Abstract
The present study describes the phenanthrene-degrading activity of Sphingomonas paucimobilis 20006FA and its ability to promote the bioavailability of phenanthrene. S. paucimobilis 20006FA was isolated from a phenanthrene-contaminated soil microcosm. The strain was able to grow in liquid mineral medium saturated with phenanthrene as the sole carbon source, showing high phenanthrene elimination (52.9% of the supplied phenanthrene within 20 days). The accumulation of 1-hydroxy-2-naphthoic acid and salicylic acid as major phenanthrene metabolites and the capacity of the strain to grow with sodium salicylate as the sole source of carbon and energy indicated that the S. paucimobilis 20006FA possesses a complete phenanthrene degradation pathway. However, under the studied conditions, the strain was able to mineralize only the 10% of the consumed phenanthrene. Investigations on the cell ability to promote bioavailability of phenanthrene showed that the S. paucimobilis strain 20006FA exhibited low cell hydrophobicity (0.13), a pronounced chemotaxis toward phenanthrene, and it was able to reduce the surface tension of mineral liquid medium supplemented with phenanthrene as sole carbon source. Scanning electron micrographs revealed that: (1) in suspension cultures, cells formed flocks and showed small vesicles on the cell surface and (2) cells were also able to adhere to phenanthrene crystals and to produce biofilms. Clearly, the strain seems to exhibit two different mechanisms to enhance phenanthrene bioavailability: biosurfactant production and adhesion to the phenanthrene crystals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Considerable effort has been focused on the isolation of microorganisms able to degrade polycyclic aromatic hydrocarbon (PAH) and use it as a source of carbon and energy [35]. Sphingomonads can be regarded as “key members” among the PAH-degrading bacterial genera in soil, having frequently been isolated from PAH-contaminated sites [2, 3, 5, 7, 14, 17, 19]. For this reason, Sphingomonas species would be expected to be a promising bacteria to bioremediation of PAH-contaminated sites by bioaugmentation.
When re-inoculating PAH-contaminated soil with PAH-degrading Sphingomonads, a high number of cells are able to establish themselves in the soil. However, this process does not necessarily cause an increase in the PAH-degradation capacity of the microbial soil community [5]. This indicates that it is not only the presence of a specific strain, which determines PAH degradation. Low substrate bioavailability [13] and inhibition by the products of PAH transformation [15] may also limit the rate and extent of PAH biodegradation.
It has been suggested that PAH-degrading strains to be used for bioaugmentation purposes need to be selected on the basis of a range of physiological properties. Some of these are thought to play a critical role in the colonization of soil environments and degradation of PAH [7]. One of these properties is the bacterial capacity to make the contaminant bioavailable. To improve bioavailability, PAH-degrading bacteria seem to have developed strategies such as substrate source attachment [3, 31], high specific substrate affinity [11, 12], or biosurfactant production [24, 25]. However, the PAH-degrading isolates belonging to the genus Sphingomonas have been characterized to a variable extent [1], and the knowledge of their mechanisms to make PAH bioavailable is quite limited.
The objective of this work was to characterize the strain Sphingomonas paucimobilis 20006FA by studying its phenanthrene-degrading activity and the cells’ ability to promote bioavailability of phenanthrene. The strain was isolated from a previous experiment of bioremediation in a soil microcosm contaminated with phenanthrene and selected for its ability to grow in different PAH (anthracene, phenanthrene, and dibenzothiophene) as sole carbon and energy source. The capacity of the strain to establish itself in phenanthrene-contaminated soil was previously described [5].
Material and Methods
Bacterial Strains
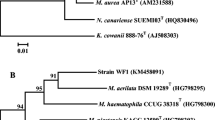
S. paucimobilis 20006FA was obtained from a PAH-contaminated soil microcosm and selected according to its ability to grow using different PAH as sole source of carbon and energy. The strain was identified according to biochemical characteristics and 16S rDNA sequence analysis [5]. To determine the phylogenetic position of the strain, the 16S rDNA was aligned with the corresponding sequences of the known PAH-degrading strains within the genus Sphingomonas. Mycobacterium sp. PYR-I was used as the out-group. The aligned sequences were analyzed with the distance matrix method. Alignments and phylogenetic trees were constructed using the MEGA program version 4 [27] (Fig. 1).
Consensus phylogenetic tree, based on a distance matrix analysis of 16S rRNA gene sequence of Sphingomonas paucimobilis 20006FA and PAH-degrading species within the genera Sphingomonas and Sphingobium. Mycobacterium sp. PYR-I was used as the out-group. The tree was constructed with selected sequences available in GenBank (http://www.ncbi.nlm.nih.gov/Genbank) using the MEGA software version 4 (Tamura et al.). The numbers at each node corresponds to the bootstrap percent values. The space bar indicates 0.02% sequence variation. The GenBank accession number for each species is indicated in brackets
For preservation, the strain was grown in R3 broth [22] at 26°C (100–150 rpm), and aliquots of 1 ml were stored in glycerol (20% v v −1) at −80°C.
Alcanivorax borkumensis SK2T was obtained from the German culture collection (DSMZ no. 11573T). The strain degrades n-alkanes and produces biosurfactants [34].
Growth Media
Depending on the experiment, S. paucimobilis 20006FA was grown either in R3 or R2 broth [22] or on R3 or R2 agar plates (R3A and R2A). When necessary, the strain was also cultivated in mineral medium (MM), which was prepared according to Vecchioli et al. [30] and supplemented with the carbon source phenanthrene (2 g/L; Carlo Erba, Italy, >99.5% purity).
A. borckumensis was routinely cultivated in marine broth (MB, Difco) at 26°C (100–150 rpm) and preserved at −80°C as described for S. paucimobilis 20006FA.
Scanning Electron Microscopy
S. paucimobilis 20006FA was grown in R3 broth or in phenanthrene-saturated liquid MM (2 g L−1). Samples of 0.3 ml were taken from the cultures, after 9, 48, 122, and 360 h of incubation. The samples were carefully dispensed onto the surface of SuperFrost Ultra Plus® microscope slides (Menzel-Gläser, Germany) and allowed to decant. They were then fixed with 4% (v v−1) glutardialdehyde at room temperature, washed with phosphate buffer (0.2 M), and dehydrated by passage through a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 100%, v v−1), subsequently dehydrated with liquid carbon dioxide and coated with platinum powder for further analysis with a scanning electron microscope (Hitachi S 3200N, Japan).
Carbon Source Utilization
Growth with phenanthrene or sodium salicylate as the sole source of carbon and energy was tested in sterile phenanthrene-saturated liquid MM (2 g phenanthrene per liter) or with the addition of 1 g L−1 sodium salicylate. Cultures were incubated at 26°C, 150 rpm for 100 h. Growth was monitored by counting colony-forming units (cfu) after spreading 0.1 ml of an appropriate dilution (about 2 × 106 cells per milliliter) on R2A medium and incubating the plates in the dark for 10 days at 30°C.
Phenanthrene Degradation and Mineralization Studies
Degradation and mineralization experiments were conducted in duplicate in 300-ml glass bioreactors (Schott), which contained 150 ml of liquid MM supplemented with 2 g L−1 phenanthrene in sterility. Cultures were started with 1.5 mL corresponding to 2 × 106 cfu mL−1 of a 24 h old pre-culture and incubated at 26°C for 20 days. One non-inoculated bioreactor was used as an abiotic control. The bioreactors had been developed in our laboratory for cultivating small culture volumes of organisms, which exhibit low respiration activities. The culture vessels were continuously stirred (150 rpm), equipped with a specific Teflon tap that permits sterile gas influx and gas efflux. It also permits taking samples and pH control without opening the tap. Gassing was performed via air sparging; the gas flow (0.5–5 NL h−1) was controlled by an electronically controlled gassing system (Krohne Messtechnik GmbH & Co. KG, Duisburg, Germany). The cultures were aerated once a day, and the CO2 concentration (as well as the O2 concentration) of the air influx as well as the gas efflux were analyzed online (CO2, infrared, UNOR 6N, Sick Maihak GmbH, Hamburg, Germany; O2, oxygen sensor, KE-25 F4, Figaro Engineering Inc. 1–5–11 Senba-nishi Mino, Osaka 562, Japan). Daily aeration and gas analysis were performed as long as was needed to obtain the same carbon dioxide and oxygen concentration in the gas efflux as in the air influx. Since the pH of the cultures was maintained constant, no bicarbonate accumulation occurred in the culture’s liquid. The O2 consumption rate (QO2) as well as the CO2 production rate (QCO2) was calculated as described by Berthe-Corti et al. [4]. The rate of mineralized phenanthrene was calculated on the basis of the produced CO2 according to the stoichiometric equation of phenanthrene mineralization:
Chemical Analysis
To analyze the phenanthrene concentration in the cultures, each culture (150 mL) was supplemented with 12 mL of an internal standard (25.2 mg n-hexadecane per milliliter n-hexane). The total culture volume was extracted three times with 100 mL of n-hexane. The organic extracts of each culture were pooled and dried with anhydrous sodium sulfate.
In order to analyze the type of phenanthrene metabolites produced by the strain, a sequential extraction was performed on the remnant aqueous phase from the n-hexane extraction. First, it was extracted with 3 vol. of ethyl acetate [neutral extractable ethyl acetate fraction (NEEF)]; subsequently, the remnant aqueous phase was acidified to pH 2.5 with concentrated HCl and re-extracted with ethyl acetate in the same way [acid extractable ethyl acetate fraction (AEEF)].
The phenanthrene concentration in the hexane extracts was determined with a gas chromatograph (Perkin-Elmer autosystem) equipped with a flame ionization detector under the following conditions: fused-silica capillary column RTX5 (30 m × 0.25 mm i.d.; Restek Corporation, Bellefonte), carrier gas helium, and gas flow rate of 2 mL min−1. The injection port was maintained at a temperature of 280°C, the detector at 340°C. The oven was set at 120°C (initial time, 2 min), then raised to 320°C at a rate of 10°C min−1, (final time, 5 min). The injection volume was 1 μl, split 1:100.
Phenanthrene metabolites in NEEF and AEEF were analyzed by reversed phase high-pressure liquid chromatography (HPLC) using a Waters® chromatograph with a Symmetry Waters ® C18 column (15 cm × 4.6 mm i.d.; bead size, 5 μm; pore size, 100 Å). The compounds were eluted using a linear gradient of 15 mM phosphoric acid in nanopure water solution and methanol (20:80 to 5:95, v v −1) over 15 min and a flow rate of 1 mL min−1. The UV absorption spectra of metabolites were obtained with a model 2996 photodiode array detector (Waters®) and analyzed with Empower software. Sample retention times and UV absorption spectra were compared to authentic standards (50 μg mL−1) for identification of phenanthrene intermediate metabolites.
Dioxygenase Activity
Ten milliliters of 24-h old cultures, either grown in phenanthrene-saturated MM supplemented with phenanthrene 2 g L−1 or MM supplemented with glucose (2 g L−1), were centrifuged and the pellet resuspended in 0.9% NaCl. A 0.1-mL sample of a tenfold dilution was spread on plates containing solid MM supplemented with indole (1 mM) as the sole carbon source and incubated at 26°C for 10 days. Development of blue colonies indicated the conversion of indole into indigo, thereby proving the activity of a ring-cleaving dioxygenase.
Analyses of the Surface Tension
Cultures of S. paucimobilis 20006FA were grown in R3 broth as well as in phenanthrene-saturated MM (supplemented with 2 g phenanthrene per liter). A. borkumensis was grown in MB with the addition of 0.1% (v v −1) of n-hexadecane. From the cultures, 10-mL samples were taken after 7 days of incubation and centrifuged (2,500×g, 10 min). In the cell-free supernatant, the surface tension was determined under standard conditions with a ring tensiometer (K12, Phywe, Germany) using the method described by Willumsen and Karlson [32]. Calibration curves were made with sodium dodecyl sulfate (SDS) dissolved in the growth media. Each measurement was repeated five times.
Cell Surface Hydrophobicity and Swarming Motility
Whole-cell surface hydrophobicity was determined, measuring the bacterial adhesion to hydrocarbon (BATH) as described by Rosenberg and Rosenberg [23].
Chemotaxis was tested applying a swarm plate assay as described by Lanfranconi et al. [16]. S. paucimobilis 20006FA was grown in R2 broth for 24 h (26°C, 100 rpm), harvested by centrifugation and resuspended in the chemotaxis buffer (10 mM potassium phosphate, pH 7, 0.1 mM EDTA) to achieve an OD(600 nm) of 0.8.
The swarm plates were prepared including the phenanthrene, previously solubilized in ethanol, in the MM agar 0.2% (w v −1). The final concentration of the tested compound phenanthrene was 0.05%. Two microliters of the cell suspension was placed into the center of these swarm plates and simultaneously into the center of control plates, which contained MM without any carbon source. After inoculation, the plates were incubated at 26°C for 7 days.
Results
Growth in Liquid MM Saturated with Phenanthrene
Due to the low water solubility of phenanthrene (1.0 mg L−1) [17], addition of 2 g L−1 to the medium resulted in an oversaturation with phenanthrene, which was present mainly in the form of crystals. S. paucimobilis 20006FA exhibited growth as free living cells (planktonic cells) as well as attached to the crystals. Figure 2 shows the growth of planktonic cells of S. paucimobilis 20006FA. At this high phenanthrene concentration, strain 20006FA showed good growth and exhibited around seven duplications within 40 h. During growth, a brownish color in the medium could be observed, indicating the accumulation of intermediates of the phenanthrene metabolism.
Figures 3 and 4 show scanning electron micrographs of cells of S. paucimobilis 20006FA growing in R3 broth (Fig. 3a) and in phenanthrene-saturated MM (Fig. 3b–e). After 24 h of incubation in R3 broth, it was possible to observe rods (0.4–0.6 × 1.5 μm) with a smooth cell surface. These cells grew as single cells or formed lax flocks, which were linked by a few filaments (Fig. 3a).
Scanning electron micrographs of S. paucimobilis 20006FA. a Cells grown in R3A after 24 h of incubation; b–e planktonic cells grown in phenanthrene-saturated liquid MM (2 g phenanthrene per liter), after 9 (b), 24 (c), 122 (d), and 360 h (e) of incubation. The dimensions of bars are given in micrometers
In liquid phenanthrene-saturated MM, cells grew as suspended cells (Fig. 3b–e) but also adhered to the crystal surfaces (Fig. 4). Suspended cells formed dense flocks, which were linked by a polymer net that increased in size with culture time (Fig. 3b, c, e), and the cell surface was covered with extracellular vesicles (Fig. 3d), which were also observed in very young cultures (9 h; Fig. 3b). Colonization of phenanthrene crystals started in very young cultures by pairs of cells that were anchored to the surface by a fiber-like structure (Fig. 4a, b). Cells that performed binary fission clearly indicated that the cells exhibited growth on the surface of the phenanthrene crystals (Fig. 4a). Two-day-old cultures revealed a net of fibers and pronounced cell patches on the crystal surfaces (Fig. 4c). After 5 days of growth, most crystals were covered by a multi-layer film of cells (Fig. 4d, e).
Cell Hydrophobicity, Swarming Motility and Surfactant Production
Cells grown in phenanthrene-saturated liquid MM exhibited low cell hydrophobicity (0.13) when tested using the method described by Rosenberg and Rosenberg [23]. Testing the swarming motility, using the method developed by Lanfranconi et al. [16], revealed a positive response of the cells toward phenanthrene, as revealed by the formation of concentric rings in the plates (Fig. 5). Incubated under the same conditions, the control swarm plate without phenanthrene did not show any rings (data no shown).
One possible mechanism to enhance the bioavailability of non-polar substrates is the production of surface active agents. The surface tension of cultures of S. paucimobilis strain 20006FA grown with phenanthrene was compared to the surface tension of cultures of A. borkumensis SK2T, a known biotenside-producing strain [34]. It was also compared to aqueous solutions of the tenside SDS. S. paucimobilis strain 20006FA, when it was cultivated with phenanthrene as a carbon source, reduced the surface tension in the same range as A. borkumensis did when it was cultivated in MB containing 0.1% n-hexadecane (Table 1). However, when S. paucimobilis strain 20006FA was cultivated in R3 broth, no surface activity was observed (Table 1). This was a clear indication that strain 20006FA produced surface active agents when grown on phenanthrene.
Phenanthrene Degradation and Mineralization
Cultivating cells in small bioreactors (150 mL working volume) made it possible to monitor oxygen consumption and carbon dioxide production as well as the cultures’ phenanthrene consumption. Cultures were started with an initial amount of 300 mg (1.7 mmol) of phenanthrene in 150 ml liquid MM. After 20 days of growth when the metabolic activity had stopped, as indicated by a stop of the cultures’ CO2 production, the residual amount of phenanthrene in the culture broth was 71 ± 13 mg (0.4 ± 0.1 mmol). Thus, around 229 ± 13 mg (1.3 ± 0.1 mmol) of phenanthrene was eliminated (76 ± 4%). At that point, 2.1 ± 0.2 mmol of oxygen were consumed and 1.3 ± 0.3 mmol of carbon dioxide were produced. The abiotic control showed a phenanthrene elimination level of 20 ± 6%, but no oxygen consumption or CO2 production could be detected.
The data about the phenanthrene elimination together with the data about the oxygen consumption and the CO2 production made it possible to estimate the carbon and oxygen flux in the cultures (Fig. 6).
The amount of phenanthrene that was taken up by the cells was 53 ± 6% (0.9 ± 0.1 mmol) of the phenanthrene, which had been supplied to the cultures. Only about 10 ± 2% of the consumed phenanthrene was completely mineralized to CO2 and water, whereas about 89 ± 11% (0.8 ± 0.1 mmol) were transformed to metabolic products and biomass. The cells consumed about 0.6 ± 0.3 mmol of dioxygen, which was not needed for mineralizing the incorporated phenanthrene and which was free for other oxidation processes. The ratio of the consumed dioxygen that is not needed for phenanthrene mineralization to the consumed phenanthrene that is not mineralized is 0.6 ± 0.3 (mmol O2) to 0.8 ± 0.1 (mmol C10H14). Therefore, one may conclude that cells consumed enough oxygen that the dioxygenases could oxidatively attack the phenanthrene, which had been consumed but not been mineralized.
Phenanthrene Metabolites
Cells pre-incubated in liquid MM, irrespective of whether this was supplemented with glucose or phenanthrene, and transferred to MM plates supplemented with indole (1 mM) produced blue colonies caused by the production of indigo blue, which is an indicator that dioxygenases have acted upon aromatic rings.
HPLC analysis of phenanthrene metabolites in NEEF revealed a major peak at 5.05 min (data no shown). HPLC analysis of AEEF revealed an additional metabolite (Fig. 7). The peak at 5.05 was identical to the HPLC retention time and UV absorption spectrum of 1-hydroxy-2-naphthoic acid, whereas the peak at 2.95 min was identical to the HPLC retention time and UV absorption spectrum of salicylic acid (Fig. 7).
HPLC analysis of phenanthrene metabolites in culture supernatants of Sphingomonas paucimobilis 20006FA grown in phenanthrene-saturated liquid MM (2 g phenanthrene per L). HPLC elution profile of acid extractable ethyl acetate fraction (-----), salicylic acid standard (- - - -), and 1-hydroxy-2-naphthoic acid standard (.......). HPLC retention times are given in minutes. Inset UV absorption spectrum of salicylic acid (peak a) and 1-hydroxy-2-naphthoic acid (peak b) produced by Sphingomonas paucimobilis 20006FA
When sodium salicylate was offered in liquid cultures as the sole carbon source, cells showed good growth. These data suggested the capacity of strain 20006FA to oxidatively open the three rings of phenanthrene as well as to oxidatively de-carboxylize salicylic acid.
Discussion
S. paucimobilis strain 20006FA was isolated by our group from a PAH-contaminated soil microcosm. In a previous bioaugmentation study, we have demonstrated that the strain rapidly established itself in soil, which had been freshly spiked with phenanthrene, and that strain also enhanced phenanthrene degradation during the first 20 days of treatment. After that, a pause in the decrease in the phenanthrene concentration was observed [5]. We hypothesized that this pause was mainly due to the accumulation of phenanthrene metabolites and not due to a lack of phenanthrene bioavailability. In order to study the factors that might limit the efficiency of S. paucimobilis 20006FA to enhance the biodegradation process in PAH-contaminated soil, the physiologic characterization of the strain was done in more depth.
PAH-degrading strains used for bioaugmentation purposes need to be selected on the basis of several traits such as (1) the capacity of performing a complete degradation pathway (so that potentially toxic metabolites do not accumulate), (2) specific physiological properties that will help to enhance bioavailability of PAH (motility, biofilm formation, and surfactants production) [7], and (3) ecological properties that contribute to the successful colonization of soil [29].
The results presented in this work show that S. paucimobilis 20006FA fulfills several of these traits. It could efficiently degrade phenanthrene (52.9% of the supplied phenanthrene) when grown in liquid MM saturated with phenanthrene as the sole carbon source. The strain produced 1-hydroxy-2-naphthoic acid and salicylic acid as major metabolites (Fig. 7). This showed that phenanthrene was metabolized by the strain via a salicylate pathway, as had been previously reported on other Sphingomonas strains [28, 33]. This evidence and the fact that S. paucimobilis 20006FA grew with sodium salicylate as the sole source of carbon and energy indicate the presence of a complete phenanthrene degradation pathway in the strain.
In cultures growing with phenanthrene as sole source of carbon, a reduction in the surface tension of the cell-free supernatant was observed (Table 1), a fact which indicated the surfactant production. In addition, the micrographs of planktonic cells grown with phenanthrene revealed small vesicles on the cell surfaces (Fig. 3b, e). Comparable vesicles on the cell surface were reported for Marinobacter hydrocarbonoclasticus growing on eicosane [8] and for a marine phenanthrene-degrading Sphingomonas species growing on 2-methyl phenanthrene [10]. The Sphingomonads have an outer gluco-sphingolipid cell membrane. The structure of these gluco-sphingolipids resembles the structure of surfactants [12]. It may be that the vesicles, which have been described for Sphingomonas species and for M. hydrocarbonoclasticus function as surfactants. Maneerat [18] proposed that extracellular membrane vesicles may partition hydrocarbons to form a microemulsion, which would play an important role in hydrocarbon uptake by microbial cells.
The capacity of S. paucimobilis 20006FA to produce vesicles as well as to reduce the surface tension of the growth medium seem to be a very unique characteristic within the genus Sphingomonas. The only report in the literature about a comparable capacity is from Gilewicz et al. [10] who showed that Sphingomonas sp. 2MPII, isolated from a marine environment, not only exhibited vesicles on the cell surface but also reduced the surface tension of the medium when grown with a non-polar carbon source. Johnsen and Karlson [12] tested S. paucimobilis EPA505 for its influence on surface tension when grown with phenanthrene, but they could not detect any similar effect.
In spite of the low hydrophobicity of the cell surface of strain 20006FA (0.13), as shown in Fig. 4, cells of S. paucimobilis strain 20006FA are also able to adhere to phenanthrene crystals, to proliferate on the crystal surfaces, and to finally produce biofilms. This suggests that strain 20006FA is highly adhesive to surfaces and may increase its PAH uptake through the direct contact of the cells with the non-polar substrate. Formation of biofilms was also reported by Cunliffe and Kertesz [6]. The authors tested nine different Sphingomonas isolates for their ability to form biofilms on slides, which were coated with phenanthrene. Using indirect proof by staining the slides with a crystal violet solution, the authors could demonstrate biofilm formation. The authors, however, did not show the development of the biofilm in time. Taking samples from growing cultures at different culture times and analyzing the phenanthrene crystal surfaces by scanning electron microscopy (SEM), we were able to make the biofilm formation visible in its different stages of development. It seems that, in young cultures, pairs of cells first adhere to the crystal’s surface by peritrich filaments, which extend from the cells (Fig. 4a). These structures were short and thin, connecting the cells very closely to the substrate. With increasing culture time, cells also connected to each other by structures that were apparently considerably longer and thicker, thus forming multilayer nets connecting more and more cells on the crystal surface (Fig. 4c). Members of the genus Sphingomonas secrete a group of structurally related exopolysaccharide (EPS) referred to as sphingans [9]. The fiber-like structures of strain 20006FA observed by SEM (Figs. 3 and 4) might be caused by the dehydratation of the EPS matrix during the sample’ treatment for SEM analysis.
The lack of a correlation between cell surface hydrophobicity and biofilm formation, which was also described by Cunliffe and Kertesz [6], could be explained on the basis of a hydrophobicity/hydrophilicity shift as a mechanism for cells to regulate their attachment to the substrate [20]. In this sense, the production of cell-bound biosurfactants may change hydrophobic cell surfaces into charged and hydrophilic ones [20]. This might explain why the vesicles on the cell surfaces of S. paucimobilis strain 20006FA were only observed in the micrographs of planktonic cells (Fig. 3e) but not in the cells attached to phenanthrene crystal surface (Fig. 4).
In short, S. paucimobilis strain 20006FA, a PAH-degrading strain within the genus Sphingomonas is able to grow with phenanthrene as sole source of carbon and energy. Apart from showing physiological properties, such as chemotaxis toward phenanthrene, the strain seems to exhibit two clearly different mechanisms to make phenanthrene bioavailable: (1) it enhances the solubility of phenanthrene by biosurfactant excretion, and (2) it enhances bioavailability by adhesion to the phenanthrene crystals. Until now, this combined capacity has not been described for PAH-degrading bacteria, which had been isolated from soil.
After 20 days of incubation under the studied conditions, the strain had mineralized only 10% of the eliminated phenanthrene but accumulated intermediates such as 1-hydroxy-2-naphthoic acid and salicylic acid. Until now, PAH metabolites have been studied only to identify novel degradation pathways or potentially toxic dead-end products [21, 26]. However, the accumulation of intermediate metabolites on the extent of biodegradation and mineralization has not been studied. Kazunga et al. [15] have demonstrated that extracellular metabolites of pyrene degradation strongly inhibited the phenanthrene degradation by Pseudomonas saccharophila and Sphingomonas yanoikuyae. Although we have not demonstrated the inhibitory effect of phenanthrene metabolites, we have demonstrated that the strain S. paucimobilis 20006FA is able to grow with phenanthrene as sole source of carbon and that it possesses a complete phenanthrene degradation pathway together with two different mechanisms to enhance phenanthrene bioavailability. In spite of this, when the strain grew in phenanthrene as sole carbon source, in excess of its aqueous solubility, the mineralization of phenanthrene was strongly limited. This evidence encourages the authors to keep their hypothesis that it is the accumulation of phenanthrene metabolites, and not the phenanthrene bioavailability, that could be one of the principal causes that limit the inoculum activity in soil freshly spiked.
References
Baboshin M, Akimov V, Baskunov B, Born TL, Khan SU, Golovlela L (2008) Conversion of polycyclic aromatic hydrocarbons by Sphingomonas sp. VKM B-2434. Biodegradation 19:567–576
Balkwill DL, Drake GR, Reeves RH, Frederikson JK, White DC (1997) Taxonomic study of aromatic-degrading bacteria from deep-terrestrial-subsurface sediments and description of Sphingomonas aromaticivorans sp. nov., Sphingomonas subterranea sp. nov., and Sphingomonas stygia sp. nov. Int J Syst Bacteriol 47:191–201
Bastiaens L, Springael D, Wattiau P, Harms H, DeWachter R, Verachtert H, Diels L (2000) Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl Environ Microbiol 66:1834–1843
Berthe-Corti L, Bruns A, Hulsch R (1997) Semi-continuous-flow cultures with marine sediment suspensions containing non-polar carbon sources—culture control by a pneumatic sediment suspension dosage system. J Microbiol Methods 29:129–137
Coppotelli BM, Ibarrolaza A, Del Panno MT, Morelli IS (2008) Effects of the inoculant strain Sphingomonas paucimobilis 20006FA on soil bacterial community biodegradation in phenanthrene-contaminated soil. Microb Ecol 55:173–183
Cunliffe M, Kertesz MA (2006) Effect of Sphingobium yanoikuyae B1 inoculation on bacterial community dynamics and polycyclic aromatic hydrocarbon degradation in aged and freshly PAH-contaminated soils. Environ Pollut 144:228–237
Cunliffe M, Kertesz MA (2006) Autecological properties of soil sphingomonads involved in the degradation of polycyclic aromatic hydrocarbons. Appl Environ Biotechnol 72:1083–1089
Fernandez-Linares LM, Acquiviva M, Bertrand JC, Gauthier M (1996) Effect of sodium chloride concentration on growth and degradation of eicosane by the marine halotolerant bacterium Marinobacter hydrocarbonoclasticus. Syst App Microbiol 19:113–121
Fialho AM, Moreira LM, Granja AT, Popescu AO, Hoffmann K, Sá-Correira I (2008) Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biotechnol 79:889–900
Gilewicz M, Nadalig NT, Budzinski H, Doumenq P, Michotey V, Bertrand JC (1997) Isolation and characterization of a marine bacterium capable of utilizing 2-methylphenanthrene. Appl Microbiol Biotechnol 48:528–533
Harms H, Bosma TNP (1997) Mass transfer limitation of microbial growth and pollutant degradation. J Ind Microbiol Biotech 18:97–105
Johnsen AR, Karlson U (2004) Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol 63:452–459
Johnsen AR, Wick LY, Harms H (2005) Principles of microbial PAH-degradation in soil. Environ Pollut 133:71–84
Kästner M, Maroh B, Weinberg (1993) Biologische Schadstoffe in Böden. Economia, Bonn
Kazunga C, Aitken MD (2000) Products of incomplete metabolism of pyrene by polycyclic aromatic hydrocarbon-degrading bacteria. Appl Environ Microbiol 66:1917–1922
Lanfranconi MP, Alvarez HM, Studdert CA (2003) A strain isolated from gas oil-contaminated soil displays chemotaxis towards gas oil and hexadecane. Environ Microbiol 5:1002–1008
Leys NM, Ryngaert A, Bastiaens L, Top EM, Verstraete W, Springael D (2005) Culture independent detection of Sphingomonas sp. EPA 505 related strains in soils contaminated with polycyclic aromatic hydrocarbons (PAHs). Microb Ecol 49:443–450
Maneerat S (2005) Biosurfactants from marine microorganisms. Songklanakarin J Sci Technol 27:1263–1272
Mueller JG, Chapman PJ, Blattmann BO, Pritchard PH (1990) Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol 56:1079–1086
Neu TR (1996) Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev 60:151–166
Pinyakong O, Habe H, Supaka N, Pinpanichkarn P, Juntongjin K, Yoshida T, Furihata K, Nojiri H, Yamane H, Omori T (2000) Identification of novel metabolites in the degradation of phenanthrene by Sphingomonas sp. strain P2. FEMS Microbiol Lett 191:115–121
Reasoner D, Geldreich E (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Rosenberg M, Rosenberg E (1981) Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol 148:51–57
Santos EC, Jacques RJS, Bento FM, Peralba MCR, Selbach PA, Sá ELS, Camargo FAO (2008) Anthracene biodegradation and surface activity by an iron-stimulated Pseudomonas sp. Bioresource Technol 99:2644–2649
Semple KT, Doick KJ, Lukas YW, Harms H (2007) Microbial interactions with organic contaminants in soil: Definitions, processes and measurement. Environ Pollut 150:166–176
Story SP, Parker SH, Hayasaka SS, Riley MB, Kline EL (2001) Convergent and divergent points in catabolic pathways involved in utilization of fluoranthene, naphthalene, anthracene, and phenanthrene by Sphingomonas paucimobilis var. EPA505. J Ind Microbiol Biotechnol 26:369–382
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tao XQ, Lee GN, Dang Z, Yang C, Yi XY (2007) A phenanthrene degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochem 42:401–408
van Veen JA, van Oberbeek LS, van Elsas JD (1997) Fate and activity of microorganisms introduced into soil. Microbiol Mol Biol Rev 61:121–135
Vecchioli GI, Del Panno MT, Panceira MT (1990) Use of selected autochthonous soil bacteria to enhance degradation of hydrocarbons in soil. Environ Pollut 67:249–258
Wick LY, de Munain AR, Springael D, Harms H (2002) Responses of Mycobacterium sp. LB501 to the low bioavailability of solid anthracene. Appl Microbiol Biotechnol 58:378–385
Willumsen PA, Karlson U (1996) Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation 7:415–423
Xia Y, Min H, Rao G, Liu ZM, Liu J, Ye YP, Duan XJ (2005) Isolation and characterization of phenanthrene-degrading Sphingomonas paucimobilis strain ZX4. Biodegradation 16:393–402
Yakimov MM, Golyshin PN, Lang S, Moore ERB, Abraham WR, Lünsdrof H, Timmis KM (1998) Alkanivorax borkumensis gen. nov., sp. nov., a new hydrocarbon-degrading and surfactant–producing marine bacterium. Int J Syst Bacteriol 48:339–348
Zhao HP, Wang L, Ren JR, Li Z, Li M, Gao HW (2008) Isolation and characterization of phenanthrene-degrading strains Sphingomonas sp. ZP1 and Tristella sp. ZP5. J Hazard Mater 152:1293–1300
Acknowledgements
We thank Dipl. Ing. Reiner Hulsch for his help with the bioreactors and Ms. Kort and Dr. Rhiel for their help in performing SEM analysis. This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT2006-884) and a DAAD scholar ship for Ms. Bibiana Coppotelli. Coppotelli B. is postdoctoral fellow of CONICET, Ibarrolaza A. is doctoral fellow of CONICET, and Dias R. is a doctoral fellow of CIC-PBA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coppotelli, B.M., Ibarrolaza, A., Dias, R.L. et al. Study of the Degradation Activity and the Strategies to Promote the Bioavailability of Phenanthrene by Sphingomonas paucimobilis Strain 20006FA. Microb Ecol 59, 266–276 (2010). https://doi.org/10.1007/s00248-009-9563-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9563-3