423–431
Sun Z, Ramsay JA, Guay M, Ramsay BA (2007a) Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl Microbiol Biotechnol 74:69–77
Sun Z, Ramsay JA, Guay M, Ramsay BA (2007b) Fermentation process development for the production of medium-chain-length poly-3-hydroxyalkanoates. Appl Microbiol Biotechnol 75:475–485
Acknowledgment
This project was supported by the Natural Science and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., Ramsay, J.A., Guay, M. et al. Fed-batch production of unsaturated medium-chain-length polyhydroxyalkanoates with controlled composition by Pseudomonas putida KT2440. Appl Microbiol Biotechnol 82, 657–662 (2009). https://doi.org/10.1007/s00253-008-1785-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1785-7