Abstract
Yellow serradella (Ornithopus compressus), a valuable pasture species in Mediterranean areas, presents a high diversity of endophytic mycoflora. In the present work, the hypothesis of a significant effect of fungal endophytic species on the parameters of forage production, nutritive value and mineral status of herbage was tested. O. compressus plants were inoculated with each of seven endophytes (four in 2012/2013 and three in 2013/2014). After inoculation, two experiments (under greenhouse and field conditions) were established. Results evidenced a certain influence of several endophytes on herbage yield, nutritive value and mineral status of O. compressus forage. Byssochlamys spectabilis increased herbage biomass yield by around 42% in the field experiment. Stemphylium sp. improved the nutritive value of forage either by increasing crude protein, digestibility and the concentration of essential minerals (such as B, Mo, P or S) or by reducing the concentration of toxic elements such as Al or Pb. In conclusion, the results presented here provide evidence that plant inoculation with endophytes could be a suitable strategy to increase forage yield and its nutritive value or to deal with potential nutrient deficiencies or potential mineral toxicity in forage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Yellow serradella (Ornithopus compressus L.) is an annual pasture species of Mediterranean origin. It is endemic to grasslands in southwestern Spain due to its adaptation to the Mediterranean climate and tolerance of sandy and acidic soils containing low levels of organic matter. The legume O. compressus has been widely used for recovering areas with degraded soils, as well as for amending soil structure and fertility [29]. Although extremely variable due to the inter-annual irregularity typical of the Mediterranean climate, herbage yield ranges between 2200 and 6000 kg dry matter (DM) ha−1 [11, 28]. This species produces forage with an excellent nutritive value, high in crude protein (CP) concentration and digestibility. Values reported at full bloom stage are CP of 217–290 g kg−1 DM, acid detergent fibre (ADF) of 188–231 g kg−1 DM and levels of metabolizable energy (ME) of 11.2–12.0 MJ kg−1 DM [11]. For this reason, O. compressus is commonly used for animal feeding. It has been used as a sown fodder crop in its native European area, but it has also been introduced to other Mediterranean climate areas, mainly Chile [10] and Australia [5, 23]. Its use is mostly seen in Australia where it is cultivated alone or mixed with pink or French serradella (O. sativus Brot.), subterranean clover (Trifolium subterraneum L.), and/or other clover and Biserrula species [2, 12].
Forage nutritive value, mineral content and forage yield of pasture species can be affected by soil, climate, plant phenology and diseases [25, 34]. Fungal endophytes, organisms which inhabit plant tissues without causing any disease symptoms, have also been shown to affect some of the productive parameters in other pasture species, such as Festuca rubra L. [37]. These endophytic fungi frequently have beneficial effects on the host plants, increasing their adaptive value, especially under stress conditions, and protecting them against different plant pathogens [8, 19, 31, 36]. These positive effects caused by endophytes have shown to bring about increments in biomass production and/or in regrowth of the plant host, as has previously been observed in endophyte-infected plants by using different endophyte-host combinations, such as Neotyphodium spp.-Lolium perenne L. or Chaetosphaeronema sp.-Trifolium subterraneum L. [13, 16].
Similarly, several endophytes, such as Epichloë festucae Leuchtm., Schardl and Siegel, have been found to increase the nutritive value of forage, for instance by increasing the organic matter digestibility in different hosts such as F. rubra [37]. Moreover, the uptake capacity of host plants and later accumulation of minerals in forage can be modified by fungal endophytes [15]. In some cases, the fungus was able to increase uptake of some minerals such as P, Ca or Mg [37]. However, the literature reports also the opposite effect, i.e. a decrease in uptake of other minerals such as Al [18] or Zn [16].
Nevertheless, the effects caused by fungal endophytes on their hosts appear to be variable and clearly dependent on species of endophyte, host genotype and environmental conditions [1]. Therefore, if the final objective is improvement of the productive parameters of forage crops by using fungal endophytes, each endophyte-host system should be specifically studied for each environmental condition. To date, most studies dealing with effects of fungal endophytes on plant biomass productive parameters have been carried out on grasses, especially on Lolium and Festuca species. Few studies on endophyte influence have been performed on leguminous species, and none on serradella. Therefore, the objective of this study was to evaluate the effect of seven fungal endophytes, isolated from pasture species, on biomass production (herbage and root biomass), nutritive value (protein, fibre, lignin and ash content) and mineral status of O. compressus forage.
Materials and Methods
Fungal and Plant Material
The effect of fungal endophytes on biomass production, nutritive value and mineral status of O. compressus forage was evaluated after the inoculation of each fungus into 2-month-old seedlings of this plant host. The seven fungal endophytes used in the experiments were previously isolated from the herbage of pasture species and identified in our laboratory by morphologic and molecular procedures (Table 1). The isolation and identification process was similar to that described by Lledó et al. [17]. Endophyte identification was first based on morphologic characteristics and then on comparison to ITS sequences in GenBank with a similarity criterion ≥99%. Four of the endophytes (E060: Fusarium sp., E071: Sordaria fimicola, E140: Stemphylium sp. and E636: Sporormiella intermedia) were selected because they had been quite frequently isolated from several pasture hosts [17], a fact which could reflect an eventual important ecological role. Selection of the other three species (E063: Mucor hiemalis, E346: Fusarium equiseti and E408: Byssochlamys spectabilis) was based on the observation of some interesting properties related to plant protection in previous experiments carried out in our laboratory (data not yet published). In order to obtain sufficient inoculum for the seedling infection, 2 months before the experiments, two plugs (5-mm diameter) of the actively growing mycelia of each fungus growing on potato dextrose agar (PDA) plates were added to 1.5-L flasks containing 1 L culture medium potato dextrose broth (PDB) and incubated at 25 °C in the dark. Flasks were shaken manually every 3 days during 5 min.
Seeds of O. compressus cv ‘Mazagón’ were surface-disinfected by immersion for 5 min in 2.5% NaClO and then washed three times with sterilized distilled water. Five sterilized seeds per pot were sown in 7 × 7 × 6 cm plastic pots containing sterilized (1 h at 121 °C, twice) soil substrate consisting of a 1:1 (v/v) mixture of perlite and peat (COMPO SANA Universal, Compo GmbH & Co. KG, Münster, Germany). On dried and homogenized soil substrate samples, pH was determined using a calibrated pH meter (ratio 10 g soil 25 mL deionized H2O), extractable P by Olsen procedure [27]; Ca and K were extracted with ammonium acetate (1N) and quantified by complexometric titration method [7] and using a K-ion-selective electrode (ISE 96 61, Crison Instruments S.A., Barcelona, Spain), respectively. Electrical conductivity (EC) was determined using an EC-meter. Total N was determined using the Kjeldahl method [6], with the aid of a Kjeltec™ K350 distillation Unit (Buchi Ltd., Flawil, Switzerland). Total Al, B, Ca, Cu, Fe, K, Li, Mg, Mn, Mo, Na, P, Pb, S, Se and Zn were determined by the Ionomics Service of the CSIC (Spanish High Centre for Science and Research) by means of inductively coupled plasma optical emission spectrometry (ICP-OES) after digestion with HNO3/H2O2 in UltraClave Microwave Milestone. The soil substrate properties are described in Table 2.
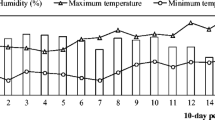
The experiment was carried out over 2 years, 2012/2013 (for endophytes E060, E071, E140 and E636) and 2013/2014 (for endophytes E063, E346 and E408). In both study years, the sowing date was in early December. After sowing, pots were placed in a greenhouse and watered to field capacity every 2 to 3 days. Maximum and minimum temperatures and relative humidity in the greenhouse during the experiments are reported in Fig. 1. In order to avoid interactions between the evaluated endophytes and those previously established naturally, which could have some effect on our results, plants were treated with a systemic fungicide before inoculations. The first fungicide treatment was applied on 1-month-old plants and was repeated twice every 7 days. Around 1 mL per pot of solution obtained by adding 1 mL of fungicide (Amistar Xtra®, Syngenta, Madrid, Spain) to 1 L of distilled water was sprayed as foliar application. In the third application, 1 mL per pot of the solution was additionally applied to the soil substrate. This procedure was similar to that used in previous works with similar objectives [37].
Inoculations
In order to verify that plants were free of endophytes, just before inoculation, four plants were randomly selected and taken to the laboratory. These plants were then surface-disinfested by one immersion for 30 s in 95% ethanol, and then another immersion for 1 min in 2% NaClO plus two drops Tween-80 per litre. Next, five 5-mm-long segments were cut from different parts of each plant and placed in a Petri dish containing PDA supplemented with 50 mg/L chloramphenicol to suppress bacterial growth. Plates were incubated at 25 °C in the dark and observed daily during the first 2 weeks and weekly during the following 2 months.
The plants of ten pots (each containing a set of five plants) were inoculated with each endophyte. An additional ten pots were inoculated only with culture medium to be used as control (thus a total of 50 pots were inoculated in 2012/2013 and 40 pots were inoculated in 2013/2014). Inoculations were carried out by following the procedure indicated by Lledó et al. [16], which had been shown to produce effective infections. Four weeks after the last fungicide application, plants were wounded by puncturing their leaves and stems with a homemade tool composed of two arms, one of which had a multiple needle structure at the end and the other a smooth surface [16]. In this way, plants were wounded sufficiently to facilitate fungal infection without serious plant damage. For fungal inoculation of the wounded plants, the actively growing mycelium of each endophyte was homogenized with the culture medium by blending at a medium speed during approximately 5 min. The homogenized inoculum was then placed in hand sprayer.
In order to evaluate the viability of the mycelium after blending, Petri dishes containing PDA were sprayed with the homogenized inoculum. New colonies of each fungus were observed after a few days. Inoculation was carried out with the aid of the hand sprayer in two doses: one half of the homogenized inoculum (i.e. 500 mL) just after plant wounding, and the other half 3 days later. Each plant received the following amount of mycelia depending on the endophytic species inoculated: E060 (Fusarium sp.) 56.4 mg plant−1, E063 (M. hiemalis) 74.0 mg plant−1, E071 (S. fimicola) 143.2 mg plant−1, E140 (Stemphylium sp.) 815.4 mg plant−1, E346 (F. equiseti) 134.0 mg plant−1, E408 (B. spectabilis) 63.8 mg plant−1, E636 (S. intermedia) 282.6 mg plant−1. During 48 h following the inoculations, plants were maintained in a high humidity atmosphere in order to maximize fungal infection.
Experimental Design and Response-Variable Determinations
The experiment was carried out under the greenhouse conditions described before. A set of five plants (i.e. one pot) was considered as the experimental unit. The pots containing the inoculated plants were arranged on greenhouse benches by following a completely randomized design (with five replicates or pots per endophyte treatment), as no environmental variations in the experimental area were expected. Pots were placed at least 5 cm apart, without direct contact, to avoid secondary infections. In order to ensure the effectiveness of the inoculations, approximately 1 month later, plant samples of each treatment were taken to the laboratory for re-isolations following the same procedure as that carried out to verify that plants were free of endophytes.
In order to evaluate eventual pathogenic behaviour of the endophytes used in the present study on O. compressus, plants were visually examined weekly (over 8 weeks starting 1 week after inoculation) to check for disease symptoms (yellowing, drying, rotting leaves, blackish spots, etc.). According to the severity of these symptoms, the plants in each pot were assigned to one category on the following scale: 1 = healthy, 2 = slightly affected, 3 = moderately affected, 4 = severely affected and 5 = dead. Disease progress curves for each pot were constructed by plotting the values of disease severity over time. The area under the disease progress curve (AUDPC) was calculated as the sum of the area of the corresponding trapezoids, considering one unit per period between two consecutive measurements. The AUDPC was used as response variable to evaluate disease severity.
Three months after inoculations (i.e. early May for both years of study, 2012/2013 and 2013/2014), herbage and roots were harvested and taken to the laboratory for processing. Roots were carefully washed with tap water to remove soil substrate. In the laboratory, samples were oven-dried (70 °C) until constant weight. After that, DM of herbage (HDM) and root (RDM) biomass production was recorded. From part of the herbage samples, the following parameters were determined: CP by multiplying biomass N × 6.25 (protein on average contains 16% N) as conversion factor (biomass N was obtained by Kjeldahl distillation (distillation unit K-350, Buchi, Flawil, Switzerland), neutral detergent fibre (NDF), ADF and acid detergent lignin (ADL) by means of a fibre analyzer (ANKOM 8-98, ANKOM Technology, Macedon, NY, USA), and total ash by ignition in a muffle furnace at 600 °C by following the official procedures [3]. The other part of the herbage samples was sent to the Ionomics Service of the CSIC for mineral determinations as described above for the soil samples.
Only five out of the ten pots inoculated per endophytic treatment were used in the greenhouse experiment. The remaining five pots were used to evaluate how transferrable the results obtained in the greenhouse were under field conditions. This evaluation was performed on the ‘herbage biomass yield’ response variable, as it is considered one of the most important parameters for farmers. Approximately 1 month after the inoculations, the second half of the pots were transported to an experimental area located in the grassland ‘Valdesequera’ owned by the regional government of Extremadura. This area is located in Badajoz, southwest Spain (UTM coordinates, zone 29 north datum X = 685,365 m; Y = 4,325,603) in Alfisol Xeralf soil (according to USDA classification). The soil of the study site had a sandy loam texture, determined gravimetrically on four representative soil samples taken before transplanting at 30 cm depth, which is the usual rooting depth of herbaceous legume species. On these soil samples, all the edaphic characteristics were determined as explained above for the substrate samples (Table 2). The climatic data during the experiments, which were taken from a weather station located close to the study site, can be observed in Fig. 1.
Before transplanting, conventional tillage was applied to prepare an appropriate environment for plants. Transplanting was made in late February, and the experimental units (a set of five plants) were arranged by following a completely randomized design with a planting layout of 50 cm × 50 cm. Two months and a half after transplanting (i.e. mid-May for both study years, 2012/2013 and 2013/2014), herbage was harvested and taken to the laboratory for processing. The herbage DM production obtained in the field experiment (FDM) was determined as explained above for the HDM.
Statistical Analysis
Under greenhouse conditions, the evaluated effects of the endophytes (five treatments, including controls in 2012/2013, and four treatments, including controls in 2013/2014) were as follows: disease severity (estimated as the area under the disease progress curve, AUDPC); herbage and root dry matter yield (HDM and RDM, respectively); nutritive value parameters (CP, NDF, ADF, ADL and ash) and herbage mineral concentration (Al, B, Ca, Cu, Fe, K, Li, Mg, Mn, Mo, Na, P, Pb, S, Se and Zn). Data were subjected to a one-way ANOVA for each year separately. Under field conditions, the effect of the endophyte treatment was also evaluated by one-way ANOVA on herbage dry matter yield (FDM). Fisher’s protected least significant difference (LSD) test for multiple comparison was used when significant differences (P < 0.05) were found in the ANOVA. In order to normalize variable distribution and to stabilize the variance of residuals, the following variable transformation was performed: Ln(x + 1) for AUDPC; and 5√x for HDM, RDM and FDM. All these analyses were performed with the Statistix v. 8.10 package.
Results
Effects on Disease Severity and Biomass Yield
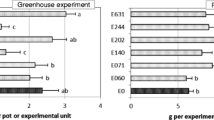
In the greenhouse experiment, none of the endophytes caused disease severity on the O. compressus plants, as the area under the disease progress curve (AUDPC) was not significantly affected by the endophyte treatment (Table 3). With regard to biomass yield, herbage production was not significantly affected by the endophyte treatment in the two study years (Table 3). Herbage dry matter ranged between (mean ± standard error) 0.68 ± 0.13 g pot−1 and 1.55 ± 0.58 g pot−1 in 2012/2013, and 0.65 ± 0.10 g pot−1 and 0.94 ± 0.12 g pot−1 in 2013/2014 (Fig. 2a). In the case of root dry matter, this was significantly affected by the endophyte treatment in 2012/2013, but not in 2013/2014 (Table 3). In the case of 2012/2013, plants inoculated with the endophyte E060 (Fusarium sp.) presented a root biomass 47% lower than that of the controls (Fig. 2a).
Effect of endophyte inoculation in 2012/2013 and 2013/2014 on a biomass yield (FDM herbage dry matter in the field experiment, HDM and RDM herbage and root DM, respectively, in the greenhouse experiment), b crude protein (CP), c fibre (NDF and ADF) and lignin (ADL) and d ashes. Vertical bars indicate means and vertical lines standard error. For each parameter and study year, averages with the same letter are not significantly different according to LSD test at a significant level of 0.05. When letters do not appear, the influence of the endophyte treatment was not significant (α > 0.05) according to ANOVA. C control, E060 Fusarium sp., E063 Mucor hiemalis, E071 Sordaria fimicola, E140 Stemphylium sp., E346 Fusarium equiseti, E408 Byssochlamys spectabilis, E636 Sporormiella intermedia
In the field experiment, herbage yield (FDM) was not significantly affected by the endophyte treatment in 2012/2013, but it was in 2013/2014 (Table 3). In the latter case, the endophyte E408 (B. spectabilis) caused an increment of about 42% in the herbage yield in comparison with the controls (Fig. 2a). On average, the herbage yield obtained in 2013/2014 was much higher (9.37 ± 0.54 g pot−1) than in 2012/2013 (2.32 ± 0.19 g pot−1).
Effects on Nutritive Value Parameters
The endophyte treatment affected significantly CP, lignin (ADL) and total ash obtained in herbage in 2012/2013 and ADF in 2013/2014 (Table 3). In 2012/2013, inoculation with endophytes E060 (Fusarium sp.), E140 (Stemphylium sp.) or E636 (S. intermedia) provided forage with higher CP values in comparison with those obtained in the controls (87.54, 62.61 and 80.87%, respectively; Fig. 2b). The forage derived from plants inoculated with any of the same three endophytes (E60, E140 or E636) presented the lowest values of ADL and total ashes (Fig. 2c, d). Plants inoculated with endophyte E140 (Stemphylium sp.) presented ADL values significantly lower (18%) than those found in the controls (Fig. 2c). In the case of total ash content, the three endophytes provided forage with values approximately 64% lower than the controls (Fig. 2d). In 2013/2014, inoculation with any of the three endophytes studied, E063 (M. hiemalis), E346 (F. equiseti) or E408 (B. spectabilis), provided forage with higher ADF than that obtained in the control plants (12.67, 17.04 and 13.45%, respectively; Fig. 2c).
Mineral Uptake and Accumulation in Herbage
The ANOVA showed that, while in 2012/2013, the endophyte treatment affected significantly the concentration in plants of Al, B, Fe, Mn, Mo, P, Pb, S and Zn (Table 4), in 2013/2014, it affected only the concentration of Al and Mg (Table 5). In 2012/2013, forage of the plants inoculated with any of the four studied endophytes presented higher concentrations of B, Mo, P, S and Zn (on average around 16, 114, 31, 35 and 38%, respectively) than in the controls (Table 4). From this general pattern, only inoculation with S. fimicola (E071) and Stemphylium sp. (E140) did not cause significant differences in the concentration of P and Zn, respectively, in comparison with the controls. Regarding the concentration of Al, Fe and Pb, the pattern observed in plants inoculated with either E060 (Fusarium sp.), E140 (Stemphylium sp.) or E636 (S. intermedia) was quite similar. In the three cases, endophyte E140 caused a strong decrease in Al, Fe and Pb concentrations in the forage (around 74, 49 and 46%, respectively) than in the controls (Table 4). Inoculation with either endophyte E060 (Fusarium sp.) or E636 (S. intermedia) also resulted in a significant but more moderate decrease in concentration of Al, Fe and Pb (on average around 51, 22 and 25%, respectively) than in the controls (Table 4). Likewise, Pb concentration in the forage was also negatively affected by S. fimicola (E071), with values 25% lower than the controls. This fungus, in addition to E636 (S. intermedia), also provoked a higher concentration of Mn (37 and 24%, respectively) in comparison with the controls (Table 4).
In 2013/2014, the forage of the plants inoculated with any of the three studied endophytes presented a lower concentration of Al than in the controls; around 31, 37 and 26%, for E063 (M. hiemalis), E346 (F. equiseti) and E408 (B. spectabilis), respectively. In the case of the Mg concentration, only plants inoculated with the endophyte E063 (M. hiemalis) presented values 11% lower than those observed in the control plants (Table 5).
Discussion
In the re-isolation process carried out before inoculations in order to check the efficiency of the systemic fungicide, no endophytic colonies were observed in the culture medium growing from any of the plant segments. The procedure used, based on fungal culturability, does not permit us to state with absolute certainty that plants were devoid of fungus, as quiescent cells or spores not culturable under the tested conditions could have persisted. However, if the fungicide treatment was able to remove the whole culturable mycobiota, which was that analysed, it seems likely that this treatment had also been efficient against the non-culturable fungi, at least at a level which could allow establishment and further spread of the inoculated endophyte. This is supported by the fact that all the isolates were positively re-isolated and identified in culture medium and also because when any of the endophytic species were inoculated, significant effects were found in at least one of the evaluated parameters (Table 6).
Removal of the pre-existing fungi was carried out to evaluate the actual effect of the studied endophytes on their host under the best possible controlled conditions, so that comparisons could be made between them. Untreated plants may have contained a high mycobiota load with a complex fungal diversity of a variable and undetermined nature. Therefore, the use of such untreated plants would have meant microbiologically uncontrolled conditions during the experiments. Likewise, the uninoculated controls would have been exposed to the effect of undetermined potentially active microbiota, thus possible either enhancing or counteracting the measured effects. It is clear that with this technique, we are creating an artificial environment where eventual competition has been mostly eliminated. Furthermore, in the present study, inoculations were performed after wounding the plants in order to facilitate infection of the endophytes. Again, with this procedure, we are creating an artificial route for endophytes to reach the inner part of plant tissues.
The results obtained, therefore, cannot be considered as directly transferrable to real in-field agriculture, since a future application of the new inoculants should not require fungicide treatment and/or previous plant wounding as a pre-requisite to observe the expected effects. As many of the endophytes used in the present study were selected because they had been very frequently isolated naturally from several pasture hosts, it seems likely that they have a strong competitive ability and a good aptitude to naturally colonize plant tissues. In any case, the study presented here should be understood as a previous necessary search for endophytic candidates, requiring further investigation in order to be used in the future as growth-promoting inoculants for plants. Future experiments should include natural conditions, to evaluate endophytes’ ability to enter plants by natural mechanisms and to produce the required effects in environments subjected to natural competition with pre-existing microorganisms. In addition, different inoculation procedures or the development of endophyte-based products (which could eventually produce the same effects) should also be tested in order to optimize the effectiveness of the application. Another interesting line of research which could be explored is the possibility of vertical transmission (i.e. from mother plant to offspring, via seeds) in the studied endophytes, since this phenomenon has been found to occur more frequently and in more non-clavicipitaceous endophyte species than commonly thought [14]. In this way, the use of endophytes in in-field agriculture on a large scale might become more feasible, as no further treatments other than seeding with already-infected seeds would be necessary for farmers.
For inoculum production, all the endophytes tested were incubated under the same conditions for 2 months. Since each endophyte presented a different growth rate, the amount of inoculum produced for each endophyte after 2 months of incubation was different. However, we decided to use the same incubation time for all of the endophytes rather than the same inoculum amount, in order to establish the comparisons between them by focusing on the inoculum production process. In any case, the inoculum produced by all of the endophytes after 2 months of incubation could be considered sufficient to infect the plant. The eventual effect caused by a fungus on the host, once it has spread inside the plant, is expected to be due to its specific aptitude to produce this effect rather than to the initial amount of inoculum of the endophyte. This is supported by studies carried out by Spiering et al. [32], which show that the effects caused by a fungal endophyte on its host were independent of in-planta endophyte concentration.
Several studies have shown a significant and positive effect of endophytes on herbage and root biomass productivity in several hosts (e.g. Assuero et al. [4], Hesse et al. [13], Lledó et al. [16]). However, most of those experiments were performed under certain limitations for plant growth: water stress, deficiencies in mineral nutrition, soil mineral toxicity, pest or disease infections, etc. Therefore, it would seem to be clear that since the endophyte can give a higher adaptive value to the plant host, the effect of the microorganism-plant interaction might be more evident under stressful conditions for plants. This fact may explain why in our study, under good environmental conditions in the greenhouse, none of the endophytes affected herbage biomass yield. However, under the less favourable conditions of the field experiment, inoculation with the endophyte E408 (B. spectabilis) produced around 42% more herbage than the controls.
To explain this increase in herbage yield caused by endophytes, several authors have proposed that these organisms could affect photosynthesis and CO2 fixation [22, 32], which could produce a higher growth rate in the plant host. On the other hand, fungal endophytes have also been found to cause a lengthening of the vegetative growth period of their plant host [37], which is the period with a more active biomass accumulation. Nevertheless, the mechanisms involved in this aspect are not fully understood. In some cases, endophyte occurrence could prevent or delay inflorescence emergence, thus postponing the beginning of the reproductive period, in which biomass production is considerably reduced. Other authors such as Assuero et al. [4] have suggested that endophyte presence may promote plant growth by producing some hormone-like substances which could delay the growth cycle of the plant, or that it may promote root growth, giving plants a higher capacity to absorb water and minerals. Regarding the production of hormone-like substances, B. spectabilis has been shown to be an important source of secondary metabolites (as summarized by Mioso et al. [20]); it has been found to produce cornexistin, a secondary metabolite with herbicidal activity [21], and several other bioactive compounds, such as viriditoxin or varioxepine, with antimicrobial properties [26, 38]. The ability of the multiple compounds produced by this fungus to generate physiological responses in its plant hosts has not been investigated. Therefore, we are not able to know with certainty whether any of these compounds could act as a hormone-like substance or whether they could promote the production of phytohormones by plant hosts. Further experiments should be specifically designed to clarify the mechanisms of this increase in herbage biomass yield.
It may also be that the increase in herbage biomass yield caused by the endophyte is due to a fertilizer effect of the inoculum applied (composed by mycelia growing in 1 L of PDB medium), rather than to the direct action of the living endophyte interacting with its host plant. However in this case, this possibility seems to be unlikely for several reasons: first, if the inoculum applied had a fertilizer effect, a higher amount of inoculum applied would have resulted in greater growth, but this was not the case. The increase in herbage production was only observed for one of the endophytes, and it was not that which had highest inoculum production during the incubation time before inoculations. Secondly, the effect on plant growth was not observed until plants were transferred to the field (Table 6); if the inoculum applied had a fertilizer effect, such an influence would also have appeared, and more intensively, in the greenhouse experiment, where plants were growing in small pots with a limited amount of substrate. Once plants were transplanted into the field, the importance of the substrate contained in the pots in the nutrition of plants might be lesser than that in the field soil, which did not receive additional nutrients. Consequently, the most probable explanation is that the observed effects on plant growth could be attributed to the activity of the living endophyte inside the plant, rather than to a fertilizer effect of the inoculum applied.
Taking into account that the quality of a forage is directly proportional to the protein content and to its digestibility (which is inversely correlated to fibre and lignin content), inoculation with the endophytes E60 (Fusarium sp.), E140 (Stemphylium sp.) or E636 (S. intermedia) provided forage with a higher nutritive value than that obtained in the controls (Table 6). These three fungi produced either a higher crude protein or reduced lignin content in the resulting forage. Authors such as Zabalgogeazcoa et al. [37], who also found a positive influence of endophytes (in this case E. festucae) on the digestibility of the organic matter in plants of Festuca rubra, F. arundinacea Schreb and L. perenne, have suggested endophytes to cause a delay in the maturity of the plant to explain this fact. Since throughout the plant life cycle, fibre and lignin values increase whereas protein values decrease [30], such a delay may have produced the higher protein content and the lower lignin content observed in the present study. However, further experiments including an exhaustive analysis of the exact growth stage of the plant after inoculations should be performed in order to confirm this hypothesis.
Changes in the nutritional status of the forage derived from plants infected with fungal endophytes has also been observed in T. subterraneum or Poa pratensis L. growing under good and controlled conditions [15, 16]. In these studies, two of the isolates evaluated in the present work, E60 (Fusarium sp.) and E140 (Stemphylium sp.), were also used. In both cases, E140 (Stemphylium sp.) was also found to cause a decrease in fibre or lignin content of the forage, but E60 (Fusarium sp.) did not. This might indicate, as suggested by other authors [1], that a significant influence of the endophyte might occur only if the proper combination of endophyte species, host genotype and environmental conditions occurs.
Considerable differences were found between the study years in several of the parameters analysed, especially in herbage biomass yield obtained in the field experiment and in protein content of the forage. It could be that these differences were due to the different endophytes inoculated in each study year. However, the same differences were also observed between the controls. To explain this fact, it is important to bear in mind that the field experiment was carried out in an area with a typical Mediterranean climate, characterized by a great inter-annual irregularity, especially in precipitations which widely affect herbage yield. The climatic conditions of 2013/2014 (Fig. 1), with higher rainfall in February and April, seemed to favour plant growth in comparison with the conditions of 2012/2013. This high inter-annual irregularity highlights the importance of the controls to be included each year and prevents comparison between the years. Furthermore, temperatures greatly affect the plant growth cycle, producing a shortening in the plant vegetative stage as temperatures increase. As is already known, protein values of herbage can decrease during the plant life cycle [30]. This fact might explain the rather lower protein content of the forage in 2012/2013, in which the temperatures were considerably warmer than in 2013/2014 (Fig. 1).
With regard to mineral concentration contained in the herbage, Al is not essential for plants and animals. In fact, high concentrations of Al in the soil, which are mainly found when pH is low (as in the present study), may be toxic for plants and animals. Consequently, in those conditions, a lower Al uptake and later accumulation in forage may be highly desirable. Although most of the endophytes were able to reduce Al in forage (Table 6), endophyte E140 (Stemphylium sp.) was especially effective in this aspect, reducing the Al concentration by almost 75% in comparison with the controls. This endophyte was also the most effective in reducing accumulation of Pb in forage when it was inoculated. Lead poisoning is one of the most frequently reported causes of poisoning in farm livestock, cattle being the most commonly affected species [33]. Therefore, in areas with high lead concentration in the soil, due for example to lead-mining activity, the use of this fungus could reduce uptake and later accumulation of Pb in the forage to a suitable level for animal feeding, which should be lower than 5 mg kg−1 DM according to the maximum permitted level [24]. The ability of Stemphylium sp. to reduce Al and Pb concentrations in the host plant has also been evidenced in other forage legume species such as T. subterraneum L. [16]. This fact seems to suggest that this endophyte may not present high host specificity, since it is able to establish effective association with different hosts and to consistently produce the indicated effect regardless of the host. This is an interesting aspect as it may allow use of this fungus for this purpose at a broader range.
Similarly, this species (Stemphylium sp.), and also E60 (Fusarium sp.), E071 (S. fimicola) and E636 (S. intermedia), produced forage with concentrations of B, Mo, P, S and Zn higher than in the controls in most of the cases (Table 6). These minerals are essential nutrients for both plants and animals. Therefore, although the concentration obtained in the present work even in the controls was higher than the recommended minimum concentration in pasture dry matter for grazing cattle and sheep [33], the increase in accumulation caused by the endophyte should be considered as positive, at least up to the maximum tolerable levels, established as 150 mg B kg−1 DM, 5 mg Mo kg−1 DM, 6 g P kg−1 DM, 5 g S kg−1 DM and 300 mg Zn kg−1 DM [24]. Several species, closely related to the studied endophytes, such as Stemphylium globuliferum (Vestergr.), Fusarium lateritium Nees or Paecilomyces variotii Bainier, have been reported to be pathogenic in several plant species in which they produce metabolites derived from their activity, which can have phytotoxic effects [9, 20, 35]. This is an important issue with regard to a future eventual application of the studied endophytes, because if they showed pathogenicity or had phytotoxic effects, their use would have to be avoided. In the present case, none of the endophytes inoculated caused disease symptoms in plants or a loss in forage productivity, thus indicating a lack of pathogenicity of the isolates used and a lack of phytotoxic effects. An additional basic requirement to an eventual in-field application of these endophytes is that they should not produce toxic forage for livestock. Although according to the literature the studied endophytes do not seem to produce toxic compounds for animals, this fact should be further verified before use.
In conclusion, the results presented here provide evidence that endophytes can affect herbage yield, nutritive value and nutrient content of yellow serradella forage. According to our results, plant inoculation with endophytes could be a suitable strategy to increase forage yield and nutritive value, or to deal with potential nutrient deficiencies or with potential mineral toxicity in forage. The suitability of each endophyte might depend on the required effect. Of the endophytes studied, B. spectabilis in particular might increase herbage biomass yield, and Stemphylium sp. might improve the nutritive value of forage either by increasing crude protein, digestibility and the concentration of essential minerals, or by reducing the concentration of toxic elements such as Al or Pb.
References
Ahlholm JU, Helander M, Lehtimäki S, Wäli P, Saikkonen K (2002) Vertically transmitted fungal endophytes: different responses of host-parasite systems to environmental conditions. Oikos 99:173–183
Anonymous 2001. Serradella. Agfact P2.5.23, Second edition. Department of Primary Industries, NSW. www.dpi.nsw.gov.au/agriculture/pastures/pastures-and-rangelands/species-varieties/factsheets/serradella; accessed 15 December 2016.
AOCS (2006) Official methods of analysis. Association of Official Analytical Chemists, Washington, D.C.
Assuero SG, Tognetti JA, Colabelli MR, Agnusdei MG, Petroni EC, Posse MA (2006) Endophyte infection accelerates morpho-physiological responses to water deficit in tall fescue. N Z J Agric Res 49:359–370
Bolland MDA, Gladstones JS (1987) Serradella (Ornithopus spp.) as a pasture legume in Australia. J Aust Inst Agric Sci 53:5–10
Bremner JM (1996) Nitrogen total. In: Sparks DL (ed) Methods of soil analysis, part 3: chemical methods. Soil Science Society of America, Madison, Wisconsin, pp. 1085–1121
Cheng KL, Bray RH (1951) Determination of calcium and magnesium in soil and plant material. Soil Sci 72:449–458
Clarke BB, White JF, Hurley H, Torres MS, Sun S, Huff DR (2006) Endophyte mediated suppression of dollar spot disease in fine fescues. Plant Dis 90:994–998
Debbab A, Aly AH, Edrada-Ebel R, Wray V, Müller WE, Totzke F, Zirrgiebel U, Schächtele C, Kubbutat MH, Lin WH, Mosaddak M, Hakiki A, Proksch P, Ebel R (2009) Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J Nat Prod 72:626–631
Del Pozo A, Ovalle C (2009) Productivity and persistence of yellow serradella (Ornithopus compressus L.) and biserrula (Biserrula pelecinus L.) in the Mediterranean climate region of central Chile. Chilean J Agric Res 69:340–349
Frame J (1998) Ornithopus compressus L. (Yellow serradella). Grassland species profiles database. FAO. www.fao.org/ag/AGP/AGPC/doc/GBASE/data/pf000489.htm; accessed 15 December 2016.
Frame J, Charlton JFL, Laidlaw AS (1998) Temperate forage legumes. CABI Publishing Series, CAB International, Wallingford
Hesse U, Schöberlein W, Wittenmayer L, Förster K, Diepenbrock W, Merbach W (2005) Influence of water supply and endophyte infection (Neotyphodium spp.) on vegetative and reproductive growth of two Lolium perenne L. genotypes. Eur J Agron 22:45–54
Hodgson S, de Cates C, Hodgson J, Morley NJ, Sutton BC, Gange AC (2014) Vertical transmission of fungal endophytes is widespread in forbs. Ecol Evol 4:1199–1208
Lledó S, Rodrigo S, Poblaciones MJ, Santamaria O (2015) Biomass yield, mineral content, and nutritive value of Poa pratensis as affected by non-clavicipitaceous fungal endophytes. Mycol Prog 14:67 online version
Lledó S, Rodrigo S, Poblaciones MJ, Santamaria O (2016a) Biomass yield, nutritive value and accumulation of minerals in Trifolium subterraneum L. as affected by fungal endophytes. Plant Soil 405:197–210
Lledó S, Santamaria O, Rodrigo S, Poblaciones MJ (2016b) Endophytic mycobiota associated with Trifolium subterraneum growing under semiarid conditions. Ann Appl Biol 168:243–254
Malinowski DP, Belesky DP (1999) Tall fescue aluminum tolerance is affected by Neotyphodium coenophialum endophyte. J Plant Nutr 22:1335–1349
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Mioso R, Toledo Marante FJ, Herrera Bravo de Laguna I (2015) The chemical diversity of the ascomycete fungus, Paecilomyces variotii. Appl Biochem Biotechnol 177:781–791
Nakajima M, Itoi K, Takamatsu Y, Sato S, Furukawa Y, Furuya K, Honma T, Kadotani J, Kozasa M, Haneishi T (1991) Cornexistin: a new fungal metabolite with herbicidal activity. J Antibiot 44:1065–1072
Newman JA, Abner ML, Dado RG, Gibson DJ, Brooking A, Parsons AJ (2003) Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: growth, photosynthesis, chemical composition and digestibility. Glob Chang Biol 9:425–437
Nichols PGH, Revell CK, Humphries AW, Howie JH, Hall EJ, Sandral GA, Ghamkhar K, Harris CA (2012) Temperate pasture legumes in Australia—their history, current use, and future prospects. Crop Pasture Sci 63:691–725
NRC (National Research Council) (2005) Mineral tolerance of animals, 2nd edn. National Academy of Sciences, Washington, DC
Oesterheld M, Loreti J, Semmartin M, Sala OE (2001) Inter-annual variation in primary production of a semi-arid grassland related to previous-year production. J Veg Sci 12:137–142
Oliveira Silva MR, Kawai K, Hosoe T, Campos Takaki GM, Buarque Gusmão N, Fukushima K (2013) Viriditoxin, an antibacterial substance produced by mangrove endophytic fungus Paecilomyces variotii. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science, technology and education, vol 2. Formatex Research Center, Badajoz, pp. 1406–1411
Olsen SR, Cole CV, Watanable FS, Dean LA (1954) Estimation of available phosphorus in soil by extraction with sodium bicarbonate. U.S. Department of Agricultural Circular 939, Whashington
Ovalle C, Arredondo S, Romero O (2006) Serradela amarilla (Ornithopus compressus) y Serradela rosada (O. sativus): dos nuevas especies de leguminosas forrajeras anuales para la Zona Mediterránea de Chile. Agricultura Técnica 66:196–209
Sandoval MA, Celis JE, Morales P (2011) Structural remediation of an alfisol by means of sewage sludge amendments in association with yellow serradela (Ornithopus compressus L.) J Soil Sci Plant Nutr 11:68–78
Santamaría O, Rodrigo S, Poblaciones MJ, Olea L (2014) Fertilizer application (P, K, S, Ca and Mg) on pasture in calcareous dehesas: effects on herbage yield, botanical composition and nutritive value. Plant Soil Environ 60:303–308
Santamaría O, Smith DR, Stanosz GR (2012) Interaction between Diplodia pinea or Diplodia scrobiculata and fungal endophytes isolated from pine shoots. Can J For Res 42:1819–1826
Spiering MJ, Greer DH, Schmid J (2006) Effects of the fungal endophyte, Neotyphodium lolii, on net photosynthesis and growth rates of perennial ryegrass (Lolium perenne) are independent of In Planta endophyte concentration. Ann Bot 98:379–387
Suttle NF (2010) Mineral nutrition of livestock, 4th edn. CABI, Wallingford, Oxfordhire
Vázquez de Aldana BR, García-Ciudad A, Pérez-Corona ME, García-Criado B (2000) Nutritional quality of semi-arid grassland in western Spain over a 10-year period: changes in chemical composition of grasses, legumes and forbs. Grass Forage Sci 55:209–220
Yun HY, Lee YW, Kim YH (2013) Stem canker of giant dogwood (Cornus controversa) caused by Fusarium lateritium in Korea. Plant Dis 97:1378
Zabalgogeazcoa I (2008) Review. Fungal endophytes and their interaction with plant pathogens. Span J Agric Res 6:138–146
Zabalgogeazcoa I, García-Ciudad A, Vázquez De Aldana BR, García-Criado B (2006) Effects of the infection by the fungal endophyte Epichloë festucae in the growth and nutrient content of Festuca rubra. Eur J Agron 24:374–384
Zhang P, Mandi A, Li XM, Du FY, Wang JN, Li X, Kurtan T, Wang BG (2014) Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org Lett 16:4834–4837
Acknowledgments
This study was funded by Project AGL2011-27454, granted by the Ministry of Economy and Competitiveness of Spain (the former Ministry of Science and Innovation) and by the European Regional Development Fund (ERDF). We would like to thank Teodoro García-White, Natalia Hernández and Pablo Romero for their invaluable help with the technical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santamaria, O., Lledó, S., Rodrigo, S. et al. Effect of Fungal Endophytes on Biomass Yield, Nutritive Value and Accumulation of Minerals in Ornithopus compressus . Microb Ecol 74, 841–852 (2017). https://doi.org/10.1007/s00248-017-1001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1001-3