Abstract
Kentucky bluegrass (Poa pratensis L.) is a very common grass species with a wide distribution, including in Mediterranean areas. The objective of this study was to investigate the effects of non-clavicipitaceous fungal endophytes on forage production by P. pratensis, and on the nutritive value and mineral status of its herbage. Plants were inoculated with each of eight endophytic species and grown under greenhouse conditions. After 3 months, the plants were harvested and dried before evaluating the following parameters: dry mass of herbage and roots, crude protein content, neutral detergent fibre content, and mineral contents in herbage. The results showed that endophytes affected the biomass yield (herbage and roots), fibre content, and mineral contents in the forage. Compared with controls, plants inoculated with Stemphylium globuliferum showed the highest herbage and root biomass values, whilst those inoculated with Embellisia leptinellae and S. globuliferum showed reduced fibre contents in herbage. Plants infected with Epicoccum nigrum and S. globuliferum showed increased herbage concentrations of calcium, magnesium, molybdenum, and titanium. These results demonstrate that inoculation of Poa pratensis with endophytes can increase its biomass yield and the nutritive value of its herbage. Therefore, endophyte inoculation may be a useful strategy to reduce nutrient deficiencies in P. pratensis forage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kentucky bluegrass or smooth-stalked meadow-grass (Poa pratensis L.) is a common perennial grass native to Europe, Asia, North America, and northern Africa. The ecological variability of this grass allows it to adapt to cold winter climates (Reader et al. 1994) and explains its ability to grow in many different conditions and at various latitudes. In Mediterranean areas, for instance, it is a naturalized species commonly found in permanent grasslands, meadows, open woodlands, prairies, and at disturbed sites. Owing to its high adaptive capacity, P. pratensis has been widely cultivated in temperate climates as forage and turf grass. As forage, its herbage yields range from 4100 to 10,400 kg ha−1, depending on the environmental conditions (Dürr et al. 2005). Several studies have focused on differences in the forage quality of P. pratensis (Dürr et al. 2005; Holman et al. 2007). The crude protein (CP) content has been reported to range from 7.6 to 26.6 %, and neutral detergent fibre (NDF) content from 43.5 to 58.7 %. However, in Mediterranean areas, the herbage productivity of P. pratensis is low, despite its high nutritive quality. As a forage crop, this grass species can be sown alone or in mixtures with other grasses or legumes such as white clover (Trifolium repens L.) (Murphy et al. 1997).
Climatic conditions, especially precipitation during the growing season, are the most important factors affecting the growth, biomass production, quality traits, persistence, and regeneration of P. pratensis (Croce et al. 2001). However, soil fertility (Hodge 2004), the plant growth stage (Holman et al. 2007), and the photoperiod (Hay and Heide 1983) can also affect these parameters. Fungal endophytes, which invade the living tissues of plants for part or all their life cycle without causing disease, can also affect the production and performance of different pasture species (Zabalgogeazcoa et al. 2006; Iannone et al. 2011). Endophytes have been shown to have some beneficial effects; for example, increasing the drought resistance of their host (Assuero et al. 2006), protecting their hosts against plant pathogens (Clarke et al. 2006; Santamaría et al. 2012), and improving forage quality (Oliveira et al. 2004). Other studies have shown that biomass production and regrowth capacity are greater in endophyte-infected plants than in uninfected plants (Hesse et al. 2005; Thom et al. 2013), especially when plants are subjected to biotic or abiotic stresses (Assuero et al. 2006; Oberhofer et al. 2014).
The beneficial effects of endophytes could be due to improved adaptive responses and increased effectiveness of physiological and developmental processes in response to water deficit mediated by the plant hormone abscisic acid (Zhang et al. 2006). Endophyte-infected plants growing in non-limiting growth conditions showed increased biomass yield (Iannone et al. 2011; de Lima Fávaro et al. 2012), but such positive effects can be missed because of the profuse growth of both infected and uninfected plants within a population. Consequently, several other studies have reported that under non-limiting conditions, herbage and root biomass yields of the host plants were unaffected or negatively affected by endophyte infection (Zabalgogeazcoa et al. 2008; Faeth et al. 2010).
Infection with the endophyte Neotyphodium resulted in higher antioxidant activity in two grasses, Stipa robusta (Vasey) Scribn. and Festuca arizonica Vasey (Hamilton and Bauerle 2012). Endophytes have also been shown to alter the mineral status of some other forage plants, including Neotyphodium coenophialum (Morgan-Jones & Gams) Glenn, Bacon & Hanlin in Festuca arundinacea Schreber (Matthews and Clay 2001). In another case, endophytes provided a solution for cropping in polluted areas where infected plants can grow and degrade contaminant compounds (Soleimani et al. 2010). Recently, Likar and Regvar (2013) demonstrated the mutualistic relationship between Phialophora endophytes and Salix plants that allowed plants to grow in cadmium-polluted soil. The endophyte-infected plants showed decreased uptake of this contaminant metal.
However, the positive effects of endophyte infection are not always detected, as they appear to be dependent on the species of the endophyte and the host, and on various environmental conditions (Ahlholm et al. 2002). For example, Oliveira et al. (2004) found that Neotyphodium lolii (Latch & Samuels) Glenn, Bacon & Hanlin did not notably affect many forage quality parameters in perennial ryegrass. Some studies have reported negative effects of endophyte infection on plant production or forage quality. For example, Epichloë typhina (Pers.) Tul. & Tul. was shown to cause ear sterility in several grass species (White 1988), and other endophytes, such as Neotyphodium lolii, produce secondary metabolites that cause ryegrass staggers disease in livestock (Fletcher et al. 1993).
Fungal endophytes can be divided into two major groups: the clavicipitaceous and non-clavicipitaceous fungi (Rodriguez et al. 2009). Most studies on plant–endophyte interactions have been conducted on grass species infected with clavicipitaceous fungi (Tanaka et al. 2012). The interactions between endophytes and their hosts appear to differ between clavicipitaceous and non-clavicipitaceous endophytes. Clavicipitaceous endophytes are transmitted vertically through seeds, they usually form a systemic intercellular infection, and their ecological and functional roles are well-defined. In contrast, most non-clavicipitaceous endophytes are horizontally transmitted, are locally restricted within plant tissues after infection, and their ecological roles are poorly defined (Rodriguez et al. 2009). The hypothesis tested in the present study was that non-clavicipitaceous endophytes affect the production and quality performance of Kentucky bluegrass. To test this hypothesis, we evaluated the effects of eight non-clavicipitaceous fungal endophytes on the biomass production (herbage and root biomass), nutritive value (protein and neutral detergent fibre contents), and mineral status of P. pratensis.
Materials and methods
Fungal and plant materials
Eight fungal endophytes that were previously isolated from pasture species and identified in our laboratory were used in these experiments (Table 1). Endophyte identification was based on comparison of the ITS region sequence with sequence data from EMBL/GenBank using BLASTn. A similarity of > 97 % was used for species identification. When several species met this criterion, they were further identified based on their morphology (colony characteristics and reproductive structures). Two months before experiments, fungi were grown at 25 °C in the dark in 1.5-L flasks containing 1-L potato dextrose broth (PDB) to obtain sufficient inoculum for plant inoculations.
Seeds of P. pratensis cv ‘Sobra’ were surface-disinfected by immersion for 2 min in 2.5 % NaClO, then washed three times with sterilized distilled water. In early December 2012, sterilized seeds (ten seeds per pot) were planted in plastic pots (7 × 7 cm, and 6 cm high) containing soil substrate (1:1 vol/vol perlite:peat; COMPO SANA Universal, COMPO GmbH & Co. KG, Münster, Germany). The pots were placed in a greenhouse and watered to field capacity every 2–3 days. The maximum and minimum temperatures and relative humidity recorded in the greenhouse during the experiments are shown in Fig. 1. When plants were 1 month old, they were treated every 10 days with three doses of Amistar Xtra® (Syngenta, Madrid, Spain), a systemic fungicide, applied as a foliar spray (approx. 1 mL per pot of a dilution of 1-mL fungicide in 1-L distilled water). At the third application, 1 mL of fungicide solution was also applied to the soil substrate in each pot.
Four soil substrate samples were analysed to determine their chemical properties. In dried and homogenised soil samples, pH was determined using a calibrated pH meter (CLP22, Crison Instruments SA, Barcelona, Spain) (10 g soil: 25 ml deionized H2O). The dried soil samples were extracted with ammonium acetate (1 N) adjusted to pH 7, and then extractable Ca and K were quantified by atomic absorption spectrophotometry (Helyos alpha, 9423-UVA, Unicam, Cambridge, UK). Extractable P was determined by the Olsen method (Olsen et al. 1954). Electrical conductivity (EC) was determined using an EC-meter (Basic30, Crison Instruments SA, Barcelona, Spain). Total N was determined using the Kjeldahl method (Bremner 1996) (Kjeltec™ K350 destillation Unit, Buchi Ltd., Flawil, Switzerland).
Inoculations and experimental design
Four weeks after the last fungicide application, plants were wounded by puncturing their leaves and stems with a home-made tool with two arms; one with multiple needles at the end, and the other with a smooth surface. In this way, plants were wounded sufficiently to facilitate fungal infection, but were not seriously damaged. The actively growing mycelia of each endophyte were homogenized in culture medium using a blender. Inoculation was carried out using a hand sprayer in two doses: half of the homogenized inoculum (i.e., 500 mL) was applied immediately after wounding the plants, and the other half was applied 3 days later. During the 48 h following inoculation, the plants were maintained under high humidity conditions to maximize fungal infection. Each set of ten plants (i.e., one pot) was inoculated with one endophyte. Plants in additional pots were inoculated only with culture medium as the control. Pots containing inoculated plants were arranged following a completely randomized design with ten replicates (pots) per treatment. To avoid secondary infections, the pots were placed at least 5 cm apart, with no direct contact, on the greenhouse benches. Plant samples collected before inoculation and approximately 1 month after inoculation were analysed to verify that they were free of endophytes before inoculation, and infected with endophytes after inoculation. Plants were surface-disinfested by immersion in 95 % ethanol for 30 s, and then in 2 % NaClO plus Tween 80 (two drops/L) for 1 min. Then, five 5-mm-long segments were cut from different parts of each plant and placed on Potato Dextrose Agar (PDA) in a Petri dish. The Petri dishes were incubated at 25 °C in the dark to allow the fungal endophytes to grow from the plant pieces.
Analyses of biomass, crude protein, biomass nitrogen, neutral detergent fibre, and mineral contents
The herbage and roots were harvested and processed 3 months after inoculation with the fungal endophytes. The roots were carefully washed with tap water to remove soil substrate. The herbage and root samples were air dried to constant weight at 70 °C before recording dry matter (DM). A portion of each herbage sample was used to analyse crude protein (CP). The CP content was calculated as follows: biomass N × 6.25 (protein on average contains 16 % N). Biomass N was determined using the Kjeldahl method, and neutral detergent fibre (NDF) was analysed with a fibre analyser (ANKOM 8-98, ANKOM Technology, Macedon, NY). Another portion of each herbage sample was analysed by the Ionomics Service of the CSIC (Spanish High Centre for Science and Research) to determine mineral determinations. After digestion with HNO3/H2O2 in an UltraClave Microwave (Milestone S.r.l., Sorisole, Italy), samples were analysed by inductively coupled plasma optical emission spectrometry (ICP-OES) to quantify Al, B, Ca, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Se, Sr, Ti, and Zn. These analyses were also conducted for the four soil substrate samples described above (Table 2).
Statistical analyses
The effects of the endophyte (nine treatments, including controls: E0, E060, E138, E140, E202, E244, E269, E361 and E631) on herbage and root dry matter yield, nutritive value parameters (CP, and NDF) and mineral contents (Al, B, Ca, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Se, Sr, Ti, Tl and Zn) were evaluated by one-way ANOVA. Fisher’s protected least significant difference (LSD) test for multiple comparisons was used when significant differences (p < 0.05) were detected in the ANOVA. Assumptions of normality and homoscedasticity were assured by Kolmogorov–Smirnov and Levene’s tests, respectively. Pearson correlation tests were performed among the different parameters. All of these analyses were conducted using Statistix v. 8.10.
Results
Soil substrate properties and effects of endophytes on herbage and root biomass
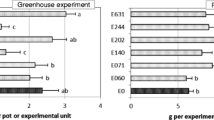
Table 2 summarizes the soil substrate properties. None of the endophytes caused plant disease symptoms during these experiments. No endophytes were detected in plant samples collected before inoculations. Five of the endophytes [E060 (Fusarium lateritium), E140 (Stemphylium globuliferum), E202 (Chaetosphaeronema sp.), E244 (Pleosporales sp.), and E631 (Epicoccum nigrum)] were positively re-isolated 1 month after inoculation. The presence of an endophyte significantly affected (P < 0.05) herbage and root biomass of P. pratensis (Table 3). The dry matter yield was nearly 40 % greater in plants inoculated with endophyte E269 (Penicillium sp.) (1.01 g pot−1) than in the control (0.63 g pot−1; Fig. 2a). The herbage biomass was also significantly greater in plants inoculated with the endophytes E140 (Stemphylium globuliferum), E202 (Chaetosphaeronema sp.), or E361 (unidentified endophyte) than in the control (Fig. 2a). The root biomass was higher in plants inoculated with E202 and E269 (2.17 and 2.41 g pot−1, respectively) than in control plants (1.67 g pot−1; Fig. 2b). None of the studied endophytes caused a reduction in herbage or root biomass (Fig. 2a, b). There was a strong correlation between herbage biomass and root biomass (r = 0.84).
Effect of endophyte infection on a dry matter (DM) of herbage b root DM, c crude protein content, and d neutral detergent fibre (NDF) content. Mean values and standard errors are shown. For each parameter, different letters above bars indicate significant differences (LSD test, p < 0.05). Absence of letters indicates no significant difference among mean values (p > 0.05; ANOVA). E060: Fusarium lateritium; E138: Embellisia leptinellae; E140: Stemphylium globuliferum; E202: Chaetosphaeronema sp.; E244: Pleosporales sp.; E269: CODE269; E361: CODE361; E631: Epicoccum nigrum
Effects of endophyte infection on crude protein and neutral detergent fibre contents
None of the endophytes significantly (p > 0.05) affected the CP content (Table 3). The CP contents ranged from 4.96 to 5.82 % (Fig. 2c). Conversely, the NDF content in plants was significantly (P ≤ 0.05) affected by some endophytes (Table 3). Plants infected with E138 (Embellisia leptinellae), E140 (Stemphylium globuliferum), E202 (Chaetosphaeronema sp.), E244 (Pleosporales sp.), or E269 (Penicillium sp.) showed considerably lower NDF contents (p ≤ 0.05) (approximately 5 % lower) than that in the control plants (56 %, Fig. 2d). None of the endophytes resulted in increased NDF content (Fig. 2c, d).
Effects of endophytes on mineral contents in herbage
The presence of endophytes significantly affected (p ≤ 0.05) the concentrations of Al, B, Ca, Cr, Fe, Li, Mg, Mn, Mo, Ni, P, Se, Sr, Ti, and Zn in herbage (Tables 3 and 4). Plants infected with E631 (Epicoccum nigrum) showed increased herbage concentrations of Al, Mo, and Ti (approx. 6, 2, and 8 times that in control plants, respectively; Table 4), and B, Fe, Li, and Ni (20, 64, 22, and 28 % higher than that in control plants, respectively; Table 4). Plants infected with E361 (unidentified) also showed increased herbage concentrations of B, Ca, Mg, Ni, and Sr (20, 12, 15, 27, and 15 % higher, respectively, than that in control plants; Table 4).
Conversely, plants infected with the endophyte E244 (belonging to the Pleosporales order) showed lower herbage concentrations of Ca, Cr, Ni, P, Se, and Zn, compared with those in control plants (Table 4). Plants inoculated with E. leptinellae (E138) showed decreased herbage concentrations of Cr, Mn, P, and Se (65, 11, 18, and 51 % lower, respectively, than those in control plants) (Table 4). The endophyte E140 (S. globuliferum) resulted in increased herbage concentrations of Ca, Mg, and Sr (0.90 g kg−1, 0.36 g kg−1 and 77.68 mg kg−1, respectively; compared with 0.78 g kg−1, 0.31 g kg−1, and 68.07 mg kg−1, respectively, in control plants). This endophyte also resulted in significantly lower herbage concentrations of Cr and Se (2.70 mg kg−1 and 1.12 mg kg−1, respectively, compared with 6.37 mg kg−1 and 1.73 mg kg−1, respectively, in control plants; Table 4).
Discussion
The lack of disease symptoms in the inoculated plants indicated that the fungi used in these experiments were non-pathogenic, at least for the duration of this experiment. This is an important and basic requirement for the eventual application of fungal endophytes in the field. We failed to re-isolate three of the eight endophytes, possibly because of the limited efficiency of culture-dependent re-isolation methods (Prior et al. 2014), rather than inefficient inoculation. All of the endophytes, even those that could not be re-isolated (E138: Embellisia leptinellae, E269: Penicillium sp., and E361: unidentified species), significantly affected (p ≤ 0.05) at least one of the evaluated parameters in P. pratensis. This suggested that the inoculation method had been sufficiently effective to achieve infection, as a failed inoculation would not be expected to result in a significant effect. The significant effects observed in this study could be a result of the endophyte growing internally in, or externally on the plant, or result from products produced by the endophyte. Nevertheless, the results obtained for the three endophytes that could not be re-isolated should be considered cautiously until effective inoculation can be confirmed in further experiments.
In our study, plants infected with the endophytes E269 (Penicillium sp.), E361 (unidentified species), E140 (Stemphylium globuliferum), or E202 (Chaetosphaeronema sp.) showed the greatest herbage biomass yields. Clavicipitaceous fungi have been shown to lengthen the vegetative growth period of their host (Zabalgogeazcoa et al. 2006), which may explain the higher herbage yield in infected plants in the present study. Further research should be conducted to determine whether non-clavicipitaceous endophytes affect the length of the growth stage of their hosts. Assuero et al. (2006) suggested that endophytes may promote plant growth by producing hormone-like substances that delay the growth cycle of the plant or promote root growth, which would increase the water and mineral absorption capacity of the plant. Our data showed that some of the endophytes positively affected root growth—the plants infected with E269 (Penicillium sp.) or E202 (Chaetosphaeronema sp.) produced significantly (p ≤ 0.05) increased herbage yields and also had considerably (p ≤ 0.05) enlarged root systems, compared with those of controls. The plants infected with E361 (unidentified species) and E140 (Stemphylium globuliferum) showed the highest herbage production and also tended to produce large root systems, although not significantly larger than that of the control. Fungal endophytes have been shown to affect photosynthesis and CO2 fixation (Spiering et al. 2006), which could also explain the differences in growth rate. However, further research should be conducted to determine whether the endophytes used here have such effects on their hosts.
Several studies have focused on fungal endophytes’ production of secondary metabolites that cause animal diseases. Such metabolites include lolitrem B, ergovaline, and peramine (Oliveira et al. 2003; Fuchs et al. 2013; Thom et al. 2013). However, fewer studies have focused on the effects of endophytes on other forage quality traits, such as CP and NDF contents. Our results showed that none of the endophytes affected the total CP content in herbage, consistent with the results reported by Oliveira et al. (2004) for endophyte-infected perennial ryegrass. Zabalgogeazcoa et al. (2006) reported a significant reduction in NDF in the herbage of a grass infected with an Epichloë species. Another study reported that the NDF content in faeces was lower for animals feeding on endophyte-infected forage than for those feeding on uninfected forage (Cripps and Edwards 2013). In our study, two endophytes resulted in decreased NDF contents in herbage. This may have been due to the production of hemicellulolytic enzymes by endophytes, as was reported to occur in Acremonium zeae infecting maize (Bischoff et al. 2009). It has been also proposed that endophytes might delay plant maturity, thus prolonging the vegetative growth phase of the host (Zabalgogeazcoa et al. 2006). This could also explain the NDF contents in infected plants, because fibre and lignin contents in forage increase with plant maturity (Santamaría et al. 2014). However, a delay in maturity could also be expected to increase the CP content in forage, which was not observed in our study. Further experiments on the effects of endophytes on the lengths of plant growth phases should be conducted to test this hypothesis.
The various endophytes had different effects on mineral contents in the herbage. Three endophytes resulted in increased concentrations of several minerals in herbage (E060, E361, E631); four endophytes resulted in decreased contents of some minerals in herbage (E138, E202, E244, E269); and one resulted in increased contents of some minerals and decreased contents of others in herbage (E140). Those that increased the contents of some minerals may have promoted root growth, resulting in greater mineral absorption capacity of the host, as reported by Assuero et al. (2006). However, our data did not support this hypothesis, because none of the endophytes that resulted in increased mineral concentrations in herbage resulted in larger root systems than those of control plants. Therefore, further research should be conducted to clarify the mechanisms involved in increased mineral contents in herbage. The decreased mineral contents in herbage caused by several of the endophytes could be explained by pathogenic behaviour; that is, negative effects of the fungus on metabolic and physiological processes in the host. We did not observe disease symptoms in any of the endophyte-infected plants, but low-level pathogenicity could cause small changes leading to lower accumulation of minerals such as Cr, Mn, P, and Se in the herbage of P. pratensis. Two of the endophytes that caused decreases in mineral contents in this study have been reported to be pathogens of other hosts, including Stemphylium globuliferum (Debbab et al. 2009) and Embellisia leptinellae (Lawrence et al. 2011). However, the other two endophyte species (E202 and E269) that resulted in decreased mineral contents in this study have not been reported as pathogens in other studies. A dilution effect might explain the decreased concentrations of some minerals; that is, these two endophytes resulted in increased herbage production compared with that of the control.
We observed that the endophyte F. lateritium resulted in increased concentrations of several minerals in P. pratensis herbage. However, this fungus has been reported to be a pathogen that causes diseases in several woody hosts such as common ash in apple trees (Przybył 2002; Weber and Dralle 2013). However, it has been reported to be an endophyte of Taxus baccata (Strobel et al. 1996), and its presence was detected in several wild grasses in Malaysia (Azliza et al. 2014). There have been more studies on the effects of F. lateritium on woody species than on pasture species. We observed that F. lateritium inoculation had no negative effects on P. pratensis plants, and that it increased Fe, Ni, and Zn contents in herbage by 31, 32, and 16 %, respectively, compared with those in the control. All of these are essential minerals for animals (Underwood 1981) and increased levels in forage crops would be desirable. For example, forage with a 32 % increase in Ni content was shown to greatly improve rumen metabolism (Suttle 2010).
An increase in Fe uptake by endophyte-infected plants has been already reported by other authors, such as Bartholdy et al. (2001) for Phialocephala fortinii Wang & Wilcox and Johnson et al. (2013) for Epichloë/Neotyphodium. This increase in Fe uptake could be due to the production of siderophores, which strongly bind Fe3+, by the endophytes. Johnson et al. (2013) showed that Epichloë/Neotyphodium fungi contain a non-ribosomal peptide synthetase gene (sidN) encoding a siderophore synthetase. In our study, E060 (F. lateritium) and E631 (E. nigrum) resulted in significantly higher Fe contents in herbage, compared with that in the control. It is possible that these two fungi were able to produce siderophores, but further experiments are required to confirm this.
The endophyte E. nigrum is a widespread ascomycete associated with plant primary decomposition and endophytism in a variety of plants that are not taxonomically related (Martini et al. 2009). This fungus is a pathogen of several plants, such as Indian bean (Mahadevakumar et al. 2014), but it is especially known for its biocontrol activity against several pathogens (Li et al. 2013). In a previous study, E. nigrum was shown to increase root growth of sugarcane (de Lima Fávaro et al. 2012). However, to our knowledge, this is the first report that it affects plant mineral uptake. In our study, plants inoculated with E. nigrum showed higher herbage concentrations of Al, B, Fe, Li, Mo, Ni, and Ti, compared with those in plants infected with other endophytes and control plants. The five-fold increase in Al content would not be desirable, owing to the antagonistic metabolism of Al and P, and the negative effects of Al on lambs’ appetite (Krueger et al. 1985).
In conclusion, infection with non-clavicipitaceous endophytes had beneficial effects on the growth of P. pratensis plants and on the nutritive value (NDF content and mineral contents) of its forage. The suitability of each endophyte may depend on the situation. Of the fungi studied, S. globuliferum, E202 (Chaetosphaeronema sp.), E269 (Penicillium sp.), and E361 (unidentified) could be used to increase forage productivity. These fungi (except for E361), as well as E. leptinellae and E244 (belonging to the Pleosporales order), also resulted in increased forage quality. The endophytes S. globuliferum and E. nigrum resulted in increased herbage concentrations of several nutrients that are essential for both plants and animals.
References
Ahlholm JU, Helander M, Lehtimäki S, Wäli P, Saikkonen K (2002) Vertically transmitted fungal endophytes: different responses of host-parasite systems to environmental conditions. Oikos 99:173–183
Assuero SG, Tognetti JA, Colabelli MR, Agnusdei MG, Petroni EC, Posse MA (2006) Endophyte infection accelerates morpho-physiological responses to water deficit in tall fescue. N Z J Agric Res 49:359–370
Azliza IN, Hafizi R, Nurhazrati M, Salleh B (2014) Production of major mycotoxins by Fusarium species isolated from wild grasses in Peninsular Malaysia. Sains Malays 43:89–94
Bartholdy BA, Berreck M, Haselwandter K (2001) Hidroxamate siderophore synthesis by Phialocephala fortinii, a typical dark septate fungal root endophyte. BioMetals 14:33–42
Bischoff KM, Wicklow DT, Jordan DB, de Rezende ST, Liu S, Hughes SR, Rich JO (2009) Extracellular hemicellulolytic enzymes from the maize endophyte Acremonium zeae. Curr Microbiol 58:499–503
Bremner JM, (1996) Chapter 37 –Nitrogen –Total: In Methods of Soil Analysis. Part 3. Chemical Methods. SSSA Book Series no. 5
Clarke BB, White JF, Hurley H, Torres MS, Sun S, Huff DR (2006) Endophyte mediated suppression of dollar spot disease in fine fescues. Plant Dis 90:994–998
Cripps MG, Edwards GR (2013) Fungal endophytes of a forage grass reduce faecal degradation rates. Basic Appl Ecol 14:146–154
Croce P, De Luca A, Mocioni M, Volterrani M, Beard JB (2001) Warm season turfgrass species and cultivar characterizations for a Mediterranean climate. Int Turfgrass Soc Res J 9:855–859
de Lima Fávaro LC, Sebastianes FL, Araújo WL (2012) Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE 7, e36826
Debbab A, Aly AH, Edrada-Ebel R, Wray V, Müller WE, Totzke F, Zirrgiebel U, Schächtele C, Kubbutat MH, Lin WH, Mosaddak M, Hakiki A, Proksch P, Ebel R (2009) Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J Nat Prod 72:626–631
Dürr GH, Kunelius HT, Drapeau R, McRae KB, Fillmore SAE (2005) Herbage yield and composition of Kentucky bluegrass (Poa pratensis L.) cultivars under two harvest systems. Can J Plant Sci 85:631–639
Faeth SH, Hayes CJ, Gardner DR (2010) Asexual endophytes in a native grass: radeoffs in mortality, growth, reproduction, and alkaloid production. Microb Ecol 60:496–504
Fletcher LR, Garthwaite I, Towers NR (1993) Ryegrass staggers in the absence of lolitrem B. In: Hume DE, Latch GCM, Easton HS (eds) Proceedings of the Second International Symposium on Acremonium/Grass Interactions. Palmerston North, New Zealand, pp 119–121
Fuchs B, Krischke M, Mueller MJ, Krauss J (2013) Peramine and lolitrem B from endophyte-grass associations cascade up the food chain. J Chem Ecol 39:1385–1389
Hamilton CE, Bauerle TL (2012) A new currency for mutualism? Fungal endophytes alter antioxidant activity in hosts responding to drought. Fungal Divers 54:39–49
Hay RKM, Heide OM (1983) Specific photoperiodic stimulation of dry matter production in a high latitude cultivar of Poa pratensis. Physiol Plant 57:135–142
Hesse U, Schöberlein W, Wittenmayer L, Förster K, Diepenbrock W, Merbach W (2005) Influence of water supply and endophyte infection (Neotyphodium spp.) on vegetative and reproductive growth of two Lolium perenne L. genotypes. Eur J Agron 22:45–54
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Holman JD, Hunt C, Thill D (2007) Structural composition, growth stage, and cultivar affects on Kentucky bluegrass forage yield and nutrient composition. Agron J 99:195–202
Iannone LJ, Pinget AD, Nagabhyru P, Schardl CL, De Battista JP (2011) Beneficial effects of Neotyphodium tembladerae and Neotyphodium pampeanum on a wild forage grass. Grass Forage Sci 67:382–390
Johnson LJ, Koulman A, Christensen M, Lane GA, Fraser K, Forester N, Johnson RD, Bryan GT, Rasmussen S (2013) An extracellular siderophore is required to maintain the mutualistic interaction of Epichloë festucae with Lolium perenne. PLoS Pathog 9, e1003332
Krueger GL, Morris TK, Suskind RR, Widner EM (1985) The health effects of aluminum compounds in mammals. Crit Rev Toxicol 13:1–24
Lawrence DP, Park MS, Pryor BM (2011) Nimbya and Embellisia revisited, with nov. comb. for Alternaria celosiae and A. perpunctulata. Mycol Prog 11:799–815
Li Y, Xia LQ, Wang YN, Liu XY, Zhang CH, Hu TL, Cao KQ (2013) The inhibitory effect of Epicoccum nigrum strain XF1 against Phytophthora infestans. Biol Control 67:462–468
Likar M, Regvar M (2013) Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 370:593–604
Mahadevakumar S, Jayaramaiah KM, Janardhana GR (2014) First report of leaf spot disease caused by Epicoccum nigrum on Lablab purpureus in India. Plant Dis 98:284
Martini M, Musetti R, Grisan S, Polizzotto R, Borselli S, Pavan F, Osler R (2009) DNA-dependent detection of the grapevine fungal endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis 93:993–998
Matthews JW, Clay K (2001) Influence of fungal endophyte infection on plant-soil feedback and community interactions. Ecology 82:500–509
Murphy WM, Silman JP, McCrory LE, Flack SE, Schmitt AL, Mzamane NM (1997) Management of natural Kentucky bluegrass-white clover pasture. Am J Altern Agric 12:140–142
Oberhofer M, Gusewell S, Leuchtmann A (2014) Effects of natural hybrid and non-hybrid Epichloë endophytes on the response of Hordelymus europaeus to drought stress. New Phytol 201:242–253
Oliveira JA, Rottinghaus GE, Gonzalez E (2003) Ergovaline concentration in perennial ryegrass infected with a lolitrem B-free fungal endophyte in north-west Spain. N Z J Agric Res 46:117–122
Oliveira JA, González E, Castro P, Costal L (2004) Effects of endophyte infection on dry matter yield, persistence and nutritive value of perennial ryegrass in Galicia (north-west Spain). Span J Agric Res 2:558–563
Olsen S, Cole C, Watanabe F, Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular Nr 939, US Gov. Print. Office, Washington, D.C
Prior R, Görges K, Yurkov A, Begerow D (2014) New isolation method for endophytes based on enzyme digestion. Mycol Prog 13:849–856
Przybył K (2002) Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For Pathol 32:387–394
Reader RJ, Wilson SD, Belcher JW, Wisheu I, Keddy PA, Tilman D, Morris EC, Grace JB, McGraw JB, Olff H, Turkington R, Kelin E, Leung Y, Shupley B, van Hulst R, Johansson ME, Nilsson C, Gurevitch J, Grigulis K, Beisner BE (1994) Plant competition in relation to neighbor biomass: an intercontinental study with Poa pratensis. Ecology 75:1753–1760
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Santamaría O, Smith DR, Stanosz GR (2012) Interaction between Diplodia pinea or Diplodia scrobiculata and fungal endophytes isolated from pine shoots. Can J For Res 42:1819–1826
Santamaría O, Rodrigo S, Poblaciones MJ, Olea L (2014) Fertilizer application (P, K, S, Ca and Mg) on pasture in calcareous dehesas: effects on herbage yield, botanical composition and nutritive value. Plant Soil Environ 60:303–308
Soleimani M, Afyuni M, Hajabbasi MA, Nourbakhsh F, Sabzalian MR, Christensen JH (2010) Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere 81:1084–1090
Spiering MJ, Greer DH, Schmid J (2006) Effects of the fungal rndophyte, Neotyphodium lolii, on net photosynthesis and growth rates of perennial ryegrass (Lolium perenne) are independent of In Planta endophyte concentration. Ann Bot 98(2):379–387
Strobel GA, Hess WM, Ford E, Sidhy RS, Yang X (1996) Taxol from fungal endophytes and the tissue of biodiversity. J Ind Microbiol 17:417–423
Suttle NF (2010) Mineral nutrition of livestock, 4th edn. CABI Publishing, Wallingford
Tanaka A, Takemoto D, Chujo T, Scott B (2012) Fungal endophytes of grasses. Curr Opin Plant Biol 15:462–468
Thom ER, Popay AJ, Waugh CD, Minnee EMK (2013) Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass Forage Sci 69:191–204
Underwood EJ (1981) The mineral nutrition of livestock, 2nd edn. Commonwealth Agricultural Bureaux, Farnham Royal
Weber RWS, Dralle N (2013) Fungi associated with blossom-end rot of apples in Germany. Eur J Hortic Sci 78:97–105
White JF Jr (1988) Endophyte-host associations in forage grasses. XI. A proposal concerning origin and evolution. Mycologia 80:442–446
Zabalgogeazcoa I, García-Ciudad A, Vázquez de Aldana BR, García-Criado B (2006) Effects of the infection by the fungal endophyte Epichloë festucae in the growth and nutrient content of Festuca rubra. Eur J Agron 24:374–384
Zabalgogeazcoa I, García-Ciudad A, Leuchtmann A, Vázquez de Aldana BR, García-Criado B (2008) Effects of choke disease in the grass Brachypodium phoenicoides. Plant Pathol 57:467–472
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res 97:111–119
Acknowledgments
This study was funded by Project AGL2011-27454, granted by the Ministry of Economy and Competitiveness of Spain (the former Ministry of Science and Innovation) and by the European Regional Development Fund (ERDF). We would like to thank Teodoro García-White, Natalia Hernández and Pablo Romero for their invaluable help with the technical work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Dominik Begerow
Rights and permissions
About this article
Cite this article
Lledó, S., Rodrigo, S., Poblaciones, M.J. et al. Biomass yield, mineral content, and nutritive value of Poa pratensis as affected by non-clavicipitaceous fungal endophytes. Mycol Progress 14, 67 (2015). https://doi.org/10.1007/s11557-015-1093-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-015-1093-4