Abstract
Background and aims
Trifolium subterraneum is a legume species which is valuable for feeding livestock and frequently used as a forage crop in countries with temperate or Mediterranean-like climates. The aim of the present work was to evaluate the effect of six leaf fungal endophytes on biomass production, nutritive value and mineral status of T. subterraneum forage.
Methods
Plants were inoculated with each of seven treatments (six endophytes + control) at two different growth stages. After inoculation, two experiments (under greenhouse and field conditions) were established.
Results
Endophytes affected biomass yield, nutritive value and mineral status of T. subterraneum forage, but effects varied between experiments and depended on fungal species. E202 (Chaetosphaeronema sp.) increased forage productivity by around 80 % in the field. Fusarium lateritium and E244 (Pleosporales) reduced Al concentration, and Epicoccum nigrum reduced Pb of the forage in the greenhouse experiment. An increase in essential nutrients, such as Zn, was mainly produced by Stemphylium globuliferum.
Conclusions
This study demonstrated that inoculation with endophytes can increase forage productivity and help reduce potential nutrient deficiencies and/or potential mineral toxicity in T. subterraneum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trifolium subterraneum L. (subterranean clover), an annual self-regenerating pasture legume of Mediterranean origin, occurs naturally in grasslands and dehesas (i.e., grasslands with scattered trees and well-developed herbaceous understory) in southwestern Spain on acidic soils. This species produces a relatively abundant and high quality forage for feeding livestock, with amounts of 2330–3550 kg ha−1 dry matter and values of crude protein around 15–19 % (García-Criado et al. 1986). In addition, subterranean clover forage exhibits a high palatability and digestibility due to its relatively low content in fiber and lignin, with average values of NDF (neutral detergent fiber), ADF (acid detergent fiber) and ADL (acid detergent lignin) of 26.8, 21.8, and 3.23 %, respectively (García-Ciudad et al. 1985).
For these reasons, T. subterraneum is one of the most important legumes used alone or in mixtures in sown pastures in Spain and many other countries with temperate or Mediterranean-like climates (Frame et al. 1998). Many factors affect growth, biomass production, quality traits, persistence and regeneration of T. subterraneum. Climatic conditions, especially precipitation during the growing season, are undoubtedly the most important factor (Bolger et al. 1993), but soil fertility (Saul et al. 1999), competition with other plant species (Conning et al. 2011), shading levels (Kyriazopoulos et al. 2012), livestock management (Ates et al. 2013), and pest and diseases (Simpson et al. 2011) can also have an important influence on these parameters.
In addition to these well-known factors, fungal endophytes, defined as organisms which for part or all of the life cycle invade the living tissues of plants without causing any disease symptoms, have been shown to affect widely the production and performance of other pasture species. Many studies have reported increases in biomass production and in re-growth capacity in endophyte-infected plants in comparison with uninfected ones (Hesse et al. 2005; Thom et al. 2013; Mahendra et al. 2014). The nutrive value of Festuca rubra L., F. arundinacea Schreb. and Lolium perenne L., measured through digestibility of organic matter, has also been shown to be improved by the endophyte Epichloë festucae Leuchtm. et al. (Zabalgogeazcoa 2008). Likewise, several foliar endophytes have been found either to increase the concentration of some minerals such as P, Ca or Mg (Zabalgogeazcoa et al. 2006), or to reduce the concentration of others such as Cu (Dennis et al. 1998; Zabalgogeazcoa et al. 2006), Al (Malinowski and Belesky 1999) or Zn (Monnet et al. 2001).
These effects could be a consequence of the substantial benefits that endophytic fungi may confer on hosting plants, such as drought tolerance (Hall et al. 2014), resistance to herbivory and pathogens (Thom et al. 2013; Romeralo et al. 2015), enhanced nutrient uptake (Yang et al. 2014), and increased competitive ability (Vázquez-de-Aldana et al. 2013). However, this influence appears to be variable and clearly dependent on the species of endophyte, the host genotype and environmental conditions (Ahlholm et al. 2002). For this reason, it is common also to find studies in which fungal endophytes have neutral or negative effects (Zabalgogeazcoa et al. 2006; Faeth et al. 2010). Consequently endophyte-plant interactions should be tested specifically for each combination of plant host, endophytic species and environmental conditions.
Most of the studies dealing with such plant-endophyte interactions, such as those already cited above, have been performed on grasses species with clavicipitaceous fungi, which are systemic fungal endophytes defined according to the classification given by Rodriguez et al. (2009). Conversely, studies dealing with non-clavicipitaceous endophytes in legume species are very limited (Dudeja et al. 2012), most of them focusing on endophytic diversity, such as those conducted on peanut (Ferreira de Souza et al. 2014), soybean (Impullitti and Malvick 2013), or on several sand dune wild legumes (Seena and Sridar 2004), rather than endophyte effects on plant production. With the aim of testing the hypothesis that non-clavicipitaceous endophytes also affect biomass production and quality of an important forage legume, this study evaluated the effect of six foliar fungal endophytes on the biomass production (herbage and root biomass), nutritive value (protein, fiber, and lignin) and mineral status of T. subterraneum forage.
Materials and methods
Fungal and plant material
Six fungal endophytes, previously isolated from pasture species and identified in our laboratory, were used in the experiments (Table 1). Endophyte identification was first based on morphologic characteristics and then on comparison to ITS sequences in GenBank with a similarity criterion >97 %. Two months before the experiments, fungi were grown at 25 °C in the dark in 1.5-L flasks containing 1 L culture medium PDB (Potato Dextrose Broth) to obtain sufficient inoculum for plant inoculations. Seeds of T. subterraneum cv ‘Valmoreno’ were surface-disinfected by immersion for 5 min in 2.5 % NaClO, and then washed three times with sterilized distilled water. Five sterilized seeds per pot were sown in 7x7x6 cm plastic pots containing soil substrate consisting of a 1:1 (vol/vol) mix of perlite and a commercial growing medium composed of peat, perlite, lime, root activator and fertilizer NPK (COMPO SANA Universal, COMPO GmbH & Co. KG, Münster Germany). On four dried and homogenized soil substrate samples, pH was determined using a calibrated pH meter (ratio 10 g soil: 25 ml deionized H2O). Electrical conductivity (EC) was determined using an EC-meter. Total N was determined using the Kjeldahl method (Bremner 1996), with the aid of a Kjeltec™ K350 destillation Unit (Buchi Ltd., Flawil, Switzerland). Extractable P was determined by the Olsen procedure, and Ca and K were extracted with ammonium acetate (1 N) and quantified by atomic absorption. Total Al, B, Ca, Cu, Fe, K, Li, Mg, Mn, Mo, Na, P, Pb, S, Se and Zn concentrations were determined by the Ionomics Service of CSIC (Spanish High Centre for Science and Research) by means of inductively coupled plasma optical emission spectrometry (ICP-OES).
Because the age of the plant seems somehow to affect fungal endophyte colonization (Arnold and Herre 2003), two different planting dates were established in order to obtain more robust and reliable results. The first planting date was in early December 2012, and the second 3 weeks later. After planting, pots were placed in a greenhouse and watered to field capacity every 2 to 3 days. Maximum and minimum temperatures and relative humidity in the greenhouse during the experiments are reported in Supplemental Information 1. When the youngest plants were 1 month old, all of them were treated with the systemic fungicide AMISTAR XTRA® (Syngenta, Madrid). Two additional treatments, ten days apart, were also applied. In each treatment, around 1 mL per pot of a solution obtained by adding 1 mL of fungicide to 1 L of distilled water was sprayed as foliar application. In the third application, 1 mL per pot of the solution was additionally applied to the soil substrate.
Inoculations
In order to verify that plants were free of culturable endophytes, just before inoculation, four plants were randomly selected and taken to the laboratory. Plants were then surface-disinfested by immersion for 30 s in 70 % ethanol, followed by one immersion for 1 min in 2 % NaClO plus two drops Tween-80 per litre, and washed three times with sterilized distilled water. Then five 5-mm-long segments were cut from different parts of each plant and placed on PDA in a Petri dish. Endophytic colonies were not observed growing out of any of the plant segments. Five weeks after the last fungicide application, plants were wounded by puncturing their leaves and stems with a home-made tool composed of two arms, one of them ending in a multiple needle structure, and the other in a smooth surface (Supplemental Information 2). In this way plants were wounded sufficiently to facilitate fungal infection without serious plant damage. For fungal inoculation of the wounded plants, the actively growing mycelium of each endophyte was homogenized with the culture medium by blending. Inoculation was carried out with the aid of a hand sprayer in two doses: one half of the homogenized inoculum (i.e., 500 mL) just after plant wounding, and the other half 3 days later. During the 48 h following the inoculations, plants were maintained in a high humidity atmosphere in order to maximize fungal infection. Ten pots or experimental units (containing five plants each) per planting date were inoculated with each of the endophytes. Ten additional pots or experimental units per planting date were inoculated only with culture medium to be used as control.
Greenhouse experiment
One half of the pots containing inoculated plants, i.e., five pots per endophyte and planting date (a total of 70 pots), were arranged in the greenhouse following a completely randomized design. Pots were placed on greenhouse benches separated at least 5 cm apart, without any direct contact, to avoid secondary infections. In order to check the effectiveness of the inoculations, approximately 1 month later, plant samples of each treatment were taken to the laboratory for re-isolations following the procedure described above. After the re-isolation process, the six endophytes were positively re-isolated and identified in culture medium. This might mean that the inoculation method was sufficiently effective to cause infection, and consequently the differences observed between treatments could be attributed to the inoculation. Three months after inoculations (i.e., early May 2013), herbage and roots were harvested and taken to the laboratory for processing. Roots were carefully washed with tap water to remove soil substrate.
Field experiment
Approximately 1 month after the inoculations, the second half of the pots were transported to an experimental area located in the Dehesa “Valdesequera” owned by the regional government of Extremadura. This Dehesa is located in Badajoz, south-west Spain (UTM Coordinates, Zone 29 North Datum: X = 685,365 m; Y = 4,325,603) in an Alfisol Xeralft soil (according to USDA classification). The soil of the study site had a sandy loam texture, determined gravimetrically on four representative soil samples taken before transplanting at 30 cm depth, which is the usual rooting depth of the clover (Pearson and Jacobs 1995). On these soil samples, all the edaphic characteristics were determined as explained above for the substrate samples. During the experiment, the climatic data, which were taken from a weather station located close to the study site, were the following: mean temperature 11.3, 14.3, and 16.9 °C; for March, April and May, respectively. Monthly precipitation was 190.5, 22.1, and 27.6 mm for March, April and May, respectively (Supplemental Information 1).
Before transplanting, conventional tillage was applied to prepare an appropriate environment for plants. Transplanting was made in late February and the experimental units (a set of five plants) were arranged by following a completely randomized design with a planting layout of 50 cm × 50 cm. Two months and a half after transplanting (i.e., mid-May 2013), herbage was harvested and taken to the laboratory for processing.
Response-variables determinations
In the laboratory, plant samples were oven dried (70 °C) until constant weight and then dry matter (DM) of herbage and root biomass production was recorded. From part of the herbage samples, the following parameters were determined: total N content using the Kjeldahl method (Kjeltec™ 8200 Auto Distillation Unit. FOSS Analytical. Hilleroed, Denmark), crude protein (CP) by multiplying the biomass N × 6.25 (protein on average contains 16 % N) as conversion factor (Sosulski and Imafidon 1990), neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) by means of a fiber analyzer (ANKOM 8–98, ANKOM Technology, Macedon, NY), by following the official procedures (AOAC 2006). Another part of the herbage samples was sent to the Ionomics Service of CSIC (Spanish High Centre for Science and Research) for mineral determinations with an ICP-OES as explained above for the soil samples.
Statistical analysis
The effect of the endophyte (seven treatments, including controls) and planting date (young and old), as well as their interaction on herbage dry matter (HDM) and root dry matter (RDM) (RDM only in the greenhouse experiment), nutritive value parameters (CP, NDF, ADF, and ADL) and mineral concentration (Al, B, Ca, Cd, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, and Zn) was evaluated by two-way ANOVA. Fisher’s protected least significant difference (LSD) test for multiple comparison was used when significant differences (P <0.05) were found in the ANOVA. In order to normalize variable distribution and to stabilize the variance of residuals the following variable transformation was performed: Ln (x + 1) for Al, B, Cu, Fe, K, Li, Mn, P, Pb, and Zn in the greenhouse experiment, and Fe, Mn, and Zn in the field experiment; 2√x for HDM in the field experiment; 3√x for HDM and RDM in the greenhouse experiment, and Cd, Li, Na, P, and Pb in the field experiment; and 5√x for Cd, Cr, Mo, and Ni in the greenhouse experiment, and ADL, Al, Cr, Mo, Ni, and Se in the field experiment. All these analyses were performed with the Statistix v. 8.10 package.
Results
Differences between experimental soils
Both types of soils, i.e., the substrate used in the greenhouse experiment and the soil used in the field experiment, presented quite low pH values (4.43 ± 0.01 and 5.35 ± 0.12, respectively). However, electrical conductivity (EC) was 17-fold higher in the field soil than in the greenhouse substrate. Because the commercial substrate had been amended with NPK fertilizer and lime, the values of total N, Ca and P and extractable Ca and K were much higher in the greenhouse soil in comparison with the field soil (Supplemental Information 3). Regarding essential micronutrients for plants and/or livestock, such as Fe, Li, Se or Zn, or toxic elements, such as Al or Pb, the field soil presented much higher values than the greenhouse substrate (Supplemental Information 3).
Effects on biomass yield (herbage and root)
Endophyte inoculation, regardless of the species, did not cause plant disease symptoms during the experiment. In the greenhouse experiment, herbage yield was significantly affected (P <0.05) by the two main effects considered (endophyte treatment and planting date) but not by the interaction (Table 2). The inoculation with the endophytes E631 (Epicoccum nigrum) and E202 (Chaetosphaeronema sp.) prompted the highest herbage production in the T. subterraneum forage, although without significant differences compared to the control plants. Conversely, two endophytes E244 (Pleosporales) and E140 (Stemphylium globuliferum) caused a significant reduction in herbage yield in comparison with controls (Fig. 1 left). In the case of root dry matter, the main effect of the endophyte treatment and the interaction between planting date and endophyte treatment were variables with a significant effect in the greenhouse experiment (Table 2). In this case, the endophytes E071 (Sordaria fimicola), E631 (Epicoccum nigrum) and E202 (Chaetosphaeronema sp.) yielded the highest root production in plants derived from the later planting date, i.e., those inoculated at an earlier growth stage and considered as ‘young’; whilst again E202 produced the highest root biomass in plants from an earlier planting date (considered as ‘old’) (Supplemental Information 4). Regarding the effect of planting date, plants considered as ‘old’ had a higher herbage production than ‘young’ plants (2.57 and 1.66 g per experimental unit, respectively) (Table 3).
Herbage dry matter of T. subterraneum forage when inoculated with each endophyte in the greenhouse experiment (left) and in the field experiment (right). Horizontal bars indicate means and horizontal lines standard error. For each experiment, averages with the same letter are not significantly different according to LSD test at a significance level of 0.05. Although the LSD test was performed on transformed variables, back-transformed values are represented to ease interpretation
In the field experiment only herbage biomass (HDM) was studied. In this case, the ANOVA showed a significant influence of the endophyte on herbage production (Table 2). In those field conditions, plants inoculated with the endophyte E202 (Chaetosphaeronema sp.) had higher herbage production compared with any other treatment, including controls (Fig. 1 right).
Effects on nutritive value parameters
In the greenhouse experiment, only the two main effects, endophyte treatment and planting date (age), affected significantly (P <0.05) neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) obtained in herbage (Table 2). In the case of NDF and ADF, none of the endophytes caused an increase of these parameters in the forage in comparison with controls. Conversely, inoculation with the endophytes E244 (Pleosporales) and E140 (S. globuliferum) provided forage with lower values of NDF and ADF, respectively, in comparison with those obtained in the controls (Fig. 2). By contrast, in the case of ADL, forage derived from plants inoculated with the endophyte E060 (F. lateritium) had significantly higher lignin values than those found in the controls (5.9 and 3.9 %, respectively) (Fig. 2). Regarding the effect of planting date, plants considered as ‘old’, i.e., those derived from the earliest planting date which were inoculated at a later growth stage, showed higher values of NDF, ADF and ADL (36.86, 25.39 and 4.60 %, respectively) in comparison with ‘young’ plants, i.e., those derived from the latest planting date (31.55, 21.73 and 3.88 %, respectively) (Table 3).
Quality parameters (CP: crude protein; NDF: neutral detergent fiber; ADF: acid detergent fiber; ADL: acid detergent lignin) of T. subterraneum forage when inoculated with each endophyte in the greenhouse and in the field experiments. Vertical bars indicate means and vertical lines standard error. For each parameter, averages with the same letter (lowercase letters in the greenhouse experiment and uppercase letters in the field experiment) are not significantly different according to LSD test at a significance level of 0.05. NS: Not significant according to ANOVA at a significance level of 0.05. Although the LSD test was performed on the transformed variable in the case of ADL, back-transformed values are represented to ease interpretation
In the field experiment, in the case of crude protein, although there were no significant differences between endophytes and/or planting dates (Table 2), values of CP were much higher than those obtained in the greenhouse experiment (17.9 ± 0.5 and 8.9 ± 0.3 %, respectively). Similar to the greenhouse experiment, the main effect of endophyte had a significant influence (P <0.05) on NDF, ADF and ADL (Table 2). None of the endophytes caused a reduction in any of these parameters in the forage in comparison with controls. By contrast, the inoculation with two endophytes, E060 (F. lateritium) and E244 (Pleosporales) provided forage with higher values of NDF, ADF and ADL than those of controls. Additionally, endophyte E140 (S. globuliferum) produced herbage with the highest values of ADL (Fig. 2). The planting date (age) only affected significantly ADL (Table 2). In this case, plants considered as ‘old’ had a higher ADL than ‘young’ plants (5.17 and 2.87 %, respectively) (Table 3).
Uptake and accumulation in the herbage of the different minerals
In the greenhouse experiment, inoculation with an endophyte significantly affected the concentration in plants of Al, Ca, Cd, Cr, Cu, Fe, K, Mn, Mo, Na, Pb and Zn (Table 2). Plants inoculated with endophyte E060 (F. lateritium) had a lower concentration of Al, Cr and Fe (approximately 66, 82 and 45 % lower, respectively) and a higher concentration of Pb (more than 3-fold higher) compared to the control (Table 4). Likewise, Stemphylium globuliferum (E140) caused a higher accumulation in the forage of Ca, Cd, Cu, Mn, Pb and Zn (more than 31, 217, 66, 14, 305 and 60 %, respectively) and lower concentrations of Al, Cr and Na (approximately 73, 88 and 33 % lower, respectively) in comparison with controls (Table 4). The concentration of Al was also lower (60 and 73 %, respectively) in plants inoculated with endophytes E202 (Chaetosphaeronema sp.) and E244 (Pleosporales) than in non-inoculated controls. However E244 also caused a lower accumulation of Fe (48 % lower) and a higher concentration of K and Pb (more than 72 and 225 % higher) compared to the control (Table 4). Finally, E631 (E. nigrum) provided forage with lower concentrations of Cd, Fe, Mo and Pb (100, 43, 82 and 80 %, respectively) in comparison with control plants. When a significant influence of planting date in the concentration of minerals was found (Table 2), in all cases it was higher in ‘young’ plants than in ‘old’ plants (Table 3).
In the field experiment, the concentration of minerals, but only in the case of Al, B, Cu, Fe, Li, Pb and Zn, was significantly affected only by the main effect endophyte inoculation (Table 2). The pattern of the observed effect was similar for Al, Fe and Li: E140 (S. globuliferum) provided forage with the highest values of those minerals and E071 (S. fimicola) with the lowest values, but in both cases without differences compared to the control plants (Table 4). For B and Cu, while none of the endophytes increased their concentration in the forage in comparison with controls, E140 (S. globuliferum) caused lower accumulation of both than in controls. S. globuliferum also provided the highest values of Pb in the forage. Finally, the highest values of Zn in the forage were provided by E202 (Chaetosphaeronema sp.), although without differences with controls, and the lowest by E244 (Pleosporales) (Table 4).
Discussion
The influence of endophytes on herbage and root biomass productivity has already been demonstrated in several hosts (Hesse et al. 2005; Thom et al. 2013; Mahendra et al. 2014). However, most of those experiments were performed on grasses using clavicipitaceous fungi, especially Epichloë/Neotyphodium species. The use of non-clavicipitaceous fungal endophytes for this purpose has been less frequently reported, and to our knowledge the interaction between subterranean clover and any of the fungal endophytes used in the present work, has not been previously studied. In the field experiment of our study, plants inoculated with the endophyte E202 (Chaetosphaeronema sp.) produced around 80 % more herbage as compared to the controls. In the greenhouse experiment, although plants inoculated with this endophyte also showed a high herbage production, there were no differences with control plants (Table 5). The higher adaptive value that endophytes can give to plant host (Malinowski and Belesky 2000) may explain why in less favourable environments, such as that found in the field in comparison with the greenhouse, the eventual effect of a microorganism-plant interaction might become more evident. Thus, many studies carried out under non-limiting conditions for plant growth (Fritz and Collins 1991; Faeth et al. 2010) have shown a neutral or negative effect of the endophyte on biomass yield.
To explain this higher herbage yield in infected plants, several authors have proposed that endophytes could cause a lengthening of the vegetative growth period (Zabalgogeazcoa et al. 2006), which is an important factor governing biomass production and accumulation. However, the mechanisms involved in this lengthened growth period are not fully known. In some cases endophytic infections could prevent or delay spike emergence, thus postponing the beginning of the reproductive period, in which the biomass production is considerably reduced. Other authors such as Assuero et al. (2006) have suggested that endophyte presence may promote plant growth by producing some hormone-like substances which could delay the growth cycle of the plant, or it may promote root growth giving plants a higher capacity to absorb water and minerals. This hypothesis is not strongly supported by our data because, although the endophytes which caused the highest herbage production tended also to produce the largest root system, differences with controls were not significant. Fungal endophytes have also been found to affect photosynthesis and CO2 fixation (Newman et al. 2003; Spiering et al. 2006), which could also explain the different growth rate. However further research should be done to determine whether our endophytes are capable of producing such an effect.
Bearing in mind that the quality of a forage in terms of nutritive value is directly proportional to its protein content and its digestibility (which is inversely correlated to fiber and lignin content), in the present study none of the endophytes was able to improve in general terms such quality in comparison with the controls. Several positive aspects of forage quality derived from the inoculation with endophytes can be found in the present study, but they were not consistent throughout the experiments (Table 5). For instance, in the greenhouse experiment, the endophyte E244 (Pleosporales) was able to reduce the neutral detergent fiber (NDF) content of the forage, but this effect was not consistent in the field experiment. Also in the greenhouse experiment, endophyte E140 (S. globuliferum) reduced acid detergent fiber (ADF), but in the field experiment the inoculation with this fungus provided forage with the highest values of lignin (ADL). The most interesting endophytes, at least from a herbage-yield promotion point of view, such as endophyte E202 (Chaetosphaeronema sp.), did not cause a deterioration in the quality of the forage, as the values of protein, fiber and lignin were similar to those of controls in both greenhouse and field experiments (Table 5).
Scarce information, and mainly focused on clavicipitaceous endophytes in grasses species, exists on the influence of endophytes on forage quality traits such as crude protein (CP) or neutral detergent fiber (NDF) content in the biomass. Even so, the lack of influence of endophytes on total CP content obtained in the present study had been previously reported by Oliveira et al. (2004) in perennial ryegrass. However, a significant effect of the endophyte infection, in this case on tall fescue, on total CP content and on distribution of the different CP fractions, was found by Newman et al. (2003). Regarding fiber, Zabalgogeazcoa et al. (2006) reported a significant reduction in NDF in grass biomass when an endophyte (Epichloë sp.) was present in plants. Another study (Cripps and Edwards 2013) on the faeces of animals fed with endophyte-infected forage also reported a reduction in NDF. This reduction in NDF could be explained by the production of hemicellulolytic enzymes by endophytes, as has already been reported in maize by Bischoff et al. (2009) working with the endophyte Acremonium zeae. However this was not observed consistently in our study. This lack of consistency between experiments (Table 5) and this lack of strong influence of endophytes on quality traits of forage could be explained by our environmental conditions, which might not be the most suitable to prompt such an effect. It has frequently been reported that significant effects of fungal endophytes might occur only under particular conditions, such as drought stress (Hesse et al. 2005) or high nutrient conditions (Newman et al. 2003), or if the proper combination of endophyte species, host genotype and environmental conditions takes place (Ahlholm et al. 2002).
Regarding mineral concentration contained in the herbage, there was also an evident lack of consistency between the results obtained in the greenhouse experiment and those in the field experiment (Table 5), which could be partially explained by the widely different concentration of these minerals in the substrate and soil where the experiments were carried out respectively. Such lack of consistency, however, was not evidenced to the same extent in all the minerals and with all the endophytes. Regarding Al, as the substrate/soil values were much higher in the field than in the greenhouse, the Al concentration in plants was also much higher under field conditions. Aluminium is not essential for plants and animals. High concentrations of Al in soil, which mainly occurs with low pH (such as that found in the present study) might be toxic for plants, might reduce the uptake of P and Ca (Huang et al. 1992) and might have negative effects on lambs’ appetite (Krueger et al. 1985). Consequently in those conditions, a reduction of Al uptake and later accumulation in forage may be highly desirable. Three endophytes, E140 (S. globuliferum), E202 (Chaetosphaeronema sp.) and E631 (E. nigrum), were able to reduce Al in forage in the greenhouse experiment, but not in the field. Therefore, those species might not be completely suitable for this purpose. However, there were two species, E060 (F. lateritium) and E244 (Pleosporales) which were able two reduce Al concentration in both experiments (although in field conditions, not significantly in comparison with controls) and thus may deserve further experimentation in specific assays including different Al concentrations in soil.
Fusarium lateritium has been previously reported as an endophyte in wild grasses (Azliza et al. 2014), but also as a pathogen in several woody crops (Yun et al. 2013). Although in our study, inoculation with this species did not cause any disease symptoms, this pathogenic character, though small, could have been the cause of the lower accumulation of Cr and Fe, both essential nutrients for plant and animals. However, inoculation with F. lateritium also caused a higher accumulation of Pb in forage. Lead is one of the most frequently reported causes of poisoning in farm livestock, cattle being the most commonly affected species (Suttle 2010). Consequently, the effect caused by this fungal species, but also by E140 (S. globuliferum) and E244 (Pleosporales), might be clearly negative if this biomass is used with a feeding purpose. However such an effect could also have positive applications, for instance, it could be used in phytoremediation programs to gradually clean lead-contaminated areas, e.g., due to lead-mining activity, by means of subsequent culturing and removal of endophyte-infected plants. S. globuliferum was the endophyte which caused this effect most consistently, as was clearly observed under both greenhouse and field conditions. Conversely, endophyte E631 (E. nigrum) seemed to reduce the Pb values of the forage, but this effect was only observed under greenhouse conditions where the Pb levels in soil were much lower. The suitability and real potential of this eventual application of endophytes should be contrasted and further investigated in lead-contaminated areas or by designing specific experiments with different lead concentrations.
Several of the endophytes used in the present study, such as S. globuliferum or F. lateritium, have been reported to be pathogenic in several plant species where they produce metabolites derived from their activity, which can have phytotoxic effects (Debbab et al. 2009; Yun et al. 2013). For a future eventual application of endophytes in the field, farmers a priori may be reluctant to use fungal species reported as pathogens in other hosts. However, in our study, the inoculation of these fungi did not cause any symptoms of disease in T. subterraneum plants, which may indicate a lack of pathogenicity of the isolates used. This is an important basic requirement for an eventual application of these fungal endophytes. An additional basic requirement is that endophytes do not produce toxic forage for livestock. This fact should be further investigated as several associations of fungal endophyte-plant host have been shown to produce secondary metabolites, such as lolitrem B, ergovaline, peramine, etc., which can cause animal diseases (Fuchs et al. 2013; Thom et al. 2013).
Another interesting aspect regarding the influence of Stemphylium globuliferum was the increase in the accumulation of Zn in forage when the fungus was inoculated. Zinc, which is an essential micronutrient for humans and animals, is taken insufficiently in approximately 30 % of the world (Hotz and Brown 2004) because of the deficient Zn concentration in soils of many areas. Soils with Zn concentration lower than 25 mg kg−1, such as those of the present study, are considered unsuitable to provide crops with sufficient Zn to accomplish intake requirements (Alloway 2009). Under these conditions, the use of fungal endophytes, which may increase Zn concentration in forage, could be a very promising strategy to remedy such deficiency and may deserve further research. In our study, the positive effect of Zn accumulation due to S. globuliferum was only observed in the greenhouse experiment, but not in the field. This fact could be explained by the three-fold higher Zn concentration in soils of field experiment in comparison with the substrate of greenhouse experiment. Because as has already been stated, in the microorganism-plant interaction the more unfavourable conditions for plants are, the more evident the observed effect (if any) might be.
In conclusion, the results presented here provide evidence that non-clavicipitaceous endophytes cause significant effects on the herbage production, nutritive value and mineral concentration of Trifolium subterraneum forage. The suitability of each endophyte may depend on the effect required. Of the endophytes studied, E202 (Chaetosphaeronema sp.) should be used to increase forage productivity. If a reduction in Al or Pb concentration is required, then either Fusarium lateritium or E244 (Pleosporales) and Epicoccum nigrum, respectively might be more adequate. An increase in essential nutrients for both plants and animals, such as Zn, was mainly produced by Stemphylium globuliferum.
References
Ahlholm JU, Helander M, Lehtimäki S, Wäli P, Saikkonen K (2002) Vertically transmitted fungal endophytes: different responses of host-parasite systems to environmental conditions. Oikos 99:173–183
Alloway BJ (2009) Zn in the soils and crop nutrition. International Zn Association
AOAC (2006) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Arlington
Arnold AE, Herre EA (2003) Canopy cover and leaf age affect colonization by tropical fungal endophytes: ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 95:388–398
Assuero SG, Tognetti JA, Colabelli MR, Agnusdei MG, Petroni EC, Posse MA (2006) Endophyte infection accelerates morpho-physiological responses to water deficit in tall fescue. N Z J Agric Res 49:359–370
Ates S, Lucas RJ, Edwards GR (2013) Effects of stocking rate and closing date on subterranean clover populations and dry matter production in dryland sheep pastures. N Z J Agric Res 56:22–36
Azliza IN, Hafizi R, Nurhazrati M, Salleh B (2014) Production of major mycotoxins by Fusarium species isolated from wild grasses in Peninsular Malaysia. Sains Malays 43:89–94
Bischoff KM, Wicklow DT, Jordan DB, de Rezende ST, Liu S, Hughes SR, Rich JO (2009) Extracellular hemicellulolytic enzymes from the maize endophyte Acremonium zeae. Curr Microbiol 58:499–503
Bolger TP, Turner NC, Leach BJ (1993) Water use and productivity of annual legume-based pasture systems in the south-west of Western Australia. In: Baker MJ (ed) Proceedings of the XVII international grassland congress I. New Zealand Grassland Association, Palmerston North, pp 274–275
Bremner JM (1996) Nitrogen total. In: Sparks DL (ed) Methods of soil analysis, part 3: chemical methods. Soil Science Society of America, Madison, pp 1085–1121
Conning SA, Renton M, Ryan MH, Nichols PGH (2011) Biserrula and subterranean clover can co-exist during the vegetative phase but are out-competed by capeweed. Crop Pasture Sci 62:236–247
Cripps MG, Edwards GR (2013) Fungal endophytes of a forage grass reduce faecal degradation rates. Basic Appl Ecol 14:146–154
Debbab A, Aly AH, Edrada-Ebel R, Wray V, Müller WE, Totzke F, Zirrgiebel U, Schächtele C, Kubbutat MH, Lin WH, Mosaddak M, Hakiki A, Proksch P, Ebel R (2009) Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J Nat Prod 72:626–631
Dennis SB, Allen VG, Saker KE, Fontenot JP, Ayad JYM, Brown CP (1998) Influence of Neotyphodium coenophialum on copper concentration in tall fescue. J Anim Sci 76:2687–2693
Dudeja SS, Giri R, Saini R, Suneja-Madan P, Kothe E (2012) Interaction of endophytic microbes with legumes. J Basic Microbiol 52:248–260
Faeth SH, Hayes CJ, Gardner DR (2010) Asexual endophytes in a native grass: radeoffs in mortality, growth, reproduction, and alkaloid production. Microb Ecol 60:496–504
Ferreira de Souza G, Galerani Mossini SA, Arrotéia CC, Kemmelmeier C, Machinski Junior M (2014) Evaluation of the mycoflora and aflatoxins from the pre-harvest to storage of peanuts: a case study. Acta Sci 36:27–33
Frame J, Charlton JFL, Laidlaw AS (1998) Temperate forage legumes. CAB International, Wallingford
Fritz JO, Collins M (1991) Yield, digestibility, and chemical composition of endophyte free and infected tall fescue. Agron J 83:537–541
Fuchs B, Krischke M, Mueller MJ, Krauss J (2013) Peramine and lolitrem B from endophyte-grass associations cascade up the food chain. J Chem Ecol 39:1385–1389
García-Ciudad A, García-Criado B, Rico Rodríguez M, García-Criado L (1985) Composición química y digestiva de cultivares de tréboles subterráneos. Pastos 15:41–52
García-Criado L, Rico Rodríguez M, García-Ciudad A, García-Criado B (1986) Introducción de cultivares de trébol subterráneo, producción y calidad. Anuario del Centro de Edafología y Biología Aplicada (CSIC Salamanca) 13:247–260
Hall SL, McCulley RL, Barney RJ, Phillips TD (2014) Does fungal endophyte infection improve tall fescue’s growth response to fire and water limitation? PLoS One 9:e86904
Hesse U, Schöberlein W, Wittenmayer L, Förster K, Warnstorff K, Diepenbrock W, Merbach W (2005) Influence of water supply and endophyte infection (Neotyphodium spp.) on vegetative and reproductive growth of two Lolium perenne L. genotypes. Eur J Agron 22:45–54
Hotz C, Brown KH (2004) Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:91–204
Huang JW, Grunes DL, Kochian LV (1992) Aluminum effects on the kinetics of calcium uptake into cells of the wheat root apex. Planta 188:414–421
Impullitti AE, Malvick DK (2013) Fungal endophyte diversity in soybean. J Appl Microbiol 114:1500–1506
Krueger GL, Morris TK, Suskind RR, Widner EM (1985) The health effects of aluminium compounds in mammals. CRC Crit Rev Toxicol 13:1–24
Kyriazopoulos AP, Abraham EM, Parissi ZM, Koukoura Z, Nastis AS (2012) Forage production and nutritive value of Dactylis glomerata and Trifolium subterraneum mixtures under different shading treatments. Grass Forage Sci 68:72–82
Mahendra R, Dnyaneshwar R, Gauravi A, Mudasir D, Brestic M, Pastore GM, Marostica Junior MR (2014) Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis 62:63–79
Malinowski DP, Belesky DP (1999) Tall fescue aluminum tolerance is affected by Neotyphodium coenophialum endophyte. J Plant Nutr 22:1335–1349
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Monnet F, Vaillant N, Vernay P, Coudret A, Sallanon H, Hitmi A (2001) Relationship between PSII activity, CO2 fixation, and Zn, Mn and Mg contents of Lolium perenne under zinc stress. Aust J Plant Physiol 158:1137–1144
Newman JA, Abner ML, Dado RG, Gibson DJ, Brooking A, Parsons AJ (2003) Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: growth, photosynthesis, chemical composition and digestibility. Global Chang Biol 9:425–437
Oliveira JA, González E, Castro P, Costal L (2004) Effects of endophyte infection on dry matter yield, persistence and nutritive value of perennial ryegrass in Galicia (north-west Spain). Span J Agric Res 2:558–563
Pearson CJ, Jacobs BC (1995) Root distribution in space and time in Trifolium subterraneum. Aust J Agric Res 36:601–614
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Romeralo C, Santamaria O, Pando V, Diez JJ (2015) Fungal endophytes reduce necrosis length produced by Gremmeniella abietina in Pinus halepensis seedlings. Biol Control 80:30–39
Saul GR, Kearney GA, Flinn PC, Lescun CL (1999) Effects of superphosphate fertiliser and stocking rate on the nutritive value of perennial ryegrass and subterranean clover herbage. Aust J Agric Res 50:537–545
Seena S, Sridar KR (2004) Endophytic fungal diversity of two sand dune wild legumes from the southwest coast of India. Can J Microbiol 50:1015–1021
Simpson RJ, Richardson AE, Riley IT, McKay AC, McKay SF, Ballard RA, Ophel-Keller K, Hartley D, O’Rourke TA, Li H, Sivasithamparam K, Ryan MH, Barbetti MJ (2011) Damage to roots of Trifolium subterraneum L. (subterranean clover), failure of seedlings to establish and the presence of root pathogens during autumn–winter. Grass Forage Sci 66:585–605
Sosulski FW, Imafidon GI (1990) Amino acid composition and nitrogento-protein conversion factors for animal and plant foods. J Agric Food Chem 38:1351–1356
Spiering MJ, Greer DH, Schmid J (2006) Effects of the fungal endophyte, Neotyphodium lolii, on net photosynthesis and growth rates of perennial ryegrass (Lolium perenne) are independent of In Planta endophyte concentration. Ann Bot 98:379–387
Suttle NF (2010) Mineral nutrition of livestock, 4th edn. CABI Publishing, Wallingford
Thom ER, Popay AJ, Waugh CD, Minneé EMK (2013) Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass Forage Sci 69:191–204
Vázquez-de-Aldana BR, García-Ciudad A, Garcíia-Criado B, Vicente-Tavera S, Zabalgogeazcoa I (2013) Fungal endophyte (Epichloe festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS One 8:e84539
Yang B, Wang X-M, Ma H-Y, Jia Y, Li X, Dai C-C (2014) Effects of the fungal endophyte Phomopsis liquidambari on nitrogen uptake and metabolism in rice. Plant Growth Regul 73:165–179
Yun HY, Lee YW, Kim YH (2013) Stem canker of giant dogwood (Cornus controversa) caused by Fusarium lateritium in Korea. Plant Dis 97:1378
Zabalgogeazcoa I (2008) Fungal endophytes and their interaction with plant pathogens. Span J Agric Res 6:138–146
Zabalgogeazcoa I, García-Ciudad A, Vázquez-de-Aldana BR, García-Criado B (2006) Effects of the infection by the fungal endophyte Epichloë festucae in the growth and nutrient content of Festuca rubra. Eur J Agron 24:374–384
Acknowledgments
This study was funded by Project AGL2011-27454, granted by the Ministry of Economy and Competitiveness of Spain (the former Ministry of Science and Innovation) and by the European Regional Development Fund (ERDF). We would like to thank Teodoro García-White, Natalia Hernández and Pablo Romero for their invaluable help with the technical work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
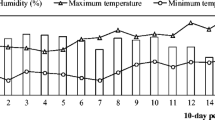
Supplemental Information 1
Mean relative humidity or monthly precipitation, and mean maximum and minimum temperatures during the greenhouse (a) and field (b) experiments (PDF 61 kb)
Supplemental Information 2
Home-made tool used for wounding leaves and stems before inoculation (PDF 330 kb)
Supplemental Information 3
Soil substrate (greenhouse experiment) and soil (field experiment) properties expressed as mean ± standard error (SE) from four samples (n = 4) (PDF 23 kb)
Supplemental Information 4
Root dry matter of T. subterraneum as affected by the interaction endophytic isolate*planting date for the greenhouse experiment. Vertical bars indicate means and vertical lines standard error. Averages with the same letter are not significantly different according to LSD test at a significance level of 0.05. Although the LSD test was performed on transformed variable, back-transformed values are represented to ease interpretation (PDF 17 kb)
Rights and permissions
About this article
Cite this article
Lledó, S., Rodrigo, S., Poblaciones, M.J. et al. Biomass yield, nutritive value and accumulation of minerals in Trifolium subterraneum L. as affected by fungal endophytes. Plant Soil 405, 197–210 (2016). https://doi.org/10.1007/s11104-015-2596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2596-0