Abstract
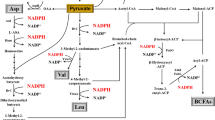
Acetolactate synthase catalyzes two molecules of pyruvates to form α-acetolactate, which is further converted to acetoin and 2,3-butanediol. In this study, by heterologous expression in Escherichia coli, the enzymatic properties of acetolactate synthase (AlsS) from Bacillus licheniformis WX-02 were characterized. Its K m and k cat for pyruvate were 3.96 mM and 514/s, respectively. It has the optimal activity at pH 6.5, 37 °C and was feedback inhibited by l-valine, l-leucine and l-isoleucine. Furthermore, the alsS-deficient strain could not produce acetoin, 2,3-butanediol, and l-valine, while the complementary strain was able to restore these capacities. The alsS overexpressing strain produced higher amounts of acetoin/2,3-butanediol (57.06 g/L) and l-valine (2.68 mM), which were 10.90 and 92.80% higher than those of the control strain, respectively. This is the first report regarding the in-depth understanding of AlsS enzymatic properties and its functions in B. licheniformis, and overexpression of AlsS can effectively improve acetoin/2,3-butanediol and l-valine production in B. licheniformis. We envision that this AlsS can also be applied in the improvement of acetoin/2,3-butanediol and l-valine production in other microbes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetoin and 2,3-butanediol (2,3-BD) are important platform compounds, and their derivative compounds are also promising valuable chemicals because of their extensive applications [1,2,3,4,5]. Branched-chain amino acids (l-valine, l-leucine, and l-isoleucine) are used in dietary products, pharmaceuticals, cosmetics, animal feed additives, or as precursors of antibiotics or herbicides [6,7,8]. Acetolactate is the direct substrate for acetoin/2,3-BD biosynthesis, and the precursor of l-valine and l-leucine, while α-acetohydroxybutyrate is the precursor of l-isoleucine [9]. There are two kinds of acetolactate synthase for synthesizing acetolactate: anabolic acetohydroxyacid synthase (AHAS) and catabolic acetolactate synthase (cALS) [9]. Both enzymes can convert two molecules of pyruvates to one acetolactate, and AHAS can also catalyze the condensation of pyruvate and α-ketobutyrate to form α-acetohydroxybutyrate. Thus, the anabolic AHAS is responsible for biosynthesis of the branched-chain amino acids, while cALS is responsible for production of acetoin/2,3-BD [10, 11].
Generally, both anabolic AHAS and cALS require thiamine diphosphate (ThDP) and a divalent metal ion (Mg2+) for catalytic activity [11]. The AHAS contains flavin adenine dinucleotide (FAD), and is inhibited by the branched-chain amino acids [12,13,14,15]. Compared to the anabolic AHAS, the cALS with a lower optimal pH at about 6.0 is FAD independent and lacks a regulatory subunit [16,17,18,19,20]. Considering that cALS exhibits higher affinity to pyruvate [1], cALS has attracted great attention and is served as a potential target of genetic engineering for bioproduct production, such as acetoin/2,3-BD and other high-value products. Recently, several recombinant strains have been developed via altering the expression levels of alsS. For example, the AlsS from Bacillus subtilis was overexpressed to improve the 2,3-BD production in Saccharomyces cerevisiae [21], and the ethanol titer was significantly improved by the koncking-out of alsS in Pyrococcus furiosus [22].
Bacillus licheniformis is a generally regarded as safe (GRAS) strain, which is capable of producing 2,3-BD at high yield [23], and its recombinant strain shows high capacity to produce l-valine [7]. However, the acetolactate synthase (AlsS) of B. licheniformis has not been characterized and whether it is involved in l-valine formation was unclear. The objective of this work was to investigate the enzymatic properties of acetolactate synthase AlsS in B. licheniformis WX-02 as well as its functions in the biosynthesis of acetoin/2,3-BD and l-valine. An in-depth understanding of AlsS characteristics and functions in B. licheniformis will help the re-direction of carbon flux distribution to improve the production of acetoin/2,3-BD and l-valine by enhancing the biosynthetic capacity of intercellular acetolactate.
Materials and methods
Bacterial strains, plasmids, and culture conditions
The strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5α and BL21 (DE3) were used for the cloning procedures and protein purification, respectively. The wild-type WX-02 (CCTCC M208065) was used as the host strain [24]. The plasmid pET-28a (+) was used for the induced expression, the plasmid pT49 was applied for construction of expression vector in B. licheniformis, and the T2 (2)-ori vector was used for gene knock-out.
Bacillus licheniformis seeds were incubated at 37 °C, 180 rpm for 8 h in 250-mL flasks containing 50 mL LB medium. The medium for acetoin and 2,3-BD production was described previously [25], consisting of (per liter): 120.00 g glucose, 33.00 g corn steep liquor, 9.00 g (NH4)2SO4, 1.50 g MgSO4·7H2O, 1.00 g K2HPO4·3H2O, 0.50 g NaCl, 0.12 g ZnCl2, 1.00 mg MnSO4·H2O, 1.00 mg FeCl3·6H2O, pH 7.0. The seed culture was inoculated 1% (v/v) into 250-mL flasks containing 30 mL acetoin/2,3-BD production medium, and then cultivated at 37 °C, 180 rpm for 48 h. The l-valine production medium was prepared according to the previous research [7], consisting of (per liter): 100.00 g glucose, 2.00 g corn steep liquor, 15.00 g (NH4)2SO4, 1.00 g KH2PO4·3H2O, 0.50 g MgSO4∙7H2O, 3.00 g CaCO3, pH 7.0. The seed culture 2% (v/v) was inoculated into a 250-mL flask containing 30 mL l-valine production medium, and then cultivated at 37 °C, 180 rpm for 84 h.
Expression and purification of AlsS protein
The alsS encoding acetolactate synthase is located in the butanediol operon, and the gene was amplified from B. licheniformis WX-02 genomic DNA using the primers alsS-F/R (Table 2). Then, the DNA fragment was sub-cloned into pET-28a (+), confirmed by PCR verification and Sanger sequencing. The recombinant plasmid was further transferred into E. coli BL21 (DE3) to construct the recombinant strain, named as E. coli BL21 (DE3)/pETalsS.
The E. coli BL21 (DE3)/pETalsS was cultivated in the 50 mL LB medium containing 20 μg/mL kanamycin at 37 °C. Cells were induced by 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) until the OD600nm value reached 1.0, and further cultivated for 6 h. Then, the culture was centrifuged at 8000 rpm, 4 °C for 10 min to collect the cell pellets. After washed twice with lysis buffer (50 mM Tris, 500 mM NaCl, 10% glycerol, 50 mM sodium dihydrogen phosphate, 20 mM imidazole, 5 mM β-Mercaptoethanol, pH 8.0), cell pellets were resuspended with 50 mL lysis buffer, and disrupted by high pressure. After centrifugation at 12,000 rpm for 30 min, the supernatant was collected for purification of AlsS using the Ni–NTA column [26]. The eluted AlsS was dialyzed in 500 mL buffer containing 20 mM Tris–HCl (pH 8.0) containing 10% glycerol, 1 mM EDTA (pH 8.0), 0.1 mM DTT and 0.002% Tritonx-100 at 4 °C for 24 h. The purified AlsS was analyzed by SDS-PAGE and quantified by Bradford protein kit according to the manual [27].
Determination of enzymatic properties of AlsS
The enzymatic activity of AlsS was determined according to the previously reported method [28]. Briefly, mixtures of 20 μL distilled water, 60 μL reaction buffer (75 mM potassium phosphate buffer containing 15 mM MgCl2 and 5 mM ThDP, pH 7.5) and 5 μL AlsS were added into 96-well microplates, followed by incubation at 37 °C for 10 min. After the addition of 5 μL 0.6286 M sodium pyruvate, the mixture was incubated at 37 °C for 30 min. The enzymatic reaction was terminated by adding 10 μL 3 M H2SO4 and incubated at 60 °C for 20 min for the decarboxylation of acetolactate. Next, the volume of 100 μL 5% (w/v) α-napthtol solution and 100 μL 0.5% creatine (w/v) was added, respectively, followed by incubating at 37 °C for 20 min. The resulting sample was measured at 525 nm using a microplate reader (Bio-Rad, USA). One unit of AlsS activity is defined as producing 1 μmol acetolactate in 1 min.
The optimum temperature and pH of the AlsS were determined by measuring the enzyme activity as described above. The reaction was carried out at temperature from 4 to 80 °C. The optimum pH was measured using reaction buffer with different pH from 2.0 to 10.0.
The kinetic parameters of AlsS were determined by measuring the enzyme activity under the condition of different substrate concentrations. The values of K m and V max for the substrate were acquired according to the Michaelis–Menten equation.
The effect of branched-chain amino acids on the AlsS activity was also analyzed by the standard assay method with different concentrations of l-valine, l-leucine, and l-isoleucine at 37 °C. The inhibition constants of three amino acids were calculated, respectively.
Construction of alsS-deficient strain in B. licheniformis
The method of markerless gene deletion used in this study has been previously described by Qiu et al. [29]. Briefly, the fragments for deleting alsS were amplified from the WX-02 genomic DNA using the primers alsS-AF/AR and alsS-BF/BR, respectively (Table 2). The two fragments were fused by splice overlap extension PCR (SOE-PCR) using the primers alsS-AF/BR, and the fused fragment was inserted into T2 (2)-ori at BamHI and SacI restriction sites, generating the plasmid T2ΔalsS. The plasmid T2ΔalsS was transformed into B. licheniformis WX-02 via electroporation [30]. The positive clone was incubated in LB medium containing 20 μg/mL kanamycin 45 °C for 8 h, and then streaked on LB agar plates with kanamycin for another 8 h to obtain the single-crossover recombinants. The single-cross clones were grown in LB medium at 37 °C with serial subcultures to promote homologous recombination, The kanamycin sensitive colonies meaning a double crossover event were verified by PCR using the corresponding primers (Table 2) and the gene-deleted clones were further verified by DNA- sequencing. The mutant strain was designated as WX-02ΔalsS.
Construction of alsS overexpressing strain and complementary strain in B. licheniformis
The alsRS fragment containing alsS, its promoter, and the positive regulatory gene of alsR [31] was amplified from WX-02 genome using primers alsS-OF/OR (Table 2). Then, the fragment was incorporated into pT49 vector at XhoI and NotI restriction sites. The recombinant plasmid was further verified by DNA sequence and designated as pT49alsRS.
The plasmid pT49alsRS was transformed into B. licheniformis WX-02 and WX-02ΔalsS by electroporation, respectively, and the transformants were selected by kanamycin (20 μg/mL), followed by PCR verification with the primers pT49-YF/YR (Table 2). In this study, the alsS overexpressing and complementary strains were designated as WX-02/pT49alsRS and WX-02ΔalsS/pT49alsRS, respectively. The recombinant strains WX-02/pT49 and WX-02ΔalsS/pT49 were served as the control strains in this research.
Analytical procedures
Cells were harvested from the fermentation broth via centrifugation at 12,000 rpm for 5 min. The separated cells were washed twice with deionized water and resuspended for determination of the cell density at 600 nm (OD600nm) with a spectrophotometer (Bio-Rad, USA). Residual glucose was measured enzymatically by a Bio-analyzer (SBA 40C, Shandong Academy of Sciences, China). The concentrations of acetoin and 2,3-BD in the supernatant were analyzed by gas chromatography [23]. l-valine was determined by gas chromatography after derivatization process with ethyl chlorocarbonate [7].
Statistics analysis
All experiments were performed in triplicate, and the data were presented as the mean ± the standard deviation for each sample point. All data were conducted to analyze the variance at P < 0.05, and an approximate Duncan’s multiple range test was applied to compare the mean values.
Results
Cloning, expression, and purification of AlsS
The 1719 bp alsS fragment was cloned into pET-28a (+) vector to form the recombinant plasmid pETalsS, which was then transformed into E. coli BL21 (DE3). The induced protein showed the size at 63 kDa on SDS-PAGE gel (lane 3) (Fig. 1a), which was consistent with the molecular weight of the AlsS of B. licheniformis WX-02, indicating that the AlsS was expressed successfully. After purification by Ni-NAT, the total intracellular proteins showed a single band of purified AlsS on the SDS-PAGE gel (Fig. 1b), and the concentration of the purified AlsS was 30.71 mg/L.
SDS-PAGE analysis of induced expression (a) and purification (b) of the AlsS in E. coli. a Induced expression of AlsS in E. coli. Lane M1: Protein Marker (116, 66.2, 45, 35, 25, 18.4 and 14.4 KDa); Lane 1: The total intracellular proteins of E. coli BL21 (DE3)/pET-28a(+) induced by IPTG; Lane 2: The total intracellular proteins of E. coli BL21 (DE3)/pETalsS non-induced by IPTG; Lane 3: The total intracellular proteins of E. coli BL21 (DE3)/pETalsS induced by IPTG. b SDS-PAGE analysis of the purified AlsS. Lane M2: Protein markers (200, 150, 120, 100, 85, 70, 60, 50, 40, 30, 25, 20, 15 and 10 kDa); Lane 1: Purified AlsS
AlsS enzymatic properties and feedback inhibitory effects of branched-chain amino acids
To obtain the optimum temperature and pH for the AlsS, the enzyme activity was measured under different temperatures (4–80 °C) and pH (2.0–10.0). Based on our results, the AlsS exhibited considerable activity in the range of pH 6.0–7.5, with a maximum activity at pH 6.5 under different pH buffer conditions measured at 37 °C (Fig. 2a). And the apparent optimum temperature for AlsS activity was 37 °C measured at pH 6.5, while the activity above 50 °C was less than 50% and then completely decreased at higher temperatures (Fig. 2b).
Effects of different temperatures and pH on the catalytic activity of theAlsS. a pH. b Temperature. The optimum pH value was measured at 37 °C using the reaction buffer with different pH. The optimum temperature was determined at different temperatures under the condition of pH 6.5. Data were expressed as mean ± standard errors of three replicates
A discontinuous colorimetric analysis was used to determine the kinetic parameters of the AlsS. The kinetic parameters of the AlsS were determined at different concentrations of pyruvate under the optimal pH and temperature. As shown in Fig. 3, the K m and V max values for pyruvate were calculated at 3.96 mM and 0.75 mM/min by Lineweaver–Burk plot, respectively.
Lineweaver–Burk plot of the AlsS. The enzymatic reaction was carried out under the optimum conditions (temperature 37 °C, pH 6.5) to measure the reaction rate of pyruvate at the different concentrations. The K m and V max value for pyruvate were calculated according to the formula: 1/ν = (K m/V max)(1/[S]) + 1/V max. Data represented the average of more than three independent measurements
The feedback inhibitory effects of branched-chain amino acids (l-valine, l-leucine, and l-isoleucine) on the AlsS activity were further measured. The inhibition constants (K i) of l-valine, l-leucine, and l-isoleucine were 2.73 × 10−3, 1.19 × 10−2, and 0.19 mM, respectively. These results implied that the AlsS, participating in production of acetoin and 2,3-BD, was also feedback inhibited by the three branched-chain amino acids, and l-valine displayed the strongest inhibitory effect on the AlsS activity.
Effects of alsS deletion on acetoin/2,3-BD and l-valine biosynthesis
To further investigate the role of the AlsS on biosynthesis of acetoin/2,3-BD and l-valine, the alsS-deficient strain WX-02ΔalsS was constructed. The wild-type strain WX-02 produced 51.61 g/L acetoin/2,3-BD and 1.53 mM l-valine in the corresponding medium, respectively, while WX-02ΔalsS could not produce any of those products under same conditions (Fig. 4a, c). Meanwhile, the complementary strain WX-02ΔalsS/pT49alsRS successfully restored the biosynthesis capacity, which could produce 9.54 g/L acetoin/2,3-BD and 0.93 mM l-valine, respectively (Fig. 4a, c). Also, the deletion of alsS resulted in a poor cell growth, which could be partially recovered by the complementation of alsS (Fig. 4b, d). Previous research has reported that acetoin and 2,3-BD biosynthesis pathways can prevent acidification through conversion of excess pyruvate into neutral compounds (acetoin and 2,3-BD) [32]. Our results indicated that the deletion of alsS could eliminate the biosynthesis of acetoin/2,3-BD and l-valine.
Effects of the alsS deficient on the acetoin/2,3-BD and l-valine metabolism in B. licheniformis. a Time course of the acetoin (solid symbols) and 2,3-BD (open symbols) produced by the alsS deficient strain (WX-02ΔalsS) (circles), the complementary strain (WX-02ΔalsS/PT49alsRS) (triangles), WX-02ΔalsS/PT49 (inverted triangles), and wild-type strain (WX-02) (squares), circles overlaps with inverted triangles. c Time course of l-valine produced by the alsS deficient strain (WX-02ΔalsS) (black circles), the complementary strain (WX-02ΔalsS/PT49alsRS) (black triangles), WX-02ΔalsS/PT49 (black inverted triangles), and wild-type strain (WX-02) (black squares), black triangles overlaps with white circles. b, d Time course of the cell growth in the alsS deficient strain (WX-02ΔalsS) (black circles), the complementary strain (WX-02ΔalsS/PT49alsRS) (black triangles), WX-02ΔalsS/PT49 (black inverted triangles), and wild-type strain (WX-02) (black squares) in the acetoin/2,3-BD and l-valine production medium, respectively. Data were expressed as mean ± standard errors of three replicates
Effects of alsS overexpression on acetoin/2,3-BD and l-valine production
Since the AlsS was proven to be essential in the biosynthesis of acetoin/2,3-BD and l-valine, the recombinant strain WX-02/pT49alsRS was constructed to enhance the acetolactate biosynthesis pathway, which might further lead to increase of acetoin/2,3-BD and l-valine yields.
The metabolite profiles were determined to evaluate the performance of WX-02/pT49alsRS on the production of acetoin/2,3-BD and l-valine. The output of acetoin has no significant difference between WX-02/pT49alsRS and the control strain at the first 16 h of the fermentation, but the acetoin titer of WX-02/pT49alsRS was higher after 16 h (Fig. 5a). The titer of 2,3-BD increased rapidly until 32 h, then decreased gradually in both WX-02/pT49alsRS and WX-02/pT49, and the output of 2,3-BD produced by WX-02/pT49alsRS was higher during the whole fermentation (Fig. 5a). The combined production of acetoin/2,3-BD produced by WX-02/pT49alsRS reached the highest titer at 57.06 g/L, which was 10.9% higher than that of WX-02/pT49 (Fig. 5a). Also, as shown in Fig. 5c, WX-02/pT49alsRS produced more l-valine during the fermentation process between 24 and 72 h, and the maximum titer of l-valine produced by WX-02/pT49alsRS reached 2.68 mM, increased by 92.8% compared with 1.39 mM l-valine produced by WX-02/pT49. However, the cell growth of WX-02/pT49alsRS was slightly impaired during the fermentation process of acetoin/2,3-BD and l-valine production (Fig. 5b, d). These results implied that overexpression of the AlsS could enhance the titers of acetoin/2,3-BD and l-valine in B. licheniformis.
Comparison of metabolite profiles among the different strains. a Acetoin yield (solid symbols)/2,3-BD (open symbols). b Cell growth in the acetoin/2,3-BD production medium. c l-valine. d Cell growth in l-valine production medium. Symbols:squares, WX-02; circles, WX-02/pT49; triangles, WX-02/pT49alsRS. Data were expressed as mean ± standard errors of three replicates
Discussion
Due to the important roles of acetolactate in microorganisms, acetolactate synthase (ALS), which condenses two molecules of pyruvate into acetolactate, is of great interests for researchers. Two different types of ALSs have been reported in microorganisms, anabolic acetohydroxyacid synthase (AHAS) and catabolic acetolactate synthase (cALS) [9, 10]. The AHAS is involved in the biosynthesis of branched-chain amino acids, and overexpression of AHAS could improve the yields of l-valine in E. coli and Corynebacterium glutamicum [6, 13, 33,34,35]. The cALS is a catabolic enzyme required for 2,3-BD fermentation, which has been employed for high-level production of acetoin/2,3-BD or diacetyl in Candida glabrata, B. subtilis, and S. cerevisiae [4, 21, 36].
In this study, the amino acid sequence of the AlsS of B. licheniformis WX-02 was compared with the previously characterized cALSs (Enterococcus faecalis, Lactococcus lactis, B. subtilis, Klebsiella pneumonia, Enterococcus aerogenes, Serratia marcescens) and AHASs (E. coli I, E. coli II, E. coli III, B. subtilis, Bacillus anthracis and S. cerevisiae) of other species. The sequence alignment showed that the AlsS of B. licheniformis WX-02 has shared about 52–74% sequence identity with cALSs and about 27–30% with AHASs from other species. The results suggest that AlsS of B. licheniformis is a catabolic acetolactate synthase. The enzymatic properties of the AlsS of WX-02 were firstly characterized. The specific activity of the AlsS of WX-02 was 35.4 U/mg, which was higher than those from other microorganisms (Table 3), indicating that the AlsS of WX-02 is more active than that in other microorganisms. The K m of AlsS was 3.96 mM with pyruvate, which apparently low, compared to 13.6 mM in B. subtilis [37], 70 mM in L. lactis (70 mM) [18] and 8 mM in K. pneumonia [20], except 1.37 mM in E. faecalis. Meanwhile, its k cat for pyruvate was 514/s, which was higher than those strains mentioned above (Table 3). The K m and k cat showed the important properties among all the enzyme kinetic parameters, and they were usually evaluated as parameters for donor gene selection for metabolic engineering [1]. The values of K m and k cat showed that the AlsS could be act as a candidate to improve the acetoin/2,3-BD production.
The previously reported catabolic ALSs were active only at pH 6.0 and anabolic ones at pH 8.0 [12, 14, 16, 19, 20]. The AlsS of B. licheniformis WX-02 was optimally active at pH 6.5, which could function well in a broad pH range (pH 6.0–7.5) (Fig. 2a). Generally, the anabolic AHAS was subjected to feedback control by branched-chain amino acids, while cALS was not [9]. In this study, l-valine, l-leucine, and l-isoleucine were found to be inhibitory to the AlsS of WX-02, and our results were consistent with the previous report in E. cloacae [38]. While, were different from other class reported that was not inhibited by the branched-chain amino acids [19]. These results indicated that the AlsS of WX-02 showed the properties of both the catabolic and anabolic enzymes. The regulatory mechanism of AlsS responsible for biosynthesis of branched-chain amino acids will be further investigated.
The functions of the AlsS on biosynthesis of the acetoin/2,3-BD and l-valine in B. licheniformis were further confirmed. Based on our results, the deficient strain WX-02ΔalsS could not produce acetoin/2,3-BD and l-valine, and the complementary strain WX-02ΔalsS/pT49alsRS partially restored its capability for the production of acetoin/2, 3-BD and l-valine. Although the complementary strain could express the alsS, the expression level or activity of alss in the complementary strain might be lower than those of wild-type strain, resulting in partially recovering the capacity. The insufficient AlsS also led to partially recovered the cell growth (Fig. 4b, d). We speculate that the activity of AlsS in cells can affect the acetoin/2,3-BD and l-valine production. Furthermore, the alsS overexpressing strain WX-02/pT49alsRS produced the highest titers of acetoin/2,3-BD and l-valine, which were 10.9 and 92.8% higher than those of the control strain WX-02/pT49, respectively. These results were consistent with previous reports in other strains such as B. subtilis and S. cerevisiae [21, 39]. The ability to accumulate acetolactate was observed in this study due to the increasing AlsS activity in the alsS overexpressing strain WX-02/pT49alsRS, and the accumulation of acetolactate led to the enhanced production of l-valine, acetoin, and 2,3-BD.
Generally, AHAS is involved in the biosynthesis of branched-chain amino acids, and it has rarely been reported for the impact of cALS on branched-chain amino acids biosynthesis. The activity of AlsS was inhibited by the branched-chain amino acids, especially l-valine, and the expression of AlsS might be positive correlation with the production of l-valine. These results indicate that AlsS is responsible for biosynthesis of l-valine. Taken together, we speculate that there is a synergy function between AHAS and cALS for branched-chain amino acids biosynthesis in B. licheniformis. In addition, this study provided a novel approach to enhance the l-valine production by enhancing the accumulation of intercellular acetolactate via AlsS overexpression.
Conclusions
In this study, the AlsS of B. licheniformis was proven to be an efficient acetolactate synthase with the properties of catabolic and anabolic acetolactate synthase, and the AlsS was proven to be involved in the biosynthesis of acetoin/2,3-BD and l-valine in vivo. Therefore, the AlsS could be used as a powerful genetic target for highly efficient production of value-added bioproducts in B. licheniformis WX-02. We have also proposed that overexpression of the AlsS could be an effective strategy to improve the production of acetoin/2,3-butanediol and l-valine in other microorganisms.
References
Xiao Z, Lu JR (2014) Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv 32:492–503
Zhang L, Liu Q, Ge Y, Li L, Gao C, Xu P, Ma C (2016) Biotechnological production of acetoin, a bio-based platform chemical, from a lignocellulosic resource by metabolically engineered Enterobacter cloacae. Green Chem 18:1560–1570
Ge Y, Li K, Li L, Gao C, Zhang L, Ma C, Xu P (2016) Contracted but effective: production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem 18:4693–4703
Kim SJ, Seo SO, Jin YS, Seo JH (2013) Production of 2,3-butanediol by engineered Saccharomyces cerevisiae. Bioresour Technol 146:274–281
Lu M, Park C, Lee S, Kim B, Oh M-K, Um Y, Kim J, Lee J (2014) The regulation of 2,3-butanediol synthesis in Klebsiella pneumoniae as revealed by gene over-expressions and metabolic flux analysis. Bioprocess Biosyst Eng 37:343–353
Blombach B, Schreiner ME, Bartek T, Oldiges M, Eikmanns BJ (2008) Corynebacterium glutamicum tailored for high-yield l-valine production. Appl Microbiol Biotechnol 79:471–479
Liang C, Huo Y, Qi G, Wei X, Wang Q, Chen S (2015) Enhancement of l-valine production in Bacillus licheniformis by blocking three branched pathways. Biotechnol Lett 37:1243–1248
Bartek T, Rudolf C, Kerßen U, Klein B, Blombach B, Lang S, Eikmanns BJ, Oldiges M (2010) Studies on substrate utilisation in l-valine-producing Corynebacterium glutamicum strains deficient in pyruvate dehydrogenase complex. Bioprocess Biosyst Eng 33:873–883
Lee S-C, Kim J, La I-J, Kim S-K, Yoon M-Y (2013) Characterization of recombinant FAD-independent catabolic acetolactate synthase from Enterococcus faecalis V583. Enzyme Microb Technol 52:54–59
Renna MC, Najimudin N, Winik LR, Zahler SA (1993) Regulation of the Bacillus subtilisalsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol 175:3863–3875
Lee S-C, Jung I-P, Baig I-A, Chien P-N, La I-J, Yoon M-Y (2015) Mutational analysis of critical residues of FAD-independent catabolic acetolactate synthase from Enterococcus faecalis V583. Int J Biol Macromol 72:104–109
Pang SS, Duggleby RG, Guddat LW (2002) Crystal structure of yeast acetohydroxyacid synthase: a target for herbicidal inhibitors. J Mol Biol 317:249–262
Park JH, Jang YS, Lee JW, Lee SY (2011) Escherichia coli W as a new platform strain for the enhanced production of l-valine by systems metabolic engineering. Biotechnol Bioeng 108:1140–1147
Chang AK, Duggleby RG (1997) Expression, purification and characterization of Arabidopsis thaliana acetohydroxyacid synthase. Biochem J 327:161–169
Duggleby RG, Pang SS (2000) Acetohydroxyacid synthase. BMB Rep 33:1–36
Holtzclaw WD, Chapman LF (1975) Degradative acetolactate synthase of Bacillus subtilis: purification and properties. J Bacteriol 121:917–922
Kisrieva IuS, Serebrennikov VM, Zagustina NA, Bezborodov AM (2000) Isolation and purification of acetolactate synthase and acetolactate decarboxylase from a Lactococcus lactis culture. Prikl Biokhim Mikrobiol 36:131–137
Snoep JL, De Mattos MT, Starrenburg MJ, Hugenholtz J (1992) Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and alpha-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol 174:4838–4841
Phalip V, Schmitt P, Diviès C (1995) Purification and characterization of the catabolic α-acetolactate synthase from Leuconostoc mesenteroides subsp. cremoris. Curr Microbiol 31:316–321
Peng HL, Wang PY, Wu CM, Hwang DC, Chang HY (1992) Cloning, sequencing and heterologous expression of a Klebsiella pneumoniae gene encoding an FAD-independent acetolactate synthase. Gene 117:125–130
Ng CY, Jung MY, Lee J, Oh MK (2012) Production of 2,3-butanediol in Saccharomyces cerevisiae by in silico aided metabolic engineering. Microb Cell Fact 11:68
Nguyen DM, Lipscomb GL, Schut GJ, Vaccaro BJ, Basen M, Kelly RM, Adams MW (2016) Temperature-dependent acetoin production by Pyrococcus furiosus is catalyzed by a biosynthetic acetolactate synthase and its deletion improves ethanol production. Metab Eng 34:71–79
Qi G, Kang Y, Li L, Xiao A, Zhang S, Wen Z, Xu D, Chen S (2014) Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnol Biofuels 7:16
Wei X, Ji Z, Chen S (2010) Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-gamma-glutamic acid. Appl Biochem Biotechnol 160:1332–1340
Liu Y, Zhang S, Yong Y-C, Ji Z, Ma X, Xu Z, Chen S (2011) Efficient production of acetoin by the newly isolated Bacillus licheniformis strain MEL09. Process Biochem 46:390–394
Tian G, Fu J, Wei X, Ji Z, Ma X, Qi G, Chen S (2014) Enhanced expression of pgdS gene for high production of poly-γ-glutamic aicd with lower molecular weight in Bacillus licheniformis WX-02. J Chem Technol Biotechnol 89:1825–1832
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Singh BK, Stidham MA, Shaner DL (1988) Assay of acetohydroxyacid synthase. Anal Biochem 171:173–179
Qiu Y, Zhang J, Li L, Wen Z, Nomura CT, Wu S, Chen S (2016) Engineering Bacillus licheniformis for the production of meso-2,3-butanediol. Biotechnol Biofuels 9:117
Xue GP, Johnson JS, Dalrymple BP (1999) High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J Microbiol Methods 34:183–191
Fradrich C, March A, Fiege K, Hartmann A, Jahn D, Hartig E (2012) The transcription factor AlsR binds and regulates the promoter of the alsSD operon responsible for acetoin formation in Bacillus subtilis. J Bacteriol 194:1100–1112
Tsau JL, Guffanti AA, Montville TJ (1992) Conversion of pyruvate to acetoin helps to maintain pH homeostasis in Lactobacillus plantarum. Appl Environ Microbiol 58:891–894
Guo Y, Han M, Xu J, Zhang W (2015) Analysis of acetohydroxyacid synthase variants from branched-chain amino acids-producing strains and their effects on the synthesis of branched-chain amino acids in Corynebacterium glutamicum. Protein Expr Purif 109:106–112
Radmacher E, Vaitsikova A, Burger U, Krumbach K, Sahm H, Eggeling L (2002) Linking central metabolism with increased pathway flux: l-valine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68:2246–2250
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA 104:7797–7802
Gao X, Xu N, Li S, Liu L (2014) Metabolic engineering of Candida glabrata for diacetyl production. PLoS One 9:e89854
Atsumi S, Li Z, Liao JC (2009) Acetolactate synthase from Bacillus subtilis serves as a 2-ketoisovalerate decarboxylase for isobutanol biosynthesis in Escherichia coli. Appl Environ Microbiol 75:6306–6311
Kaushal A, Pabbi S, Sharma P (2003) Characterization of 2,3-butanediol-forming and valine-sensitive α-acetolactate synthase of Enterobacter cloacae. World J Microbiol Biotechnol 19:487–493
Zhu Y, Chen X, Chen T, Zhao X (2007) Enhancement of riboflavin production by overexpression of acetolactate synthase in a pta mutant of Bacillus subtilis. FEMS Microbiol Lett 266:224–230
Acknowledgements
This work was supported by National Program on Key Basic Research Project (973 Program, No. 2015CB150505), the Science and Technology Program of Wuhan (20160201010086), rural areas of the national science and technology plan in the 12th five-year plan of China (No. 2013AA102801-52).
Author information
Authors and Affiliations
Contributions
SC designed and supervised the study. YH and YZ performed the experiments. YH, YZ, QW, SL, SY, CN, CW and SC analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Huo, Y., Zhan, Y., Wang, Q. et al. Acetolactate synthase (AlsS) in Bacillus licheniformis WX-02: enzymatic properties and efficient functions for acetoin/butanediol and l-valine biosynthesis. Bioprocess Biosyst Eng 41, 87–96 (2018). https://doi.org/10.1007/s00449-017-1847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1847-2