Abstract
Brazzein is an intensely sweet-tasting plant protein with good stability, which makes it an attractive alternative to sucrose. A brazzein gene has been designed, synthesized, and expressed in Escherichia coli at 30 °C to yield brazzein in a soluble form and in considerable quantity. Antibodies have been produced using brazzein fused to His-tag. Brazzein without the tag was sweet and resembled closely the taste of its native counterpart. The brazzein gene was also expressed in Lactococcus lactis, using a nisin-controlled expression system, to produce sweet-tasting lactic acid bacteria. The low level of expression was detected with anti-brazzein antibodies. Secretion of brazzein into the medium has not led to significant yield increase. Surprisingly, optimizing the codon usage for Lactococcus lactis led to a decrease in the yield of brazzein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sucrose intake has been implicated in many health problems including obesity, caries, and diabetes mellitus. Some of these problems could be alleviated by greater use of alternative sweeteners that have more intense sweet taste than sucrose but with lower caloric intake. Growing awareness of the need to use safe, healthy, and natural products has increased the need for alternative sweeteners of natural origin.
Sweet-tasting proteins constitute a desirable group of natural sweeteners. They are the sweet ingredient of some edible fruits, which have been consumed by the indigenous people for centuries. They are several thousand times sweeter than sucrose on a weight basis. Six sweet-tasting proteins are known: thaumatin, monellin, mabinlin, pentadin, brazzein, and curculin (Faus 2000). Of these, brazzein possesses better pH and thermal stabilities and a pleasant profile of sweet taste (Ming and Hellekant 1994).
Brazzein was isolated from the fruit of the West African plant Pentadiplandra brazzeana Bailon (Ming and Hellekant 1994). It consists of 54 amino acids and is the smallest known sweet-tasting protein (6,473 Da) (Caldwell et al. 1998; Ming and Hellekant 1994). It is reported to be 500 times sweeter than sucrose on a weight basis. It exists in two forms that differ in sweetness. The majority of the brazzein contains pyroglutamate on the N terminus and the remainder has no pyroglutamate (Assadi-Porter et al. 2000a). Its structure consists of one short alpha helix and a three-strand beta sheet. It is highly compact and contains four intramolecular disulfide bonds (Caldwell et al. 1998). Parts of the molecule that are particularly important for the sweet taste have been defined (Assadi-Porter et al. 2003, 2000b; Jin et al. 2003a,b).
Extraction of brazzein from its natural source is expensive and, therefore, not applicable. Recombinant DNA technology and biotechnology provide an alternative option for cheaper mass production (Faus 2000). Recombinant brazzein has been, so far, successfully expressed in E. coli (Assadi-Porter et al. 2000a) and in maize (Lamphear et al. 2005).
Lactic acid bacteria (LAB) have recently received much attention due to their “generally recognized as safe” (GRAS) status and to their potential health-promoting effects as probiotics. For these reasons, they represent attractive host cells for recombinant protein expression. LAB can serve as live vectors for oral delivery of recombinant vaccines (Nouaille et al. 2003), such as tetanus toxin (Robinson et al. 1997) and HIV envelope protein (Xin et al. 2003), or for recombinant production of heterologous proteins with potential therapeutic relevance, e.g., lysostaphin (Mierau et al. 2005). Further, metabolic engineering has been used to increase their production of vitamins (riboflavin) and amino acids (l-alanine) (Burgess et al. 2004; Hols et al. 1999; Hugenholtz et al. 2002). In this study, we propose a new use for LAB by expressing sweet-tasting protein in situ in the dairy product, thereby removing the need for the addition of sugar and contributing to the value of LAB as producers of functional food (Hasler 2000). Moreover, by using recombinant LAB, there would be no need to isolate the brazzein.
Lactococcus lactis is generally used as a model LAB due to the wealth of information available and well-established genetic tools. A nisin-controlled expression system (NICE) (de Ruyter et al. 1996) has been a successful and widely applied system for heterologous protein expression in LAB. Its uses have been reviewed by Mierau and Kleerebezem (2005).
We have performed the preliminary expression of brazzein in E. coli to isolate sufficient quantities for testing and antibody production, and subsequently in L. lactis in an attempt to produce sweet phenotype LAB.
Materials and methods
Bacterial strains, media and culture conditions
Bacterial strains used in this study are shown in Table 1. E. coli strains were grown in Luria–Bertani (LB) medium with aeration at 37 or 30 °C. The LB medium was supplemented with 100 μg/ml kanamycin, 100 μg/ml ampicillin, 34 μg/ml chloramphenicol, or 350 μg/ml erythromycin where appropriate. L. lactis strain was grown in M-17 medium (Merck) supplemented with 0.5 % glucose (GM-17) at 30 °C without aeration. Erythromycin was used at a concentration of 10 μg/ml where appropriate.
Gene construction and synthesis
Two brazzein gene analogues (Fig. 1) were synthesized using two different approaches. The first brazzein gene (bra) analogue was designed on the basis of the amino acid sequence of brazzein, with codon usage optimized for the expression in E. coli. The nucleotide sequence was divided into eight partially overlapping oligonucleotides (designated BRA1 to BRA8, Table 1). Successive pairs of oligonucleotides were partially complementary. These pairs were annealed at 65 °C to form DNA double strands and were filled using Klenow fragment (Promega). This produced four consecutive double-stranded “blocks” which were ligated using relevant restriction enzyme recognition sites (NcoI, PvuII, BspEI, PstI, and NotI) at their ends.
Alignment of the bra-ht and bra-ht-opt genes and the amino acid sequence of brazzein with His-tag (brazzein-tag). The codon usages of bra-ht and bra-ht-opt genes were optimized for E. coli and L. lactis, respectively. Differences in nucleotide sequence are highlighted in grey. Bra-ht consists of synthesized bra gene and His-tag coding sequence, which is underlined
The second brazzein gene analogue (bra-ht-opt) had a codon usage optimized for expression in L. lactis (Fuglsang 2003) and was synthesized using two large oligonucleotides BRA1-LL-OPT and BRA2-LL-OPT (Table 1). Modified touchdown PCR was performed with 12.5 pmol of each oligonucleotide. An initial 5-min denaturation step was followed by ten PCR cycles which were performed with a 30-s denaturation step at 94 °C, 1 min annealing step at 63 °C (decreasing by 1 °C in each cycle) and 1 min elongation step at 72 °C. At this point, 10 pmol of primers BRA1-LL-NCO and BRA2-LL-XBA (Table 1) were added to the reaction mix. PCR was continued for 25 cycles with a 30-s denaturation step at 94 °C, 1 min annealing step at 50 °C and 1 min elongation step at 72 °C. PCR was completed with a final elongation step (72 °C, 5 min).
DNA manipulation and plasmid construction
E. coli plasmid DNA was isolated using Wizard Plus Minipreps DNA Purification System (Promega). Electroporation was performed according to Holo and Nes (1995) for L. lactis and to Sambrook et al. (1989) for E. coli, using Gene Pulser II apparatus (Biorad). Restriction enzymes and T4 DNA ligase were from New England Biolabs or Fermentas and were used according to the manufacturer’s instructions. General cloning procedures were performed according to Sambrook et al. (1989). PCR amplifications were performed using Taq polymerase (Fermentas) or Pfu DNA polymerase (Promega) on a Gene Amp PCR System 2700 (Applied Biosystems) thermocycler. PCR products were routinely ligated in pGEM-T Easy plasmid (Promega) for sequencing and further cloning procedures. Nucleotide sequencing was performed either on Abi Prism 310 (Applied Biosystems) or samples were sent to MWG Biotech (Germany). All primers (Thermo Electron) and plasmids used are listed in Table 1.
Bra gene was PCR amplified using BRA1 and BRA8 primers and ligated to pGEM-T Easy plasmid yielding pGEM::Bra. Bra gene was ligated to NcoI/NotI cut pET22b(+) and pET28a(+), yielding pET22::Bra and pET28::Bra. pGEM::Bra was used as a template for PCR amplification of bra gene using BRA1a/BRAC2, BRA1a/BRA-XBA or BRA1a/BRA-NO-STOP primers, thereby introducing NcoI/XmaI and NcoI/XbaI sites into the bra gene and removing the stop codon, respectively. These products were ligated to pIVEX2.3, pET22b(+), pET28a(+), and pMSP3545, yielding pIVEX2.3::Bra, pET22::Bra-ns, pET28::Bra-ns and pMSP3545::Bra-ht, respectively. pIVEX2.3::Bra was used to PCR amplify bra gene downstream fused to His-tag (bra-ht), with primers BRA1a and BRA-HIS that introduced NcoI/XbaI restriction sites. Bra-ht was ligated to NcoI/XbaI-digested pMSP3545, yielding pMSP3545::Bra-ht.
Bra-ht-opt was PCR-amplified with BRA1-LL-NCO and BRA2-LL-XBA primers and ligated to pGEM-T Easy. Due to problems with XbaI site mutation, NcoI and PstI (unique restriction site on pGEM-T Easy downstream of inserted gene) were used to clone bra-ht-opt to pMSP3545, yielding pMSP3545::Bra-ht-opt.
Usp45 signal peptide sequence (sp Usp) (Dieye et al. 2001; van Asseldonk et al. 1990) was PCR-amplified with USP1-NCO and USPR-NCO primers, using colony PCR on L. lactis IL 1403 colonies and cloned in pGEM-T Easy to obtain pGEM::Usp. NcoI-cut sp Usp fragment was ligated into NcoI-cut pMSP3545::Bra-ht to obtain pMSP3545::Usp-bra-ht.
Expression of brazzein in E. coli and L. lactis
Brazzein was expressed in two forms: wild-type brazzein (abbreviated brazzein-wild) and brazzein fused to His-tag (abbreviated brazzein-tag). The term “brazzein” is used to refer to brazzein in general or to both forms used in this study.
For the expression of brazzein, overnight cultures of E. coli (harboring pET22::Bra, pET22::Bra-ns, pET28::Bra or pET28::Bra-ns) or L. lactis (harboring pMSP3545::Bra, pMSP3545::Bra-ht, pMSP3545::Usp-bra-ht, or pMSP3545::Bra-ht-opt) were diluted (1:100) in fresh medium and grown to optical density A 600=0.4–1.0. E. coli cultures were induced using 0.5–1 mM IPTG and L. lactis cultures using 10–50 ng/ml nisin, or cultures were left uninduced for control. The cells were usually grown for another 3 h.
Ten-milliliter cultures were usually grown for optimization experiments. In each growth, cells were harvested by centrifugation at 5,000×g for 10 min and were resuspended in potassium phosphate buffer (pH 7.0) to the same cell optical density (A 600). Medium was collected where appropriate. Cells were frozen, thawed on ice, and briefly sonicated.
E. coli cultures for brazzein isolation were grown in 1,600-ml volume for 3 h after induction with 1 mM IPTG, and harvested by centrifugation (15 min; 5,000×g). Cells were resuspended either in 30-ml affinity chromatography buffer (50 mM sodium phosphate, 30 mM NaCl, pH 7.0) when expressing brazzein-tag, or in 30-ml gel exclusion chromatography buffer (0.1 M acetic acid, 0.3 M NaCl, 0.001 M EDTA, pH 6.0) when expressing brazzein-wild. Cells were frozen, thawed on ice, and sonicated with Sonifier W-450 (Branson) using 30 % amplitude, 15-s pulses, 25-s intermediate pauses for 15 min on ice. L. lactis culture for brazzein isolation was grown in 2-l volume for 3 h after induction with 25 ng/ml nisin. Cells were harvested, resuspended in gel exclusion chromatography buffer, and disrupted as described for E. coli.
Purification of brazzein
Brazzein-tag and brazzein-wild were isolated from cytoplasm of both E. coli and L. lactis. Disrupted cells were centrifuged three times for 20 min at 10,000×g and the pellet was discarded each time. The supernatant was filtered on 0.2-μm Minisart (Sartorius) to remove any remaining larger particles.
Brazzein-tag containing supernatant was fractionated on a 1-ml BD Talon metal affinity resin (BD Biosciences) according to manufacturer’s instructions, using pH elution. Fractions containing pure brazzein-tag were further purified by reverse-phase HPLC (Series 1100, Hewlett-Packard) using a Brownlee Aquapore BU 300 C4 column (Perkin-Elmer).
The culture medium (50 ml) was first filtered on 0.2-μM Minisart (Sartorius), then ultrafiltered through a 10-K Omega membrane (Pall) and finally, concentrated on a 3-K Omega membrane (Pall) to approximately 1 ml.
Brazzein-wild containing supernatant was fractionated on a Sepharose S200 column (4×110 cm) equilibrated with gel exclusion chromatography buffer. Fractions containing brazzein-wild were concentrated by ultrafiltration using an Amicon membrane (YM1) and dialyzed at the same time by diluting with dH2O in approximately 1:1,000 ratios. Concentrated brazzein-wild was filter-sterilized (0.2-μm Minisart, Sartorius) and used for sensory analysis.
Determination of N-terminal sequence
For brazzein-wild the N-terminal amino acid sequence was determined by automated Edman degradation using an Applied Biosystems 492 Protein sequencer.
Production of brazzein antibodies
Two rabbits were used for brazzein antibody production. Pre-immune serum was taken before immunization to serve as negative control. They were immunized three times (every 3 weeks) with 0.3 mg of purified brazzein-tag mixed with Freund’s adjuvant in 1:1 (v/v) ratio. Serum was taken 3 weeks after the last immunization.
SDS-PAGE and Western blot analysis
SDS-PAGE was performed on 12 or 15 % (w/v) polyacrylamide gels according to Laemmli (1970), using a mini-Protean II apparatus (Bio-Rad). Multi-Mark Multi-Colored standard (Invitrogen) was used for molecular weight comparison. Samples were denatured by heating at 100 °C in the presence of DTT before loading. Proteins were stained with Coomassie Brilliant Blue R-250 or transferred to a polyvinylidene fluoride Immobilon-P membrane (Millipore). The membrane was blocked for 2 h in 1 % Western blocking solution (Roche) and incubated overnight at 4 °C with appropriate antibodies (anti-brazzein serum, dilution 1:2,000 for brazzein-tag or 1:1,000 for brazzein-wild; Anti-His6-Peroxidase (Roche), dilution 1:5,000; all in 0.5 % Western blocking solution). After washing with TBST (50 mM Tris–HCl, 150 mM NaCl, 0.05 % Tween 20, pH 7.5), membranes were incubated with horseradish peroxidase-conjugated goat anti-rabit IgG (Dianova) at 1:10,000 dilution for 1 h at room temperature. They were then washed as above and Lumi-LightPLUS Western Blotting Substrate (Roche) was used for chemiluminescence detection on Hyperfilm ECL (Amersham). In the case of Anti-His6-Peroxidase antibodies, proteins were detected, as above, immediately after incubation and washing.
Sensory analysis of brazzein
Recombinant brazzein-wild was subjected to sensory analysis. The tasting protocol was designed according to Suzuki et al. (2004) and Jin et al. (2003a). Ten volunteers first tasted a series of standard sucrose solutions (0.000, 0.025, 0.050, 0.100, 0.250, and 0.500 M) for comparison with the brazzein-wild solution. One-hundred-fifty-microliter samples were applied to the anterior part of the tongue. The mouth was rinsed with tap water after each test.
Results
Constructs for brazzein expression in E. coli and L. lactis
A synthetic gene coding for brazzein was prepared on the basis of the amino acid sequence (Ming and Hellekant 1994) and optimized for expression in E. coli (Fig. 1). Ala was added to the N terminus in front of Gln1 for cloning purposes. This gene was cloned in two pET plasmids [pET22b(+) and pET28a(+)], yielding constructs enabling (pET22::Bra-ns, pET28::Bra-ns) or disabling (pET22::Bra, pET28::Bra) transcriptional fusion to His-tag. Similarly, two brazzein gene constructs, with and without His-tag, were cloned to pMSP3545, yielding pMSP3545::Bra and pMSP3545::Bra-ht for expression in L. lactis.
An additional synthetic brazzein gene optimized for L. lactis was constructed in an attempt to achieve higher brazzein yields (bra-ht-opt, Fig. 1). Codons for ten amino acid residues were changed and N-terminal Ala and Gln were removed. The gene optimized for L. lactis was again cloned in pMSP3545 (pMSP3545::Bra-ht-opt). Usp45 signal peptide sequence (sp Usp) was inserted in front of bra-ht in pMSP3545::Bra-ht to give pMSP3545::Usp-bra-ht and enable brazzein secretion. All sequences were confirmed by nucleotide sequencing. The plasmids are listed in Table 1.
Expression of brazzein in E. coli
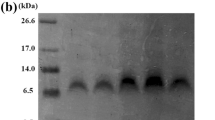
Brazzein constructs (pET22::Bra, pET22::Bra-ns, pET28::Bra, pET28::Bra-ns) were tested using different expression conditions (optical density A 600 at induction, IPTG concentration, temperature of growth, E. coli strain). Both brazzein-tag and brazzein-wild were expressed in E. coli BL21 DE3 pLysS in soluble form at 30 °C with 1 mM IPTG induction at optical density A 600=0.5 and yielded 5–10 mg/l and 30–35 mg/l, respectively.
Brazzein-tag was purified using immobilized metal affinity chromatography and reverse phase HPLC and used for production of antibodies, which were shown to react with both brazzein-tag and brazzein-wild, however requiring slightly higher concentrations with the latter.
Brazzein-wild, purified using gel exclusion chromatography and 90 % pure as judged from SDS-PAGE was used for sensory analysis. The sweetness intensity of 0.1 mM brazzein-wild solution was comparable to that of 0.1–0.5 M sucrose solution (0.2 M on average), which is less than reported previously for brazzein; the sweetness profile was, however, the same (Ming and Hellekant 1994).
The N-terminal amino acid sequence, M-A-Q-D-K, corresponds to that deduced from the brazzein gene.
Expression of brazzein in L. lactis
pMSP3545::Bra-ht was used to express brazzein-tag in L. lactis under different conditions, including time after induction and nisin concentration (Fig. 2). The yield of brazzein-tag was low under all conditions tested and it could not be detected with Coomassie Brilliant Blue, but only with immunodetection after Western blotting using anti-brazzein serum or anti-His6 antibodies. The induction was well-controlled and no brazzein-tag was detected without the addition of nisin. This served as a negative control (Fig. 2). We observed that brazzein-tag concentration peaked at 2–3 h after induction and then gradually decreased. It was highest with 25 and 40 ng/ml of nisin but, at concentrations of 40 ng/ml and above, began to inhibit cell growth significantly. The optical density at induction was not a determining factor. The following conditions were chosen as optimal among those tested: 25 ng/ml nisin for induction at optical density A 600=0.5, and 3 h of growth after induction.
Detection by Western blot of brazzein-tag and brazzein-wild in crude cell lysates of L. lactis IL 1403 containing pMSP3545::Bra or pMSP3545::Bra-ht. Cultures were induced with nisin (10, 25, 40 ng/ml). Cells were harvested 2, 3, or 4 h after induction. a Detection of brazzein-tag with anti-brazzein serum. b Detection of brazzein-tag with anti-His6 antibodies. c Detection of brazzein-wild with anti-brazzein serum
The brazzein gene optimized for L. lactis (pMSP3545::Bra-ht-opt) was tested, together with the gene optimized for E. coli (pMSP3545::Bra-ht), under identical conditions. To our surprise, the yield of brazzein-tag was lower when using the optimized gene (pMSP3545::Bra-ht-opt) (Fig. 3).
Comparison of brazzein yields in L. lactis using L. lactis or E. coli codon-optimized genes. Cultures of L. lactis IL 1403 with plasmids pMSP3545::Bra-ht-opt (colonies 1–2) or pMSP3545::Bra-ht (colonies 3–4) were induced with 25 ng/ml nisin or left uninduced as a control. Cells were harvested 3 h after induction and brazzein-tag was detected after Western blotting using anti-brazzein serum
Brazzein-tag was also expressed and directed to medium using SPUsp signal peptide (pMSP3545::Usp-bra-ht). Brazzein-tag without the signal peptide (pMSP3545::Bra-ht) was expressed and treated in an identical manner to serve as a control. Brazzein-tag was detected in both brazzein-secreting and control cells. In the former, two forms of brazzein were detected (the majority with signal peptide and the rest without). In concentrated medium, brazzein-tag was detected only with cells containing pMSP3545::Usp-bra-ht. The overall yield, however, did not improve significantly (Fig. 4).
Secretion of brazzein from L. lactis into the growth medium. Usp45 signal peptide (SPUsp) was used to enable secretion (plasmid pMSP3545::Usp-bra-ht; lanes 2, 4 and 6), with plasmid pMSP3545::Bra-ht as a control (lanes 1, 3, and 5). Uninduced L. lactis cells acted as a negative control (lanes 1 and 2). Brazzein expression was induced with 25 ng/ml nisin and cells grown for 3 h after induction. Cell (lanes 3 and 4) and medium (lanes 5 and 6) fractions were tested. Brazzein-tag was detected after Western blotting using anti-brazzein serum
Brazzein-wild was detected in L. lactis using anti-brazzein serum. The yield was low, similar to that of brazzein-tag, but peaked at 2 h after induction (Fig. 2). Brazzein-wild was expressed on a large scale under optimal conditions and isolated in a manner analogous to brazzein-wild from E. coli. After isolation, the product was detected but in quantities insufficient to be perceived as sweet.
Discussion
Sweet-tasting brazzein has been used mainly for the study of sweet taste reception and transduction and for characterization of its biochemical properties. It has not been reported to have been put into general use, despite being a natural and safe alternative sweetener whose greater use would have a positive health impact.
Brazzein gene was expressed as wild-type brazzein (brazzein-wild) and as brazzein fused to His-tag (brazzein-tag). Both were successfully expressed in E. coli in soluble form at 30 °C using pET22b(+) and pET28a(+) plasmids. We expected brazzein-tag not to be completely functional, because the C terminus has been shown to be an important sweet taste determinant (Assadi-Porter et al. 2000b). We, therefore, used the construct solely for optimizing expression and for antibody production because of the ease of its purification and detection.
Antibodies raised against purified brazzein-tag reacted with brazzein-wild. The higher concentrations of antibody required was probably due to the His-tag, which is a strong antigenic determinant.
Brazzein-wild was found to be sweet, with a sweet profile as reported in the literature (Assadi-Porter et al. 2000b; Ming and Hellekant 1994). The sweetness intensity was lower than reported, which could be due to the additional N-terminal alanine or to only partially correct folding. The identity of the brazzein was confirmed by the N-terminal amino acid sequence, which corresponded to that deduced from the nucleotide sequence.
Expression of brazzein in E. coli is feasible for research purposes, but large-scale production and isolation from this source would probably not be cost effective. In situ production and use without isolation is a more attractive option and has the potential for widespread use. An example is the expression of brazzein in maize and direct use of the germ flour (Lamphear et al. 2005). In situ production in lactic acid bacteria would provide a low-calorie sweet-tasting product that would be a significant addition to food functionality (Hugenholtz et al. 2002).
The main aim of this study was to express brazzein in L. lactis as a model lactic acid bacterium. Brazzein was expressed using nisin-controlled expression, which is a frequently used and well-characterized inducible expression system in L. lactis (Mierau and Kleerebezem 2005). We expressed both brazzein-wild and brazzein-tag, but the quantities were low, regardless of expression conditions. Brazzein was not detectable by Coomassie staining but only with anti-brazzein or anti-His6 antibodies. The induction with nisin was well-controlled and no brazzein was observed without the addition of nisin. The highest expression yield was achieved 2 to 3 h after induction and then gradually decreased. This could be due to proteolytic activity in L. lactis or to reduced availability of nisin resulting from its adsorption on producer cells. Brazzein-wild was expressed under optimal conditions and isolated in a manner analogous to that for E. coli¸ but the quantity was too low to enable thorough taste evaluation.
To increase expression yield, and given the significant difference in codon usage between E. coli and L. lactis, we prepared a new synthetic gene with codon usage optimized for L. lactis according to Fuglsang (2003). The genome of E. coli contains a higher GC content than that of L. lactis. The first two amino acid codons (for Ala and Gln) were excluded from the gene, as they could be the reason for lower sweetness intensity (Assadi-Porter et al. 2000b). However, the yield obtained using the L. lactis optimized gene, under otherwise identical conditions, was surprisingly lower. Thus, codon optimization by itself is not enough to improve yield. This may be due to the mRNA secondary structure stabilities, which differ significantly when modeled according to Mathews et al. (1999) and Zuker (2003) (data not shown).
The strategy of secreting protein into the medium was also tested, as it was reported to increase yield (Le Loir et al. 2005). Usp45 signal peptide sequence (sp Usp) was inserted in front of the brazzein-tag gene. Brazzein-tag was secreted to the medium, which could have practical benefits. However, the overall yield increased only slightly.
In conclusion, brazzein expression was optimized in E. coli, in quantities enabling anti-brazzein antibodies to be prepared and the sweet taste of purified brazzein-wild to be confirmed. The first steps have been made towards the production of sweet lactic acid bacteria by expressing brazzein in L. lactis.
References
Assadi-Porter FM, Aceti DJ, Cheng H, Markley JL (2000a) Efficient production of recombinant brazzein, a small, heat-stable, sweet-tasting protein of plant origin. Arch Biochem Biophys 376:252–258
Assadi-Porter FM, Aceti DJ, Markley JL (2000b) Sweetness determinant sites of brazzein, a small, heat-stable, sweet-tasting protein. Arch Biochem Biophys 376:259–265
Assadi-Porter FM, Abildgaard F, Blad H, Markley JL (2003) Correlation of the sweetness of variants of the protein brazzein with patterns of hydrogen bonds detected by NMR spectroscopy. J Biol Chem 278:31331–31339
Bryan EM, Bae T, Kleerebezem M, Dunny GM (2000) Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183–190
Burgess C, O’Connell-Motherway M, Sybesma W, Hugenholtz J, van Sinderen D (2004) Riboflavin production in Lactococcus lactis: potential for in situ production of vitamin-enriched foods. Appl Environ Microbiol 70:5769–5777
Caldwell JE, Abildgaard F, Dzakula Z, Ming D, Hellekant G, Markley JL (1998) Solution structure of the thermostable sweet-tasting protein brazzein. Nat Struct Biol 5:427–431
Chopin A, Chopin MC, Moillo-Batt A, Langella P (1984) Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260–263
de Ruyter PG, Kuipers OP, de Vos WM (1996) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62:3662–3667
Dieye Y, Usai S, Clier F, Gruss A, Piard JC (2001) Design of a protein-targeting system for lactic acid bacteria. J Bacteriol 183:4157–4166
Faus I (2000) Recent developments in the characterization and biotechnological production of sweet-tasting proteins. Appl Microbiol Biotechnol 53:145–151
Fuglsang A (2003) Lactic acid bacteria as prime candidates for codon optimization. Biochem Biophys Res Commun 312:285–291
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hasler CM (2000) The changing face of functional foods. J Am Coll Nutr 19:499S–506S
Holo H, Nes IF (1995) Transformation of Lactococcus by electroporation. In: Nickoloff JA (ed) Electroporation protocols for microorganisms. Humana, Totowa, New Jersey
Hols P, Kleerebezem M, Schanck AN, Ferain T, Hugenholtz J, Delcour J, de Vos WM (1999) Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat Biotechnol 17:588–592
Hugenholtz J, Sybesma W, Groot MN, Wisselink W, Ladero V, Burgess K, van Sinderen D, Piard JC, Eggink G, Smid EJ, Savoy G, Sesma F, Jansen T, Hols P, Kleerebezem M (2002) Metabolic engineering of lactic acid bacteria for the production of nutraceuticals. Antonie Van Leeuwenhoek 82:217–235
Jin Z, Danilova V, Assadi-Porter FM, Aceti DJ, Markley JL, Hellekant G (2003a) Critical regions for the sweetness of brazzein. FEBS Lett 544:33–37
Jin Z, Danilova V, Assadi-Porter FM, Markley JL, Hellekant G (2003b) Monkey electrophysiological and human psychophysical responses to mutants of the sweet protein brazzein: delineating brazzein sweetness. Chem Senses 28:491–498
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lamphear BJ, Barker DK, Brooks CA, Delaney DE, Lane JR, Beifuss K, Love R, Thompson K, Mayor J, Clough R, Harkey R, Poage M, Drees C, Horn ME, Streatfield SJ, Nikolov Z, Woodard SL, Hood EE, Jilka JM, Howard JA (2005) Expression of the sweet protein brazzein in maize for production of a new commercial sweetener. J Plant Biotechnol 3:103–114
Le Loir Y, Azevedo V, Oliveira SC, Freitas DA, Miyoshi A, Bermudez-Humaran LG, Nouaille S, Ribeiro LA, Leclercq S, Gabriel JE, Guimaraes VD, Oliveira MN, Charlier C, Gautier M, Langella P (2005) Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb Cell Fact 4:2
Mathews DH, Sabina J, Zuker M, Turner DH (1999) Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol 288:911–940
Mierau I, Kleerebezem M (2005) 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:705–717
Mierau I, Leij P, van Swam I, Blommestein B, Floris E, Mond J, Smid EJ (2005) Industrial-scale production and purification of a heterologous protein in Lactococcus lactis using the nisin-controlled gene expression system NICE: the case of lysostaphin. Microb Cell Fact 4:15
Ming D, Hellekant G (1994) Brazzein, a new high-potency thermostable sweet protein from Pentadiplandra brazzeana B. FEBS Lett 355:106–108
Nouaille S, Ribeiro LA, Miyoshi A, Pontes D, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2003) Heterologous protein production and delivery systems for Lactococcus lactis. Genet Mol Res 2:102–111
Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW (1997) Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol 15:653–657
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Suzuki M, Kurimoto E, Nirasawa S, Masuda Y, Hori K, Kurihara Y, Shimba N, Kawai M, Suzuki E, Kato K (2004) Recombinant curculin heterodimer exhibits taste-modifying and sweet-tasting activities. FEBS Lett 573:135–138
van Asseldonk M, Rutten G, Oteman M, Siezen RJ, de Vos WM, Simons G (1990) Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene 95:155–160
Xin KQ, Hoshino Y, Toda Y, Igimi S, Kojima Y, Jounai N, Ohba K, Kushiro A, Kiwaki M, Hamajima K, Klinman D, Okuda K (2003) Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 102:223–228
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgements
This work was supported by the Slovenian Research Agency Grant No. P4-0127. We are grateful to Prof. Gary M. Dunny for kindly providing the pMSP3545 plasmid and to Prof. Roger Pain for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berlec, A., Jevnikar, Z., Majhenič, A.Č. et al. Expression of the sweet-tasting plant protein brazzein in Escherichia coli and Lactococcus lactis: a path toward sweet lactic acid bacteria. Appl Microbiol Biotechnol 73, 158–165 (2006). https://doi.org/10.1007/s00253-006-0438-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0438-y