Abstract
Sweet plant proteins, which are safe, natural, low-calorie sweeteners, may be suitable replacements for sugars in the food and beverage industries. Mabinlin II, a sweet plant protein, shows the most pronounced heat stability and acid resistance of any of the six known types of plant sweet proteins. However, mabinlin II is difficult to extract from the Capparis masaikai plant, which is itself becoming increasingly scarce. This limits the use of naturally acquired mabinlin II. In this study, recombinant mabinlin II proteins were expressed and purified in Escherichia coli and in food-grade Lactococcus lactis. Recombinant mabinlin II proteins MBL-BH (containing the B-chains of mabinlin II downstream fused with His-tag) and MBL-ABH (containing the A- and B-chains of mabinlin II downstream fused with His-tag) were expressed in E. coli in the form of inclusion bodies. They were then purified and renatured. The refolded MBL-BH was found to be 100 times sweeter than sucrose by weight, but it was not heat-stable. Refolded MBL-ABH was neither sweet nor heat-stable. Recombinant mabinlin II proteins were secreted and expressed intracellularly in food-grade L. lactis, in which the concentrated cell samples and culture medium samples were detected using enzyme-linked immunosorbent assay and Western blotting analysis with anti-mabinlin II polyclonal antibody. This study demonstrated that the single B chain of mabinlin II has a sweet taste. The recombinant mabinlin II proteins have been successfully expressed in food-grade L. lactis, which is a crucial step in the production of mabinlin II through microorganism expression systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there has been an obvious increase in the prevalence of diseases relating to sugar intake, including obesity, diabetes, hyperglycemia, and caries. Safe, healthy, natural sweeteners have seen increasing demand. Sweet proteins can be used as natural, low-calorie sweeteners without triggering any demand for insulin in the individuals who consume them, unlike sucrose. In this way, they may be suitable replacements for artificial sweeteners and sugars in the food and beverage industries. Currently, six types of sweet proteins from plants, thaumatin, monellin, mabinlin, pentadin, brazzein, and curculin, have been found around the world (Faus 2000). Among them, only mabinlin is stored in seeds (Hu et al. 1985a).
Mabinlin is isolated from the seeds of Capparis masaikai Levl., which grows in subtropical regions within China. Local residents chew the seeds for their unique sweet taste and long aftertaste. Immediately after chewing, a bitter and astringent stimulus remains behind, followed by a prolonged sensation of sweetness. The mabinlin family has four sweet proteins, mabinlins I, II, III, and IV (Ding and Hu 1986; Hu and He 1983; Hu et al. 1985b; Nirasawa et al. 1994). Among them, mabinlin II possesses the most interesting properties, such as being around 400 times sweeter than sucrose on molar basis (Liu et al. 1993; Nirasawa et al. 1994), and its activity remains unchanged after 48 h of incubation at boiling temperatures (Ding and Hu 1986). Mabinlin II consists of an A-chain with 33 amino acid residues and a B-chain with 72 residues; and has a total molecular weight of 12.4 kDa (Liu et al. 1993). The B-chain is crosslinked with the A-chain through the two interchain disulfide bridges. The encoded precursor mabinlin II is composed of 155 aa residues, including a signal sequence of 20 aa, an N-terminal extension peptide of 15 aa, a linker peptide of 14 aa, and a single-residue C-terminal extension (Nirasawa et al. 1996).
The C. masaikai plant is becoming increasingly scarce, and the extraction of mabinlin II from the raw plant is expensive and complicated. This is why the use of naturally acquired sweet mabinlin II proteins in the food industry is limited. Recombinant DNA technology may be a suitable alternative option for cheaper mass production. The latest research has shown that the sweet protein mabinlin II can interact with the sweet-taste receptor hT1R2/T1R3 (Li et al. 2002b) and produce the sensation of sweetness. Studies have also shown that the B-chain with its unique [NL/I] tetralet motif is essential to this interaction. The A-chain may play a role in the long aftertaste of mabinlin II. Evaluations of sweetness have shown that the separated B-chain of mabinlin II can elicit sweetness alone, but the A-chain cannot (Li et al. 2008).
Lactic acid bacteria (LAB) have recently drawn much attention because of their “generally recognized as safe” (GRAS) status and their potential health-promoting effects as probiotics. For these reasons, they are used as host cells for the expression of recombinant proteins. Lactococcus lactis is generally used as a model LAB because of the wealth of information available and the well-established genetic tools usable for the expression of biologically useful proteins in fermentation or directly in food. Furthermore, there is a plasmid-free strain of L. lactis that does not secrete proteases or proteins in any considerable quantity. For this reason, the nisin-controlled expression system (NICE) has seen widespread use in the heterologous protein expression in LABs (de Ruyter et al. 1996).
Our aim is to develop a new application for LABs by creating a system in which they express sweet-tasting recombinant mabinlin II proteins in situ in dairy products, thereby removing the need for added sugar and contributing to the value of LABs as producers of functional foods. In this study, we first performed the expression of recombinant mabinlin II proteins in E. coli, and the expression products were collected and used to assess the sweet-tasting activities of these recombinant gene forms. Then, the active products of the recombinant mabinlin II proteins were expressed in food-grade L. lactis in an attempt to produce sweet-phenotype LABs.
Materials and methods
Bacterial strains and plasmids
E. coli Top10 and BL21 (DE3) and expression vector pET30a (+) were purchased from Invitrogen (Carlsbad, CA, US). Food-grade L. lactis NZ3900 and L. lactis expression vector pNZ8149 were purchased from NIZO (Kernhemseweg, Netherlands). Top10 was used for routine cloning, and BL21 (DE3) and L. lactis NZ3900 were used for gene expression. All bacterial strains and plasmids used in this study are listed in Table 1.
Cell growth media and plant materials
Top10 and BL21 (DE3) were cultured in Luria–Bertani (LB) medium at 37 °C with aeration. Fifty micrograms per milliliter of kanamycin was added to the media if necessary. L. lactis NZ3900 was cultured in M-17 medium (Oxoid, Basingstoke, UK) supplemented with 0.5 % (w/v) glucose (GM-17) at 30 °C without aeration. L. lactis NZ3900 transformants were selected on Elliker medium (Elliker et al. 1956). Nisin powder (Sigma, St Louis, MO, US) was dissolved in 0.05 % acetic acid for obtaining a final concentration of 1 mg/mL nisin solution, stored in aliquoted vials at −20 °C, and diluted 1,000-fold in sterile water just before use. The seeds and leaves of C. masaikai were collected from Yunnan Province, China.
DNA manipulation
Plasmid preparation, restriction endonuclease digestion, genomic DNA preparation, DNA ligation, and other recombinant DNA techniques were carried out using standard methods (Sambrook and Russell 2001). Plasmid DNA transformation of Top10 and BL21 (DE3) was performed by using the calcium chloride method. The preparation of competent cells and electroporation of L. lactis was performed using the protocol included with the MicroPulser (Bio-Rad, CA, US) (Holo and Nes 1995). Total genomic DNA of C. masaikai was extracted from the leaves using the hexadecyl trimethyl ammonium bromide (CTAB) method (Hu and He 1983).
Mabinlin II gene cloning
There is no intron in the mabinlin II gene (Genbank accession no. D83997) (Nirasawa et al. 1996); therefore, it was directly amplified by PCR from the genomic DNA of C. masaikai using primers MBL-1 and MBL-2. The PCR product was inserted into pMD18-T simple vector, renamed pMD18-T-MBL II and sequenced. The recombinant B-chain of mabinlin II downstream fused with His-tag was amplified by PCR from mabinlin II to generate recombinant gene MBL-BH using primers MBL-BH-1 and MBL-BH-2. The recombinant single chain containing the A- and B-chains of mabinlin II downstream fused with His-tag was amplified by overlapping PCR from mabinlin II to generate the recombinant gene MBL-ABH using primers MBL-ABH-1, MBL-ABH-2, MBL-ABH-3, and MBL-ABH-4. The recombinant genes MBL-BH and MBL-ABH were amplified by PCR using primers LLBH-1, LLBH-2, LLABH-1, and LLABH-2, which generated the recombinant genes MBL-LLBH and MBL-LLABH, which contained Nco I/Xba I restriction sites. The recombinant genes MBL-BH and MBL-ABH fused upstream with Usp45 signal peptide were amplified by overlapping PCR to generate Usp45-MBL-BH and Usp45-MBL-ABH using primers Usp45-1, Usp45-2, Usp45-3, Usp45-4, Usp45-5, and Usp45-6 (Fig. 1). All primers used in this study are shown in Table 2.
Expression plasmid construction and transformation to E. coli and L. lactis
The recombinant genes MBL-BH and MBL-ABH were digested with Nde I and Xho I and ligated to plasmid pET30a that had been digested with the same restriction endonucleases to produce the recombinant plasmids pET30a-MBL-BH and pET30a-MBL-ABH. These were then transformed into BL21 (DE3)-competent cells. The PCR products of the recombinant genes MBL-LLBH, MBL-LLABH, Usp45-MBL-BH, and Usp45-MBL-ABH were ligated to pMD18-T simple vector and named pMD18-T-MBL-LLBH, pMD18-T-MBL-LLABH, pMD18-T-Usp45-MBL-BH, and pMD18-T-Usp45-MBL-ABH for sequencing. These plasmids were digested with Nco I and Xba I to produce MBL-LLBH, MBL-LLABH, Usp45-MBL-BH and Usp45-MBL-ABH, and then ligated to pNZ8149 that was digested with Nco I and Xba I to produce the recombinant expression plasmids pNZ8149-MBL-LLBH, pNZ8149-MBL-LLABH, pNZ8149-Usp45-MBL-BH, and pNZ8149-Usp45-MBL-ABH. These expression plasmids were electrotransformed into L. lactis NZ3900 competent cells. The transformants were immediately diluted with GM17-MC broth (GM17 + 20 mM MgCl2 + 2 mM CaCl2), incubated for 1 h at 30 °C, and plated onto Elliker medium plates. After incubation for 48 h at 30 °C, the yellow colonies were selected for isolation of plasmid DNA from the transformed L. lactis. The recombinant plasmids were identified by PCR and digestion with Nco I and Xba I.
Anti-mabinlin II polyclonal antibody preparation and detection
Sweet protein mabinlin II was extracted from the seeds of C. masaikai as described previously (Hu and He 1983). Carboxymethyl-Sepharose FAST FLOW (GE Healthcare, Uppsala, Sweden) was applied to isolate mabinlin II. The salts were removed by ultrafiltration using Amicon Ultra-15 (Merck Millipore, Boston, MA, US). Two rabbits were used for mabinlin II antibody production. The preimmune serum was taken before immunization and served as a negative control. The rabbits were immunized three times every 3 weeks with 0.5 mg purified mabinlin II and Freund’s adjuvant in 1:1 (v/v). The serum was taken after 3 weeks of the final immunization and then purified using antigen affinity purification. Sepharose 4B (GE Healthcare, Uppsala, Sweden) coupled with mabinlin II was applied to isolate anti-mabinlin II polyclonal antibody. The anti-mabinlin II polyclonal antibody was measured using indirect ELISA. All samples were divided into pairs and tested in triplicate.
The recombinant expressions of mabinlin II in E. coli
Recombinant BL21 (DE3) strains harboring plasmid pET30a-MBL-BH and pET30a-MBL-ABH were used for the expression of the recombinant mabinlin II genes MBL-BH and MBL-ABH. These two strains were cultured overnight in LB medium containing 50 μg/mL kanamycin at 37 °C, and the bacterial cells were diluted (1:100) with fresh medium containing 50 μg/mL kanamycin. They were cultured continuously to cell density OD600 0.5–1.0, then induced using 0.6–1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and cultured for another 3–6 h. The uninduced cultures were used as controls. The bacterial cells were harvested by centrifugation at 10,000 rpm for 10 min, and resuspended in potassium phosphate buffer (pH 7.0) to produce the same cell density (OD600). The cell samples were sonicated by ultrasonic cell disruption apparatus with extraction buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 8.0), and centrifuged at 12,000 rpm for 20 min to isolate the soluble and insoluble fractions. The total proteins, soluble fraction and insoluble fraction were analyzed by Tricine–sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Tricine–SDS-PAGE) to identify the expression of the objective proteins. Protein samples were mixed in SDS loading buffer (12 % SDS, 6 % mercaptoethanol, 30 % glycerol, 0.05 % bromophenol blue in 1 M Tris–HCl, pH 6.8) in a 4:1 (v/v) ratio of sample to loading buffer and heated at 95 °C for 10 min. Samples were analyzed by Tricine–SDS-PAGE gels at 100 V for 1.5 h. Protein gels were stained with Coomassie Blue R-250. Tricine–SDS-PAGE was performed on polyacrylamide gels using a mini-Protean II apparatus (Bio-Rad, CA, US) (Schägger 2006). PageRuler Unstained Low Range Protein Ladder (Fermentas, Shanghai, China) was used to compare the molecular weights of the products.
Western blot detections of the recombinant mabinlin II proteins expressed in E. coli
Western blotting was performed in accordance with AEC chromogenic kit (Sangon bioech, Shanghai, China) manufacturer’s instructions. Spectra Multicolor Low Range Protein Ladder (Fermentas, Shanghai, China) was used to evaluate molecular weights. Total protein samples were denatured by heating at 100 °C for 10 min in SDS loading buffer and then loaded onto the gels. The gels were electrotransferred to polyvinylidene fluoride Immobilon-PSQ transfer membrane (Millipore, MA, US) using Fastblot (Biometra, Goettingen, Germany). The membranes were washed three times with Tris buffered saline with Tween 20 (TBST) and blocked in 5 % skimmed milk powder in TBST for 1 h at room temperature with gentle shaking. They were then washed three times with TBST, as before, and incubated with a primary anti-mabinlin polyclonal antibody (diluted 1:2,000) overnight at 4 °C. They were then washed three times with TBST, as before, and incubated with horseradish-peroxidase-conjugated goat anti-rabbit IgG secondary antibody (diluted 1:5,000) for 1 h at room temperature and washed three times, as above. Protein detection was visualized using 3-amino-9-ethyl-carbazole (AEC).
MALDI-TOF-TOF analysis of the recombinant mabinlin II proteins expressed in E. coli
The purified recombinant proteins (MBL-BH and MBL-ABH) were cut from the stained gels and identified by MALDI-TOF-TOF analysis (Beijing Protein Innovation, Beijing, China).
Purification and renaturation of the recombinant mabinlin II proteins expressed in E. coli
The insoluble objective proteins (MBL-BH and MBL-ABH) were washed with inclusion body purgation buffer (2 M urea, 50 mM Tris, 100 mM NaCl, 1 % Triton, pH 8.0) and dissolved in inclusion body denaturation reagent (6 M guanidine hydrochloride, 50 mM NaH2PO4, 300 mM NaCl, pH 8.0). Then the insoluble proteins were purified by Ni–NTA-Sefinose columns (BioBasic, Toronto, Canada). The soluble fractions were filtered with a 0.22 μm filter (Millipore) and loaded onto a pre-equilibrated Ni–NTA column. The objective proteins were eluted with elution buffer (8 M urea, 50 mM NaH2PO4, 300 mM NaCl, and 20–500 mM imidazole, pH 8.0) and the protein concentrations were determined using the Bradford method. The purified denatured recombinant proteins were filtered with a 0.22 μm filter and refolded by dialysis against urea concentration gradient refolding buffer. Different concentrations of refolding protein (0.10, 0.20, 0.30, 0.40, 0.50, and 0.60 mg/mL), pH (7.0, 7.5, 8.0, 8.5, 9.0 and 9.5), glycine concentrations (20, 40, 60, 80, and 100 mM), arginine concentrations (100, 200, 300, 400, and 500 mM), and oxidation reduction potential of refolding buffer (2:1, 4:1, 6:1, 8:1, and 10:1) were chosen to optimize the refolding conditions. The soluble fractions from refolding solutions were collected by centrifugation at 10,000 rpm for 10 min and analyzed using Tricine–SDS-PAGE gels. The protein concentrations of the soluble fractions were determined using the Bradford method. The renaturation ratios of the two recombinant proteins were calculated as the proportions of the refolded protein concentrations and the purified denatured protein concentrations. The refolding experiments of the two recombinant proteins were repeated three times.
Sweet-taste activity testing of refolded recombinant mabinlin II proteins from E. coli
The refolded recombinant mabinlin II protein solutions were dialyzed to sterile distilled water at 4 °C for 8 h with 4 replications. The solutions were concentrated to 0.1 mg/mL protein concentration by vacuum lyophilizer (Scientz, Ningbo, China). Double blind tests were used to identify the sweet activity of the refolded recombinant mabinlin II proteins. The tests were performed as follows: 0.1 mg/mL natural mabinlin II dissolved in water was used as a positive control, and sterile distilled water served as a negative control. Four kinds of sucrose concentration (1, 2, 3, and 4 %, m/v) were used as sweetness standards. Ten testers participated in this sweetness tests; they were not privy to any information regarding the identities of these solutions. Informed consent was obtained from all participants. Every tester tasted 1 mL solution every time and rinsed his or her mouth with distilled water. The experiments were repeated five times. The responses of all the testers were recorded and counted.
Heat-stability assays of the refolded recombinant mabinlin II proteins from E. coli
The heat stabilities of the refolded recombinant mabinlin II proteins (0.1 mg/mL of protein–water solution) were determined by directly heating the solutions. The natural mabinlin II protein solution (0.1 mg/mL) served as a positive control and distilled water served as a negative control. Both solutions were heated in a water bath at 80 °C. After heating for 1, 12, and 24 h, the solutions were sampled and cooled down to room temperature for evaluation of sweet-tasting activity.
Recombinant expression of mabinlin II in L. lactis
The recombinant L. lactis strains harboring plasmid pNZ8149-MBL-LLBH, pNZ8149-MBL-LLABH, pNZ8149-Usp45-MBL-BH, and pNZ8149-Usp45-MBL-ABH were used for the expression of the recombinant mabinlin II genes. These four L. lactis strains were cultured overnight at 30 °C without aeration and diluted (1:100) with fresh medium. Different concentrations of induction nisin (10, 20, 30, 40, 50, 60, 70, and 80 ng/mL), L. lactis cell concentration at OD600 (0.10, 0.20, 0.30, 0.40, 0.50, 0.60, 0.70, and 0.80), and the duration of incubation after induction (1, 2, 3, 4, 5, 6, 7, and 8 h) were chosen to optimize the expression conditions for the recombinant mabinlin II proteins (Zhang et al. 2009). Uninduced cultures were used as controls. After induction by Nisin, 100 mL cultures of the two recombinant strains harboring plasmid pNZ8149-MBL-LLBH and pNZ8149-MBL-LLABH were collected. The cells were harvested by centrifugation at 10,000 rpm for 10 min and then resuspended in 10 mL of 0.1 M potassium phosphate buffer (pH 7.0) and sonicated. Total soluble cell proteins were prepared by centrifugation of the lysates at 12,000 rpm for 20 min to produce the soluble fraction. The cultured supernatants of the other two recombinant strains harboring plasmid pNZ8149-Usp45-MBL-BH and pNZ8149-Usp45-MBL-ABH were collected by centrifugation at 10,000 rpm for 20 min and filtration with a 0.22 μm filter. They were then concentrated tenfold. All the samples, including concentrated total soluble cell proteins and cultured supernatants were analyzed by Tricine–SDS-PAGE.
Quantification and concentration of the recombinant mabinlin II proteins by ELISA
HisSorb Ni–NTA plates (Qiagen, Hilden, Germany) were used for immobilization of the recombinant mabinlin II proteins, as previously described (Berlec et al. 2008). ELISA was performed for the quantification of the recombinant protein samples, including concentrated total soluble proteins and cultured supernatants, in accordance with the manufacturer’s instructions. All the samples were divided into pairs and tested in triplicate. Uninduced samples were used as negative controls. A standard curve was created using recombinant mabinlin II containing his-tag purified from E. coli on the same plate, at molar concentrations ranging from 0.4 to 4 μM based under the same conditions, and the yields of the expressed recombinant proteins were calculated by molar concentration and mass concentration conversion.
Identification of the recombinant mabinlin II proteins expressed in L. lactis
Western blotting was performed for identification of the recombinant mabinlin II proteins expressed in L. lactis. The recombinant protein samples, including total soluble cell protein and concentrated cultured supernatants were mixed with SDS-loading buffer and loaded on gels at 150 V for 1 h, then electrotransferred to the polyvinylidene fluoride Immobilon-PSQ transfer membrane for Western blot analysis. The experimental methods were the same as those described above.
Results
Construction of expression plasmids for the recombinant mabinlin II proteins
Two recombinant forms of the mabinlin II gene, with six expression forms, were constructed, MBL-BH, MBL-ABH, MBL-LLBH, MBL-LLABH, Usp45-MBL-LLBH, and Usp45-MBL-LLBH. MBL-BH and MBL-ABH were not fused to any amino acid residues at the N-terminal. They were inserted into plasmid pET30a to produce pET30a-MBL-BH and pET30a-MBL-ABH and expressed directly by the T7 promoter in E. coli. These recombinant plasmids were identified by PCR and sequencing. The recombinant mabinlin II gene forms (MBL-LLBH, MBL-LLABH, Usp45-MBL-LLBH, and Usp45-MBL-LLBH) were inserted into pNZ8149, producing the recombinant plasmids pNZ8149-MBL-LLBH, pNZ8149-MBL-LLABH, pNZ8149-Usp45-MBL-BH, and pNZ8149-Usp45-MBL-ABH. The plasmids pNZ8149-MBL-LLBH and pNZ8149-MBL-LLABH were used for the intracellular expression of food-grade L. lactis, and pNZ8149-Usp45-MBL-BH and pNZ8149-Usp45-MBL-ABH were used for the expression of secreted proteins. These plasmids were identified by PCR and enzymatic digestion.
Preparation of anti-mabinlin II polyclonal antibody
Antiserum of the natural mabinlin II protein was extracted from two rabbits and purified. The titer of the purified antiserum reached 1:16,000 as determined by ELISA. The results indicated that anti-mabinlin II polyclonal antibody was produced successfully and that it could be used to detect the recombinant mabinlin II proteins expressed in E. coli and L. lactis.
Expression of the recombinant mabinlin II in E. coli
The induced expression conditions of the recombinant strains BL21 (DE3)/pET30a-MBL-BH and BL21 (DE3)/pET30a-MBL-ABH were optimized. The IPTG concentrations for the two strains were 0.8 and 1.0 mM. The OD600 values at induction of the two strains were 0.8 and 1.0, respectively. After induction, the two strains were both incubated for 5 h. Tricine–SDS-PAGE analysis indicated that recombinant mabinlin II proteins were expressed in the two recombinant stains after IPTG induction. However, no additional proteins were found in the two strains not subjected to IPTG induction (Fig. 2a). The recombinant mabinlin II proteins expressed in these two strains were insoluble and formed inclusion bodies (Fig. 2b). Western blotting experiments showed that the proteins induced by IPTG in E. coli were specifically recognized by anti-mabinlin II polyclonal antibody, but no cross-reaction occurred with any of the whole proteins from E. coli BL21/pET-30a-MBL-BH or BL21/pET-30a-MBL-ABH not subjected to induction. In this way, the extra expressed proteins were identified as recombinant mabinlin II proteins (MBL-BH and MBL-ABH) (Fig. 2c).
Expression of the recombinant mabinlin II proteins (MBL-BH and MBL-ABH) in E. coli. a The SDS-PAGE analyses of the recombinant mabinlin II proteins. Lane 1 total cell proteins of BL21/pET-30a-MBL-BH; lane 2 total cell proteins of BL21/pET-30a-MBL-BH subjected to IPTG induction; lane 3 total cell proteins of BL21/pET-30a-MBL-ABH; lane 4 BL21/pET-30a-MBL-ABH subjected to IPTG induction; lane M protein marker. b Expression analysis of recombinant mabinlin II proteins. Lanes 1–3 total cell proteins, soluble proteins, and insoluble proteins of BL21/pET-30a-MBL-BH subjected to IPTG induction; lanes 4–6 total cell proteins, the soluble proteins, and insoluble proteins of BL21/pET-30a-MBL-ABH subjected to IPTG induction; lane M protein marker. c Western blot analysis of the recombinant mabinlin II proteins MBL-BH and MBL-ABH. Lane 1 total cell proteins of BL21/pET-30a-MBL-BH; lane 2 total cell proteins of BL21/pET-30a-MBL-BH subjected to IPTG induction; lane 3 total cell proteins of BL21/pET-30a-MBL-ABH; lane 4 total cell proteins of BL21/pET-30a-MBL-ABH subjected to IPTG induction; Lane M protein marker
Purification and renaturation of the recombinant mabinlin II proteins from E. coli
After being washed with inclusion body purgation buffer, the inclusion body proteins were denatured with inclusion body denature reagent. The denatured recombinant mabinlin II proteins were purified with Ni–NTA-Sefinose columns (Fig. 3a). The optimal concentration of elution buffer was found to be 250 mM imidazole for the two recombinant mabinlin II proteins according to Tricine–SDS-PAGE. The purified recombinant proteins (MBL-BH and MBL-ABH) were identified as mabinlin II by MALDI-TOF-TOF. The purified and denatured recombinant mabinlin II proteins at concentrations of 0.2 mg/mL were refolded optimally by dialysis against urea concentration gradient refolding buffer with 40 mM glycine and 300 mM arginine at an oxidation reduction potential of 10:1 and pH 8. After refolding, the soluble fractions were collected from the refolding solutions by centrifugation and analyzed with Tricine–SDS-PAGE (Fig. 3b). The protein concentrations of the soluble fractions were determined using the Bradford method. After purification and refolding of the recombinant mabinlin II (MBL-BH and MBL-ABH), the yields of the dissoluble recombinant mabinlin II proteins were 32.5 and 59.1 mg per 1 L culture, respectively, and the refolding ratios of these two proteins were 76.4 and 83.2 %.
Identification of the biological function of recombinant mabinlin II proteins from E. coli
The sweet-tasting activities of the recombinant mabinlin II proteins expressed in E. coli were determined by double blind testing. Nine of ten testers felt that the recombinant protein MBL-BH and natural mabinlin II were sweet; but they did not find the recombinant protein MBL-ABH or distilled water to be sweet. Eight out of ten testers felt that the intensity of the sweetness of 0.1 mg/mL MBL-BH solution was similar to that of a 1 % sucrose water solution. The recombinant mabinlin II proteins were heated at 80 °C to assess their heat stability. After heating for 1 h, the two recombinant protein solutions began to appear muddy, and nine of the ten testers did not find the two recombinant proteins sweet after heating. However, all testers felt that the natural mabinlin II solutions were sweet, even after heating for 24 h. These results indicated that the recombinant protein MBL-BH possessed sweet-tasting activity at an intensity around 100 times that of sucrose by weight, but it did not have heat stability. The recombinant protein MBL-ABH did not possess sweet-tasting activity or heat stability.
Expression of the recombinant mabinlin II in L. lactis
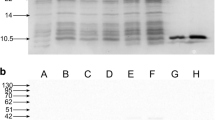
The recombinant L. lactis NZ3900/pNZ8149-MBL-LLBH, NZ3900/pNZ8149-MBL-LLABH, NZ3900/pNZ8149-Usp45-MBL-BH and NZ3900/pNZ8149-Usp45-MBL-BH were used to express the recombinant mabinlin II proteins. The concentrated total cell protein samples and culture medium samples were analyzed using Tricine–SDS-PAGE, and no additional protein bands were observed by Coomassie blue staining or silver staining. However, the recombinant mabinlin II proteins in these samples were recognized by anti-mabinlin II polyclonal antibody and Western blot analysis, but no bands were found in the negative controls (Fig. 4). These results indicated that the recombinant mabinlin II proteins were successfully expressed in the food-grade L. lactis expression system.
The Western blotting detection of recombinant mabinlin II proteins expressed in L. lactis. Lane M protein marker; lane 1 concentrated total cell proteins of L. lactis NZ3900/pNZ8149-MBL-LLBH; lane 2 concentrated total cell proteins of L. lactis NZ3900/pNZ8149-MBL-LLBH subjected to nisin induction; lane 3 concentrated total cell proteins of L. lactis NZ3900/pNZ8149-MBL-LLABH; lane 4 concentrated total cell proteins of L. lactis NZ3900/pNZ8149-MBL-LLABH subjected to nisin induction; lane 5 concentrated culture medium supernatants of NZ3900/pNZ8149-Usp45-MBL-BH; lane 6 concentrated culture medium supernatants of NZ3900/pNZ8149-Usp45-MBL-BH subjected to nisin induction; lane 7 concentrated culture medium supernatants of NZ3900/pNZ8149-Usp45-MBL-ABH; lane 8 concentrated culture medium supernatants of NZ3900/pNZ8149-Usp45-MBL-ABH subjected to nisin induction
The yields of the recombinant mabinlin II proteins expressed in L. lactis under different conditions were measured using HisSorb Ni–NTA plates and ELISA, which was recently developed to accurately quantify the difference. The yields of the recombinant mabinlin II proteins expressed in all four recombinant strains were low, and the yields of the recombinant MBL-LLBH and Usp45-MBL-BH were lower than those of the recombinant MBL-LLABH and Usp45-MBL-ABH under all induced conditions. However, no recombinant proteins were detected in cells not subjected to nisin induction. Under optimized expression conditions, the yields of the recombinant mabinlin II proteins MBL-LLBH, MBL-LLABH, Usp45-MBL-BH, and Usp45-MBL-ABH were highest in cells that were induced with 30 ng/mL nisin, but when the concentration of nisin was ≥30 ng/mL, cell growth was significantly inhibited (Fig. 5a). The yields of the recombinant mabinlin II proteins MBL-LLBH and MBL-LLABH were highest when the cells were induced at OD600 0.6, but it was OD600 0.5 in Usp45-MBL-BH and Usp45-MBL-ABH (Fig. 5b). The yields of the recombinant mabinlin II proteins MBL-LLBH and MBL-LLABH peaked 4 h after nisin induction and then gradually decreased, but the yields of Usp45-MBL-BH and Usp45-MBL-ABH peaked at 3 h and then gradually decreased (Fig. 5c). Under these conditions, the maximum yields of the recombinant mabinlin II proteins MBL-LLBH, MBL-LLABH, Usp45-MBL-BH, and Usp45-MBL-ABH were 2.21, 2.72, 1.28, and 1.59 mg per 1 L culture. The yields of these recombinant mabinlin II proteins were too low for effective purification and testing of sweet-tasting activity.
Discussion
Sweet-tasting mabinlin II has mainly been studied in sweet taste reception, transduction, and characterization of its biochemical properties. There are no reports of successful mabinlin expression in any in vitro system or reports of its general use as a sweetener, even though it both natural and safe and is believed to have a positive impact on human health. The use of a protein sweetener like mabinlin II protein in dairy food products would reduce or eliminate the need for added sugar or other alternative sweeteners, which would facilitate a healthier diet. Some other sweet proteins have been successfully expressed in microorganism systems. For example, the sweet protein brazzein has been expressed in E. coli and L. lactis (Assadi-Porter et al. 2000; Berlec et al. 2006). The sweet protein thaumatin II has been expressed in E. coli and the recombinant protein has been renatured from inclusion bodies to yield a purified protein preparation that is indistinguishable from native thaumatin in all biochemical, spectroscopic, and organoleptic respects (Daniell et al. 2000). In addition, thaumatin II was secreted by the methylotrophic yeast Pichia pastoris, and recombinant thaumatin II elicited a sweet taste similar to that of thaumatin II extracted from the Thaumatococcus danielli Benth plants (Masuda et al. 2004).The sweet protein monellin was expressed in E. coli, food yeast Candida utilis, and Bacillus subtilis, and a maximum secreted monellin yield of 0.29 mg/mL was extracted from the supernatant of B. subtilis (Kondo et al. 1997; Chen et al. 2005, 2007).
In this study, we attempted to express the recombinant mabinlin II gene in microorganism expression system and produce sweet-tasting recombinant mabinlin II proteins. E. coli is considered a simple and easy means of expressing the products of foreign genes. In this study, the E. coli expression plasmid pET30a, carrying a strong T7 promoter, was used for expression of recombinant mabinlin II genes. The recombinant mabinlin II genes were fused downstream with a 6-His tag for easy to purify. The purified recombinant mabinlin II proteins from E. coli BL21 (DE3) were tested to verify their structural activities. Two kinds of recombinant mabinlin II genes, MBL-BH and MBL-ABH, were introduced into the E. coli BL21 (DE3) strain through plasmid pET30a. The results indicated that the recombinant MBL-BH protein possessed sweet-tasting activity; and the recombinant MBL-ABH protein did not. This was possibly because of an inactive structure or incorrect refolding. The recombinant mabinlin II proteins were found to be insoluble when expressed in E. coli, and the renaturation of these recombinant proteins was difficult. This suggests that the recombinant mabinlin II genes should be fused with a small ubiquitin-related modifier (SUMO) or a thioredoxin to improve their solubility in E. coli.
One of the prominent advantages of the L. lactis expression system is that the bacteria and plasmids compatible with this system are all food-grade and known to be safe. No endotoxins or inclusion bodies are formed, which makes these genetic tools easy to handle. Finally, simple nonaerated fermentation could make a direct scale-up from 1 to 1,000 (Mierau et al. 2005). The most successful expression system of L. lactis is NICE, in which the food-grade L. lactis NZ3900 is commonly used as the host strain. The strain is a lacF deletion mutant. The expression vector pNZ8149, using the lacF gene as a food-grade selection marker, was here used to select the transformants L. lactis NZ3900. In this study, we preliminarily showed that recombinant mabinlin II proteins could be expressed in this food-grade expression system (L. lactis NZ3900/pNZ8149). The recombinant mabinlin II proteins were detectable with anti-mabinlin II polyclonal antibody immunodetection by ELISA and Western blot analysis. However, the quantities of the recombinant mabinlin II proteins were low, as in previous reports of the expression of brazzein expression in L. lactis (Berlec et al. 2006). The production of brazzein induced by bacteriocins, could be improved in L. lactis by changing the components of the growth medium, the growth conditions, or the pH of the fermentation environment (Berlec et al. 2008; Bogovic-Matijasic et al. 2001; Cheigh et al. 2002; Li et al. 2002a). This suggested that gene expression was favored at pH levels below those optimal for growth. Codon optimization was found to have an important impact on the expression of the foreign gene in L. lactis. These are both avenues that merit exploration in further studies into the improvement the production of the recombinant mabinlin II proteins through optimization of the growth parameters of L. lactis and of the codons of the recombinant mabinlin II genes.
References
Assadi-Porter FM, Aceti DJ, Cheng H, Markley JL (2000) Efficient production of recombinant brazzein, a small, heat-stable, sweet-tasting protein of plant origin. Arch Biochem Biophys 376(2):252–258
Berlec A, Jevnikar Z, Majhenic AC, Rogelj I, Strukelj B (2006) Expression of the sweet-tasting plant protein brazzein in Escherichia coli and Lactococcus lactis: a path toward sweet lactic acid bacteria. Appl Microbiol Biotechnol 73(1):158–163
Berlec A, Tompa G, Slapar N, Fonovic UP, Rogelj I, Strukelj B (2008) Optimization of fermentation conditions for the expression of sweet-tasting protein brazzein in Lactococcus lactis. Lett Appl Microbiol 46(2):227–231
Bogovic-Matijasic B, Rogelj I, Batic M, Raspor P (2001) Influence of pH on bacteriocin production by Lactobacillus K7 during batch fermentation. Period Biol 103(2):163–167
Cheigh CI, Choi HJ, Park H, Kim SB, Kook MC, Kim TS, Hwang JK, Pyun YR (2002) Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol 95(3):225–235
Chen Z, Cai H, Lu F, Du L (2005) High-level expression of a synthetic gene encoding a sweet protein, monellin, in Escherichia coli. Biotechnol Lett 27(22):1745–1749
Chen Z, Heng C, Li Z, Liang X, Xinchen S (2007) Expression and secretion of a single-chain sweet protein monellin in Bacillus subtilis by sacB promoter and signal peptide. Appl Microbiol Biotechnol 73:1377–1381
Daniell S, Mellits KH, Faus I, Connerton I (2000) Refolding the sweet-tasting protein thaumatin II from insoluble inclusion bodies synthesised in Escherichia coli. Food Chem 71(1):105–110
de Ruyter PG, Kuipers OP, de Vos WM (1996) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62(10):3662–3667
Ding M, Hu Z (1986) Study on mabinlin, a sweet protein from the seeds of C. masaikai Levl. IV: stability and denaturation. Acta Bot Yunanica 8(2):181–192
Elliker PR, Anderson AW, Hannesson G (1956) An agar medium for lactic acid streptococci and lactobacilli. J Dairy Sci 39(11):1611–1612
Faus I (2000) Recent developments in the characterization and biotechnological production of sweet-tasting proteins. Appl Microbiol Biotechnol 53(2):145–151
Holo H, Nes IF (1995) Transformation of Lactococcus by electroporation. In: Nickoloff JA (ed) Methods in molecular biology, vol 47., Electroporation protocols for microorganismsHumana Press, Totowa, pp 195–199
Hu Z, He M (1983) Study on mabinlin, a sweet protein from the seeds of C. masaikai Levl. I: extraction, purification and certain characteristics. Acta Bot Yunanica 5(2):207–212
Hu Z, Liang HX, Liu XZ (1985a) Study on mabinlin, a sweet protein from the seeds of C. masaikai Levl. III: storage site and state. Acta Bot Yunanica 7(2):187–194
Hu Z, Peng LP, He M (1985b) Study on mabinlin, a sweet protein from the seeds of C. masaikai Levl. II: comparison of mabinlin I to mabinlin II. Acta Bot Yunanica 7(1):1–10
Kondo K, Miura Y, Sone H, Kobayashi K, Iijima H (1997) High-level expression of a sweet protein, monellin, in the food yeast Candida utilis. Nat Biotechnol 15:453–457
Li C, Bai JH, Cai ZL, Fan OY (2002a) Optimization of a culture medium for bacteriocin production by Lactococcus lactis using response surface methodology. J Biotechnol 93(1):27–34
Li XD, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002b) Human receptors for sweet and umami taste. PNAS 99(7):4692–4696
Li DF, Jiang PH, Zhu DY, Hu YL, Max M, Wang DC (2008) Crystal structure of mabinlin II: a novel structural type of sweet proteins and the main structural basis for its sweetness. J Struct Biol 162(1):50–62
Liu XZ, Maeda S, Hu Z, Aiuchi T, Nakaya K, Kurihara Y (1993) Purification, complete amino acid sequence and structural characterization of the heat-stable sweet protein, mabinlin II. Eur J Biochem 211(1–2):281–287
Masuda T, Tamaki S, Kaneko R, Wada R, Fujita Y, Mehta A, Kitabatake N (2004) Cloning, expression, and characterization of recombinant sweet-protein thaumatin II using the methylotrophic yeast Pichia pastoris. Biotechnol Bioeng 85(7):761–769
Mierau I, Olieman K, Mond J, Smid EJ (2005) Optimization of the Lactococcus lactis nisin-controlled gene expression system NICE for industrial applications. Microb Cell Fact 4:16
Nirasawa S, Nishino T, Katahira M, Uesugi S, Hu Z, Kurihara Y (1994) Structures of heat-stable and unstable homologues of the sweet protein mabinlin. The difference in the heat stability is due to replacement of a single amino acid residue. Eur J Biochem 223(3):989–995
Nirasawa S, Masuda Y, Nakaya K, Kurihara Y (1996) Cloning and sequencing of a cDNA encoding a heat-stable sweet protein, mabinlin II. Gene 181(1–2):225–227
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schägger H (2006) Tricine–SDS-PAGE. Nat Protoc 1(1):16–22
Zhang XJ, Duan GC, Zhang RG (2009) Optimized expression of Helicobacter pylori ureB gene in the Lactococcus lactis nisin-controlled gene expression (NICE) system and experimental study of its immunoreactivity. Curr Microbiol 58(4):308–314
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 31060123) and the Major Technology Project of Hainan (ZDZX2013023-1).
Ethical standard
All procedures performed in studies involving animals were in accordance with the ethical standards of the Ethics Committee of the Affiliated Hospital of Hainan University (Hainan, China). All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Affiliated Hospital of Hainan University (Hainan, China) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gu, W., Xia, Q., Yao, J. et al. Recombinant expressions of sweet plant protein mabinlin II in Escherichia coli and food-grade Lactococcus lactis . World J Microbiol Biotechnol 31, 557–567 (2015). https://doi.org/10.1007/s11274-015-1809-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1809-2