Abstract
Hyaluronic acid (HA) has been industrially produced using the gram-positive bacterium Streptococcus zooepidemicus. Large amount of lactic acid formation was one of the important factors that restricted cell growth and HA productivity and lowered the substrate to HA conversion efficiency in a fermentor. In this study, polyhydroxybutyrate (PHB) synthesis genes (phbCAB) of Ralstonia eutropha were cloned from the plasmid pBHR68 and were inserted into the plasmid pEU308, an expression vector for gram-positive bacteria. The plasmid was transformed into S. zooepidemicus by electroporation. β-Ketothiolase (PhbA), acetoacetyl-CoA reductase (PhbB), and polyhydroxyalkanoate (PHA) synthase (PhbC) activity assays were carried out to demonstrate the expression of these genes. The PhbA and PhbB activities were 3.13 and 1.23 U mg−1, respectively. No PhbC activities were detected. In shake flask studies, there was no obvious difference between the wild-type and recombinant S. zooepidemicus harboring phbCAB genes in terms of lactic acid and HA formation. However, in fermentor studies, the recombinant produced only 40 g L−1 lactic acid and 7.5 g L−1 HA, whereas the wild type produced 65 g L−1 lactic acid and 5.5 g L−1 HA. These results suggested that expression of phbCAB genes in S. zooepidemicus could help regulate HA production metabolism. Because the lactic acid formation in S. zooepidemicus was sensitive to cellular oxidation/reduction potential, it is proposed that the PHB synthesis pathway could act as a regulator to adjust the cellular oxidation/reduction potential. This is the first study demonstrating that PHA synthesis related to energy and carbon metabolism could be employed as a pathway to regulate other cellular metabolism and possibly to regulate the production of other metabolic products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus equi subsp. zooepidemicus is a Lancefield group C Streptococcus and a common animal pathogen. It infects domestic and laboratory animals and has been found to cause respiratory infections in horses and, infrequently, mastitis in cows (Kester et al. 1993). In addition, its nonpathogenic mutant is widely used in industrial hyaluronic acid (HA) production (Nimrod 1988).
HA is a high molecular mass glycosaminoglycan composed of d-glucuronic acid and N-acetyl-d-glucosamine. It is present in various connective tissues of animals, such as skin and cartilage (O’Regan et al. 1994). In addition, HA is produced by various microorganisms such as streptococci types A and C. About 10% of the consumed carbon is used for the biosynthesis of HA (Holmstrom and Ricica 1967). Construction of one repeat unit needs two NAD factors, five ATP equivalents, and one acetyl-CoA group.
In fermentation processes, S. zooepidemicus produces a large amount of lactic acid, which consumes a great deal of carbon source and is harmful to the cells; yet, this is necessary for the bacterium to reduce the toxicity of pyruvate accumulation (Jeffrey and Jacky 1996). Two NADHs are oxidized to NAD when two pyruvates are converted to lactic acid by lactic acid dehydrogenase. Deletion of this enzyme is lethal to the bacterium (Chen et al. 1994). Overexpression of the NADH oxidase gene can reduce the lactic acid formation (Chong and Nielsen 2003a,b). In fermentation studies, it was found that lactic acid concentration was reduced when the dissolved oxygen (DO) was maintained at a higher level compared with that at a lower level (Gao 1999). This suggests that the lactic acid pathway plays an important role in generating cellular oxidation potential.
The synthesis of polyhydroxybutyrate (PHB) in Ralstonia eutropha has been investigated for many years. The operon for the PHB synthesis is composed of three genes, phbA, phbB, and phbC, which respectively encode the enzymes β-ketothiolase (PhbA), acetoacetyl-CoA reductase (PhbB), and PHB synthase (PhbC). NADPH is oxidized to NADP during PHB synthesis (Lee et al. 1999). This pathway could be used to provide oxidation/reduction potential for the cells, and it could possibly affect microbial metabolic products.
For the first time, we used the PHB synthesis operon to regulate lactic acid and HA production by S. zooepidemicus.
Materials and methods
Bacterial strains, plasmids, and cultivation conditions
S. zooepidemicus was cultivated in THYB [Todd–Hewitt (TH) broth (OXOID) supplemented with 0.5% yeast extract] at 37°C. Escherichia coli cells were grown in Luria–Bertani (LB) medium at 37°C. The concentration of spectinomycin was 100 μg mL−1 for S. zooepidemicus and E. coli. The concentration of isopropyl-l-thio-B-d-galactopyranoside (IPTG) was 20 mM. Plasmid pBHR68 (Spiekermann et al. 1999), containing the entire phbCAB operon of R. eutropha, and gram-positive bacterial expression vector pEU308 (Eichenbaum et al. 1998) were used in this study.
The fermentation experiments were conducted in a chemically defined medium (Gao 1999). The carbon sources for flask fermentation and fermentor studies were 40 and 70 g L−1 sucrose, respectively. Cells from the THYB plate were inoculated into a seed medium. After incubation at 37°C, they were used to inoculate the fermentor (Bioflo 3000, New Brunswick Scientific Co. Inc., NJ, USA) during the early exponential growth phase.
A 7.5-L Bioflo 3000 fermentor (New Brunswick Scientific) with an operating volume of 3 L was used. The pH in the culture was controlled at 7.0 by automatic addition of 5 mol L−1 sodium hydroxide solution. The agitation rate was 200 rpm at the beginning. When the DO was decreased to 0, the agitation rate increased to 500 rpm. When the DO was again reduced to 0, the agitation rate increased to 650 rpm and was so maintained up to the end of the fermentation. The fermentor was sparged with a continuous supply of air (5 L min−1) during cultivation.
Plasmid construction
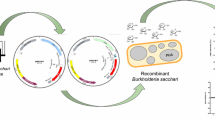
The plasmid construction is shown in Fig. 1. phbCAB genes were amplified from pBHR68 by PCR using the following primers: PphbCAB-up, ATAAAGCTT (HindIII) AAGGAGGATGGCGACCGGCAAAGGC; PphbCAB-down, GTTATCGAT (ClaI) CGGCAGGTCAGCCCATAT. The conditions for PCR amplification using Taq DNA polymerase with the primers were as follows: 96°C for 4 min, 30 cycles of 96°C for 45 s, 66°C for 1 min, and 72°C for 4.5 min, followed by 72°C for 10 min on a thermal cycler (Mastercycler gradient, Eppendorf, Germany). Plasmid pEUHB was generated by ligating the HindIII–ClaI fragment of the PCR product to plasmid pEU308 cut with HindIII and ClaI, creating a transcriptional fusion of the Spac promoter to the phbCAB gene. Plasmid pEUHBNOI was generated by self-ligation of pEUHB after digestion with SphI, which removed the lacIq repressor gene (Yansura and Henner 1984).
Competent E. coli JM109 cells were prepared according to the method described by Sambrook and Russell (2001). Competent cells were transformed with ligation mixtures and selected on LB agar plates containing spectinomycin (100 μg mL−1). The newly constructed plasmid was identified by restriction endonuclease digestion and agarose gel electrophoresis.
Electroporation of S. zooepidemicus
S. zooepidemicus cells were grown in THYB. After 14 h of incubation, cells were inoculated into 50 mL fresh THYB to less than 0.05 at OD 660 nm. The cells were further grown to about 0.25 at OD 660 nm. Approximately 30 min prior to harvest, 20 mg of hyaluronidase was added, followed by collection of the pellet cells at 8,000×g and 4°C for 10 min. The supernatant was discarded, and the pellets were washed in 20 mL of 0.5 M sucrose solution. After centrifugation, the cells were washed again in 1 mL of 0.5 M sucrose solution. Finally, the cells were resuspended in 250 μL of 10% glycerol and 0.5 M sucrose solution, followed by dividing into aliquots of useful lots. Competent cells were stored at −80°C.
About 500–5,000 ng plasmids was added into 200 μL of competent cells. The mixture was removed immediately and placed into an ice-cold cuvette. The DNA should be in a very low salt buffer. The voltage for the transformation was 1.67 kV mm−1. After application of the electric pulse, the mixture was immediately placed into 10 mL of fresh THYB and incubated on ice for 30–60 min. Then, it was incubated in 37°C water bath for 1 h. After incubation, the cells were concentrated by centrifugation at 6,000×g and 14°C for 10 min, followed by resuspension in 1 mL of THYB and plating on selective media. Colonies (colony-forming units) should be present in 24 h.
Assay of enzyme activities of β-ketothiolase, acetoacetyl-CoA reductase, and polyhydroxyalkanoates synthase
For analysis of the enzyme activity of PhbA and PhbB, cells were harvested by centrifugation after 12 h of cultivation, washed once with 0.1 mol L−1 Tris–HCl (pH 7.5), and resuspended in a 10% volume of the same buffer. Crude extract was prepared from the supernatant after cells were disrupted by sonication and centrifugation. Protein content in the crude extract was determined by the method of Bradford using Bio-Rad (Hercules, CA, USA) protein assay solution and bovine serum albumin as the standard (Bradford 1976).
The activity of PhbA was determined by a photometer assay at 25°C, calculating the decrease of absorption of acetoacetyl-CoA at 303 nm, as described by Nishimura et al. (1978). PhbB reductase activity was determined by a photometer assay at 25°C based on the decrease of absorption of NADPH at 340 nm (Senior and Dawes 1973), and PhbC activity was determined spectrophotometrically by monitoring the release of CoA at 412 nm, as described by Rehm et al. (2002).
Quantitation of hyaluronic and lactic acid
HA concentration was determined by the Bitter–Muir method (Bitter and Muri 1962). The concentration of lactic acid in the supernatant was determined by ion exchange HPLC (AS3000, SpectraSYSTEM, USA) equipped with a 7.8×300 mm HPX-87H ion exclusion column (Bio-Rad). The mobile phase consisted of 0.005 M H2SO4 pumped at a flow rate of 0.5 mL min−1. The column was maintained at 40°C. The peak elution profile was monitored with a refractive index detector (RI-150, SpectraSYSTEM).
Results
Expression of phbCAB gene in S. zooepidemicus
The plasmids harboring phbCAB genes were transformed into S. zooepidemicus by electroporation. In the preparation of competent cells, the addition of glycine dramatically increased the transformation efficiency; yet, it led to a sharp decline in HA production. Thus, glycine was omitted in the experiments. To verify the PhbA and PhbB enzyme activities, S. zooepidemicus harboring the plasmid pEUHB was incubated with 20 mM IPTG for 12 h. The enzyme activities were studied with the procedure described in “Materials and methods.” The results are shown in Table 1. The wild-type strain did not show any PhbA and PhbB activity; in comparison, the activities of these two enzymes in recombinant strain were 3.13 and 1.23 U mg−1, respectively. Interestingly, no PhbC activity was detected either in the wild-type or in the recombinant strain. As a result, no PHB accumulation in the recombinant was detected by gas chromatography.
Influence of phbCAB expression on the production of hyaluronic acid and lactic acid in shake flask cultures
S. zooepidemicus was cultured in sucrose (40 g L−1) at 37°C for 14 h. There was almost no difference between the wild-type and the recombinant strains in terms of cell dry weight and production of lactic acid and HA. These data showed that there was no remarkable influence of phbCAB expression under flask cultivation conditions (Table 2).
Influence of phbCAB expression on the production of hyaluronic acid and lactic acid in fermentor cultures
S. zooepidemicus was cultured in sucrose (70 g L−1) at 37°C for 14–16 h. The pH was controlled at approximately 7.0. The fermentation was terminated when the DO increased from zero to more than 50%. Compared with the wild-type strain, the recombinant strain took two more hours to complete the fermentation (Fig. 2). At 14 h, the wild type completed the fermentation circle, producing 65 g L−1 lactic acid and 5.4 g L−1 HA. At the same time, the lactic acid and HA produced by the recombinant strain were 38 and 5.6 g L−1, respectively. Compared with the wild-type strain, lactic acid formation was decreased by 42% under the same conditions. After 16 h, the recombinant strain completed the fermentation; it produced 41 g L−1 lactic acid, significantly less than that produced by the wild type. The final HA concentration produced by the recombinant strain was 7.3 g L−1, a 34% increase compared with that of the wild type. The cell densities of the two strains were approximately the same (Fig. 2).
Discussion
To express phbCAB in S. zooepidemicus, the gene was cloned and inserted under the Spac promoter. The activities of PhbA and PhbB in the recombinant strain were 3.13 and 1.23 U mg−1, respectively. The enzyme activities in E. coli harboring pBHR69 were 3.25 and 1.27 U mg−1, respectively (Chen et al. 2004). This indicated that PhbA and PhbB had prominent activities in the recombinant S. zooepidemicus. These results suggested that the genes had been successfully expressed in S. zooepidemicus (Table 1). NADPH can be transformed to NADP by the action of PhbB. Thus, the expression of phbAB could be sufficient to obtain the oxidation potential. Interestingly, no PhbC activity and no PHB accumulation were detected. Because phbA and phbB had been successfully transcribed and translated, phbC, the first gene under the Spac promoter, should also be immediately transcribed. To investigate the reasons for the lack of PhbC activity, phaPQRBC gene, including its own promoter from gram-positive Bacillus megaterium, was cloned and transformed into S. zooepidemicus (McCool and Cannon 1999). After coexpression of phbA from R. eutropha with phaPQRBC from B. megaterium, about 2% PHB was accumulated in E. coli, yet no PHB accumulation was observed in S. zooepidemicus. PhbA and PhbB are both intracellular proteins; however, PhbC is a membrane-bound protein. These results could suggest that S. zooepidemicus had a special mechanism for the insertion of membrane proteins. The action of PhbAB could provide oxidation potential for the cells. At the same time, acetyl-CoA was transformed to 3-hydroxybutyryl-CoA. This substance cannot be accumulated in cells. It should be consumed by certain metabolic pathways such as rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate (Rehm et al. 2001). Because the genetic background of S. zooepidemicus is not very clear, more research is needed to find out the metabolic flux of 3-hydroxybutyryl-CoA.
In shake flask cultivation, the pH could not be maintained constantly at about 7; it reached 4–5 during the stationary phase. Thus, the cell density was low (about 0.8 g L−1), and only 0.6 g L−1 HA was produced (Table 2). This low concentration of HA did not create the high viscosity and low DO level that prevent cells from growing. Under these conditions, NAD could be regenerated mainly from two pathways. The first pathway is reoxidation via the NADH oxidase (NOX) of NADH, which catalyzes the four-electron reduction of molecular oxygen to water (Ross and Claiborne 1992). The second pathway is reoxidation of NADH via lactic acid dehydrogenase, which catalyzes the reduction of pyruvate to lactic acid. The former pathway also protects the cells from the damaging effects of oxygen by reducing it to water (Stanton and Sellwood 1999). These metabolic pathways are necessary for the survival of the aerotolerant Streptococci. When phbCAB gene is introduced into S. zooepidemicus as a NAD regeneration pathway, the two pathways should be able to influence each other. Under the oxygen-rich condition, the NOX pathway oxidizes a good part of the NADH (Chong and Nielsen 2003a,b). This could compensate for the consumption of oxidation potential related to the phbCAB pathway. However, due to the rapid reduction of pH from 7 to 4, cells grew very little; thus, the cell density and lactic acid production were both low in flask cultivation. Therefore, the effect of introducing the heterogenous NAD regeneration pathway into lactic acid and HA formation was not obvious (Table 2).
In the fermentor cultivation, the lag phase of the recombinant strain was 2 h longer than that of the wild type. This phenomenon also occurred in the fermentations of the strain harboring other plasmids. It could be attributed to the existence of antibiotics. However, there was no significant difference in the exponential and stationary phases between the two strains. The recombinant strain had a 2-h delay in ending the fermentation compared with the wild type. In the fermentor studies, lactic acid production by the recombinant strain was significantly reduced compared with that of the wild type (Fig. 2), as the viscosity generated by HA was much higher in a fermentor in which cells grew much better under constant pH. Under high viscosity, cells quickly entered an oxygen limitation stage (DO=0), which was nearly an anaerobic environment. The oxygen limitation led to a reduced activity of the NOX pathway. The NAD regeneration in the cells mainly relied on the lactic acid pathway: More than 75% of carbon source used was metabolized to lactic acid (Johns et al. 1994; David and Michael 1997). Lactic acid formation is sensitive to the cellular oxidation/reduction level (Chong and Nielsen 2003a,b). Overexpressing NADH oxidase gene or increasing the DO level can decrease lactic acid production (Chong and Nielsen 2003a,b; Gao 1999). In fact, lactate dehydrogenase is sensitive to cellular NAD/NADH level (Garrigues et al. 1997). In the fermentor cultivation process, lactate formation by the recombinant strain was sharply decreased. This phenomenon provided strong evidence for the introduction of phbCAB genes that had increased the cellular NAD/NADH level. The expression of phbCAB gene in S. zooepidemicus provided a heterogenous NAD regeneration pathway for the cells, which could have a significant influence on lactic acid formation (Fig. 2a). Acting as a pathway for the generation of cellular oxidation potential, the phbCAB pathway reduced the pressure of the lactic acid pathway. At the same time, the decreased carbon flux to lactic acid allowed more carbon flux to go to other metabolic pathways; the increase of HA production was supposed to be one of the effects of the changing carbon flux (Fig. 2b).
In this study, for the first time, PHB synthesis genes (phbCAB) were used to increase the cellular oxidation potential in the HA-producing bacterium S. zooepidemicus. This recombinant pathway significantly decreased the flux to the original NADH reoxidation pathway, the lactic acid pathway of S. zooepidemicus. Compared with other NAD regeneration pathways, such as glutamate dehydrogenase, yeast alcohol dehydrogenase, lactic acid dehydrogenase, and NADH oxidase (Geueke et al. 2003), there are no harmful by-products formed. This indicates that the PHB synthesis pathway could be employed as a global regulator to adjust the cellular oxidation/reduction potential.
PHAs have been investigated as a final product, and various methods were used to regulate the PHA synthesis. This is the first study demonstrating that PHA synthesis related to energy and carbon metabolism could be employed as a pathway to regulate other cellular metabolism and possibly to regulate the production of other desired metabolic products.
References
Bitter T, Muri HM (1962) A modified uronic acid carbazol reaction. Anal Biochem 4:330–334
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Chen AP, Hillman JD, Duncan M (1994) l-(+)-Lactic acid dehydrogenase deficiency is lethal in Streptococcus mutans. J Bacteriol 176:1542–1545
Chen JY, Liu T, Zheng Z, Chen JC, Chen GQ (2004) Polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas stutzeri 1317 had different substrate specificities. FEMS Microbiol Lett 234:231–237
Chong BF, Nielsen LK (2003a) Amplifying the cellular reduction potential of Streptococcus zooepidemicus. J Biotechnol 100:33–41
Chong BF, Nielsen LK (2003b) Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase. Biochem Eng J 16:153–162
David CA, Michael RJ (1997) Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Appl Environ Microbiol 63:2759–2764
Eichenbaum Z, Federle MJ, Marra D, de Vos W, Kuipers OP, Kleerebezem M, Scott JR (1998) Use of the lactococcal nisA promoter to regulate gene expression in gram-positive bacteria: comparison of induction level and promoter strength. Appl Environ Microbiol 64:2763–2769
Gao HJ (1999) Process optimization and metabolic network analysis of hyaluronic acid synthesis by Streptococcus zooepidemicus. Dissertation, Southern Yangtze University
Garrigues C, Loubiere P, Lindley ND, Cocaign-Bousquet M (1997) Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J Bacteriol 179:5282–5287
Geueke B, Riebel B, Hummel W (2003) NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enzyme Microb Technol 32:205–211
Holmstrom B, Ricica J (1967) Production of hyaluronic acid by a streptococcal strain in batch culture. Appl Microbiol 15:1409–1413
Jeffrey D, Jacky L (1996) Genetic and physiological analysis of the lethal effect of lactic acid dehydrogenase deficiency in Streptococcus mutans: complementation by alcohol dehydrogenase from Zymomonas mobilis. Infect Immun 64:4319–4323
Johns MR, Goh LT, Oeggerli A (1994) Effect of pH, agitation and aeration on hyaluronic acid produced by Streptococcus zooepidemicus. Biotechnol Lett 15:507–512
Kester RM, Lesser S, Dowd LL (1993) Bacteria isolated from equine respiratory cultures. Equine Pract 15:33–36
Lee SY, Choi J, Wong HH (1999) Recent Advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review. Int J Biol Macromol 25:31–36
McCool GJ, Cannon MC (1999) Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592
Nimrod A (1988) Method of producing high molecular weight sodium hyallronate by fermentation of streptococcus. US Patent 4,780,414
Nishimura T, Saito T, Tomita K (1978) Purification and properties of β-ketothiolase from Zoogloea ramigera. Arch Microbiol 116:21–27
O’Regan M, Martini I, Crescenzi F, de Luca C, Lansing M (1994) Molecular mechanisms and genetics of hyaluronan biosynthesis. Int J Biol Macromol 16:283–286
Rehm BHA, Mitsky TA, Steinbüchel A (2001) Role of fatty acid de novo biosynthesis in polyhydroxyalkanoic acid (PHA) and rhamnolipid synthesis by pseudomonads: establishment of the transacylase (PhaG)-mediated pathway for PHA biosynthesis in Escherichia coli. Appl Environ Microbiol 67:3102–3109
Rehm BHA, Antonio RV, Spiekermann P, Amara AA, Steinbüchel A (2002) Molecular characterization of the poly(3-hydroxybutyrate) (PHB) synthase from Ralstonia eutropha: in vitro evolution, site-specific mutagenesis and development of a PHB synthase protein model. Biochim Biophys Acta 1594:178–190
Ross RP, Claiborne A (1992) Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J Mol Biol 227:658–671
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Senior PJ, Dawes EA (1973) The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J 134:238–255
Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbüchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other storage compounds. Arch Microbiol 171:73–80
Stanton TB, Sellwood R (1999) Cloning and characteristics of a gene encoding NADH oxidase, a major mechanism for oxygen metabolism by the anaerobic spirochete, Brachyspira (Serpulina) hyodysenteriae. Anaerobe 5:539–546
Yansura DG, Henner DJ (1984) Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A 81:439–443
Acknowledgements
We are grateful to Professor A. Steinbüchel of the University of Muenster/Germany for the kind donation of plasmid pBHR68 and to Dr. R. Scott for providing the plasmid pEU308. Thanks are also extended to Drs. L.K. Nielsen and J.R. Scott for their advice on transformation of S. zooepidemicus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Hao, N. & Chen, GQ. Effect of expressing polyhydroxybutyrate synthesis genes (phbCAB) in Streptococcus zooepidemicus on production of lactic acid and hyaluronic acid. Appl Microbiol Biotechnol 71, 222–227 (2006). https://doi.org/10.1007/s00253-005-0164-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0164-x