Abstract
When performing contrast-enhanced ultrasound (CEUS), ultrasound (US) scanner settings, examination technique, and contrast agent dose and administration must be optimized to ensure that high-quality, diagnostic and reproducible images are acquired for qualitative and quantitative interpretations. When carrying out CEUS in children, examination settings should be tailored to their body size and specific indications, similar to B-mode US. This review article details the basic background knowledge that is needed to perform CEUS optimally in children, including considerations related to US scanner settings and US contrast agent dose selection and administration techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contrast-enhanced ultrasound (CEUS) is an advanced imaging modality that is performed with US and requires the administration of US contrast agents (UCAs). UCAs act as strong reflectors of US waves, backscattering a bright echo signal that is detected by using contrast-specific US software [1,2,3].
The most commonly used commercial UCAs consist of microbubbles of inert gases stabilized by protein or lipid shells. While the microbubbles of the different UCAs vary in terms of mean bubble size and concentration, they are all smaller than red blood cells. The microbubbles expand and contract when interacting with the US beam, returning non-linear signals to the transducer [2, 3]. Because they are sensitive to the acoustic pressure of a US wave, administration techniques and scanning parameters need to be optimized to maximize the lifespan of the contrast agent for diagnostic imaging and interventional procedures.
UCAs that are administered intravenously remain confined within the blood pool. CEUS detects, in real time, the circulating microbubbles as strong echoes moving within the vessels, thus providing micro- and macro-vascular information that is used to assess the vascular perfusion of a lesion or organ [3, 4]. UCAs can also be administered into body cavities for diagnostic imaging and interventional procedures. The UCA is delivered directly through tubes or catheters in a similar manner to normal saline, iodinated- or gadolinium-based contrast agents [5,6,7].
Three currently available UCAs have been used in children: SonoVue/Lumason (sulfur hexafluoride lipid-type A microspheres, Bracco Imaging, Milan, Italy, and Bracco Diagnostics, Monroe Township, NJ, respectively); Optison (perflutren protein-type A microspheres, GE Healthcare, Princeton, NJ); and Definity (perflutren liquid microspheres, Lantheus, North Billerica, MA) [8,9,10]. Lumason is approved by the United States Food and Drug Administration (FDA) for intravenous (IV) use in children to evaluate focal liver lesions and to improve visualization of the ventricular wall in echocardiography. It is also FDA-approved for intravesical administration in the evaluation of vesicoureteral reflux in children. Lumason is known outside the USA as SonoVue. SonoVue is approved by the European Medicines Agency and the Chinese National Medical Products Administration for vesicoureteral reflux detection in children. Any other use of UCAs in children remains off-label [11].
Although commercial UCAs and US manufacturers have recommendations and default scanning settings for optimal CEUS performance, understanding the effect of various imaging parameters, transducer selection and UCA administration set-up and technique is essential background knowledge for anyone using CEUS [12,13,14]. The aim of this review article is to present the general background information needed for performing CEUS in children, specifically in terms of current practices related to CEUS imaging parameters, basic examination technique, and UCA preparation, dosing and administration.
Ultrasound scanner settings for contrast-enhanced ultrasonography
Acquisition of high-quality and reproducible CEUS images is crucial for providing accurate clinical diagnosis. The majority of commercially available US scanners today have the capability to perform CEUS using a contrast-specific imaging mode. Here we outline the basic technical requirements for a US system to be able to perform a CEUS study.
Mechanical index
Tissues and microbubbles are sensitive to the acoustic power or insonation power of a US wave. The mechanical index (MI) provides an estimate of the acoustic energy that is transferred in tissues and microbubbles during a US examination. The MI is defined as peak negative pressure divided by the square root of the frequency of the US wave. It is a unitless number, and although it is displayed on all US monitors, it is not uniform across all US manufacturers; therefore, it is not possible to compare the MI directly among different US units because the optimal MI on one US unit may not be the same on another unit [15, 16]. A high acoustic power or high MI can cause the microbubbles to become unstable and rupture. When this happens, the gas from the microbubbles diffuses and the microbubbles are no longer effectively scattering echoes. A low MI is ideal for imaging the microbubbles over a considerably longer period of time.
The FDA-approved limit of MI for postnatal diagnostic US imaging with harmonic B-mode or Doppler modes is 1.9 [17]. However, for contrast imaging with microbubble-based contrast agents, most modern US systems use contrast-specific software that is capable of generating contrast images at a very low MI, far lower than 0.3 [18]. Even at these low MI values, microbubbles can destabilize after continuous prolonged scanning because of repetitive expansion and contraction. In lesions and body parts with slower blood flow, the microbubbles traverse the capillary beds more slowly and, as a result, resonate longer than in high-flow regions. This predisposes them to rupture even with contrast-specific settings. Unintentional rupture of microbubbles can cause a pseudo-washout effect, in which contrast signal appears falsely reduced because of the destruction of microbubbles and not from actual dissipation, potentially leading to interpretation errors [19].
In other cases, however, intentional destruction of microbubbles is necessary. For example, when preparing for a repeat contrast injection, clearance of existing microbubbles might be needed. This can be achieved using the flash mode, which is a transient use of a high-power pulse that results in instant destruction of microbubbles within the imaging plane [20]. Alternatively, returning to diagnostic B-mode or Doppler imaging modes, which inherently use higher MI, similarly results in microbubble destruction. Increasing the MI during an examination can also be used as a troubleshooting technique to enhance the signal from microbubbles in the far field at the expense of microbubble destruction in the near field [12, 13].
Contrast-specific imaging modes
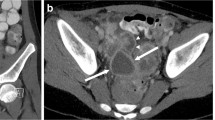
These modes separate the microbubble signal from that of the surrounding blood and tissues using a combination of non-linear imaging and pulse inversion or other proprietary pulsing schemes to produce an image that displays the microbubble-only signal [21]. Occasionally the examiner might struggle to maintain the probe position on the desired region of interest when using the contrast-specific mode because of the lack of tissue landmarks. To overcome this challenge, the CEUS mode has a dual-display option to simultaneously view a reference B-mode image side-by-side with the contrast-only image (Fig. 1). It is important to note that the reference B-mode image that is generated during CEUS imaging has lower image resolution compared to the conventional B-mode US images of the same structures because it is a modified B-mode image that is based on a low MI and adjusted pulsing scheme to prevent microbubble disruption [12, 22]. Some US scanners allow the separate manipulation of the scanning parameters of the B-mode and contrast-only images to reduce this limitation.
Contrast-enhanced US settings. Transverse dual image with simultaneous display of B-mode image (left) and contrast image (right) of a hepatoblastoma (arrow) in a 13-month-old girl. The mechanical index, displayed on the image (open rectangle), is 0.15. The focal zone (open triangle) is set deeper in the image than the area of interest. A timer (T1) is started and displays the time since the contrast agent was injected. The administered contrast dose is annotated on the image (open circle)
Several US systems also provide a color overlay feature, which superimposes the contrast image on the modified B-mode image (Fig. 2). This is sometimes helpful for localizing smaller anatomical structures that would otherwise be challenging to identify without a reference tissue landmark [12, 21]. Additionally, dual calipers that appear on the reference B-mode image as well as on the contrast image help to localize small lesions, to ensure that the correct region is interpreted.
Contrast-enhanced ultrasound color overlay on reference B-mode image of a hepatic hemangioma in a 48-year-old woman. a Sagittal contrast-only image. The lesion shows the typical peripheral nodular enhancement in the early post-contrast phases. b Sagittal color overlay image. The lesion is localized in the parenchyma when the contrast-only image is overlaid on the B-mode image
Contrast-enhanced US is a dynamic imaging modality that provides real-time visualization of blood flow. In order to study the temporal behavior of the UCA kinetics within a region of interest, the US system has a visible timer on the US screen that should be activated after the contrast injection and before the normal saline flush. It provides a reference to identify particular time points throughout the examination period until the microbubbles eventually dissipate. The timer is necessary for some intravenous CEUS examinations, and knowing the elapsed time since contrast injection assists with characterizing the different tissues during specific periods of enhancement. It also identifies any abnormal delays in the contrast arrival to a specific site, and thus it can be used for troubleshooting during the administration of the UCA.
Focal zone
The focal zone is the point of maximal acoustic pressure and therefore maximal resolution in the area of insonation [12, 19, 22]. When scanning a particular structure in B-mode US, the focal zone should be placed at the level of interest. When performing CEUS imaging, however, the focal zone should be positioned deep to the desired region of interest. This allows for a more uniform acoustic pressure field, which improves the sensitivity to microbubble signal and reduces the possibility of microbubble disruption [23, 24]. Some US systems are now capable of providing a fully focused image without requiring manual adjustment or positioning of focal zones while in contrast mode.
Imaging depth
Imaging at greater tissue depths is challenging in CEUS, just as it is in conventional B-mode US. To improve visualization, the center frequency of the probe should be adjusted to balance between resolution and penetration. A lower imaging frequency might enable the signal to penetrate more deeply; however, the trade-off is lower spatial resolution, which could result in suboptimal imaging of smaller vessels [12, 19, 22].
Increasing the MI can improve signal penetration at greater depths, and this practice is commonly used in B-mode US. However, in contrast-specific imaging, increasing the MI should be considered more carefully because it will cause microbubble destruction, especially in the near-field, and therefore decrease the late enhancement effect (Fig. 3) [13]. Before adjusting the MI, the area of interest should be positioned as close to the transducer as possible to minimize challenges of penetration at greater depths and improve temporal and spatial resolution [13].
Unintentional loss of contrast in the near field in contrast-enhanced ultrasound of the liver. Transverse dual image with simultaneous display of the B-mode (left) and the contrast (right) images in a 12-year-old girl show a hypoattenuating band (arrow) within the liver in the near field. This can occur as the mechanical index (MI) is optimized for far-field visualization, or if the transducer remains stationary over the same region for too long. Lowering the MI or moving the probe improves contrast visualization in this region
Gain
Image gain, which is the electronic amplification of the US signal after it is returned to the transducer, refers to the overall brightness of the image [13]. The amount of gain is usually shown to the side of the image in the form of a line or curve. Because different tissues attenuate US waves to a different degree depending on their distance from the transducer, adjustments of the curve are necessary to increase sound attenuation in the deep tissues.
US systems that provide a dual imaging mode for CEUS commonly offer the ability to independently adjust the gain for the reference B-mode and the contrast image. The gain should be set prior to contrast administration and should not be manipulated after contrast agent is given. Doing so during the examination can lead to misinterpretation of enhancement or washout if the gain is increased or decreased, respectively.
It is generally agreed that for CEUS imaging the gain should be set slightly above the noise floor before the microbubbles arrive [22]. The image is dark with a very low level of visible noise (Fig. 4). If the gain is set too low, then the weaker microbubble signals are missed, and only larger vessels or high microbubble concentrations are visualized. Alternatively, if the gain is set too high and the image pre-contrast is too bright or grainy, then the microbubble signal is quickly saturated upon arrival in the tissue and the full range of signal enhancement cannot be appreciated (Fig. 5) [13, 19, 22].
Gain setting in intravenous contrast-enhanced US. Transverse dual image of the liver with simultaneous display of the contrast (left) and B-mode (right) images of a metastatic lesion in a 58-year-old man. a Pre-contrast image with correct gain setting. Signal from the liver is suppressed. A strong interface between the liver and lung is seen (arrowheads). b At 26 s following intravenous contrast administration, there is heterogeneous enhancement and washout (solid arrow) of the lesion compared with the background liver (open arrow). Imaging findings are compatible with metastatic disease
Gain setting in contrast-enhanced voiding ultrasonography. Sagittal dual-image of the right kidney with simultaneous display of the B-mode (left) and contrast image (right) during contrast-enhanced voiding urosonography in a 4-year-old girl. a Gain setting is too low. On the contrast-only image the anatomy is not discernable. b Gain setting is too high. There is too much background noise, which can confound the signal from contrast agent on the image if reflux occurs. c Gain setting is optimal. Echoes from the renal sinus fat (solid arrow) are visible, as are strong interfaces, such as the renal capsule (open arrow). Background noise is minimized
Additionally, if a lesion is intrinsically echogenic, it can be beneficial to reduce the gain; however, using the flash mode can also be useful to confirm whether microbubbles are present in the lesion [19]. Some newer systems have the capability to adjust the gain settings by manipulating the raw data after the study is completed. This allows the image to be adjusted for better contrast visualization.
Temporal resolution
Similar to B-mode US, CEUS requires image acquisition at a temporal resolution that is adequate to capture real-time changes in the enhancement characteristics. The ability of a US system to distinguish between instantaneous events of moving structures is determined by its temporal resolution, which is described by the number of US images (or frames) displayed per second (s) and is expressed in Hertz (Hz) [25, 26].
The frame rate is usually indicated on the image as frames per second (FPS) or frame rate. Higher frame rates give greater temporal resolution. A trade-off with increasing the frame rate, however, is microbubble destruction. Frame rates are reduced in CEUS to decrease loss of microbubbles and improve detection of the non-linear microbubble signal [13]. In most cases, a frame rate of 10 Hz or higher is recommended for capturing the enhancement patterns within a region of interest [12]. However, in highly vascularized small lesions or contrast-enhanced echocardiography, a higher frame rate might be necessary to appreciate the rapid and dynamic behavior of the microbubbles [13]. If the temporal resolution is sub-optimal, crucial periods of the enhancement behavior and small lesions could be missed while sweeping through organs.
Strategies to improve the temporal resolution include (1) positioning the probe as close to the region of interest as possible to reduce the depth of penetration and the distance for US pulses to travel, (2) reducing the imaging area or modifying the region of interest to focus on the target location in order to reduce the scan lines per frame, (3) reducing the number of focal points and thereby reducing duplication of scan lines and (4) reducing line density.
Three-dimensional (3-D) CEUS is available on some US systems but currently suffers from poor temporal resolution (1–3 Hz). Development of newer matrix-array transducers is likely to improve 3-D CEUS performance at a temporal resolution that is more comparable to 2-D US.
Spatial resolution
The ability of a US system to distinguish between two points at a particular depth in tissue is known as spatial resolution. Axial resolution is the minimum distance that can be differentiated between two points parallel to the US signal. Lateral resolution is the minimum distance that can be distinguished between two points perpendicular to the US signal. Spatial resolution is predominantly determined by the transducer, with low-frequency transducers having generally lower spatial resolution compared to high-frequency transducers [25].
During a CEUS exam, a single dose of contrast agent consists of millions of microbubbles. In this scenario, the ability to distinguish between two microbubbles might not be as important as the ability to simply detect the bubble signal. Therefore, a transducer with a lower spatial resolution does not necessarily preclude its use for CEUS if it is able to provide a contrast signal either from a single or a large group of microbubbles. To visualize individual microbubbles, however, a transducer with a high spatial resolution is required.
Dynamic range
The dynamic range, also known as compression, defines the span of echo intensities displayed on the image. It is one of the most essential parameters that determines the image quality, and it is manually adjusted by the examiner. In B-mode US, a low dynamic range reduces the span of echo intensities, and as a result fewer shades of gray are displayed on the image, which appears over-contrasted. On the contrary, an image with a high dynamic range leads to too many shades of gray on the monitor, and the image appears smoother [25].
In CEUS imaging, the dynamic range should be optimized according to the expected enhancement pattern. A low dynamic range decreases the span of microbubble signals on the image and therefore increases the visual contrast resolution. This might be helpful if the clinical question is differentiation between lesional and parenchymal enhancement, such as in trauma cases. However, in a vascular lesion, lowering the dynamic range can limit the differentiation between areas of variable enhancement [12]. A higher dynamic range is generally recommended when the objective of CEUS examination is to perform quantification to avoid signal saturation. If acquiring a series of CEUS examinations that require comparison to one another, it is advantageous to keep the dynamic range and other post-processing settings similar between the studies [12].
Transducer selection
Transducer selection is based on the size of the child, location of the lesion or organ examined, and type of clinical study performed [12, 13]. Convex transducers with lower frequencies (ranging 1–5 MHz) are preferred for better penetration when imaging the abdomen and deep structures in older or obese children. For neonates and infants or superficial regions of interest, linear transducers with higher frequencies (ranging 7–15 MHz) are preferred. When a higher-frequency transducer is selected, the resonance frequency of the microbubbles shifts away from its natural resonant frequency, resulting in reduced microbubble signal response. To compensate for this signal reduction, a higher dose of the UCA might be needed [27, 28].
Most modern US scanners use broad bandwidth transducers, which allow selection of frequencies on the same transducers. These transducers use specific pre- and post-processing algorithms to boost the microbubble signal and therefore might not require compensation with dose modifications when using higher-frequency linear transducers. When in contrast-imaging mode, the US examiner can also toggle between settings on the transducer, from higher frequencies for greater resolution to lower frequencies for greater penetration, or change transducers depending on the indication (Fig. 6).
Depth attenuation. Transverse dual image of the liver with simultaneous display of the B-mode (left) and contrast (right) images in a 3-year-old boy. A linear 9-MHz transducer was used to evaluate a liver lesion. When sweeping through the liver to assess for additional lesions, attenuation of contrast is seen in the far field (arrow). This can be corrected by toggling to a penetration setting on this transducer, selecting a lower-frequency convex probe, or imaging from a window that brings this region of the liver closer to the probe
Contrast-enhanced ultrasound examination techniques
Pre-contrast imaging
Prior to contrast administration, B-mode and Doppler US assessment of the region or organ of interest is performed. A pre-contrast dual-display image is used to set the imaging parameters. The MI, focal zone, gain and frame rate can be optimized, noting trade-offs related to the image quality and microbubble destruction described above [19]. After these parameters are optimized, they can be saved as presets to minimize manipulation between examinations on the same US system.
Current US units allow the reference B-mode image to be optimized separately from the contrast image. In the contrast image, suppression of echoes produced by strong interfaces may be incomplete. However these echoes can be used as a reference to identify organ capsules and keep the examined areas in view during imaging.
Standardized image annotations describing the injection number and the contrast agent dose can be helpful during off-line image review and quality analysis within a US section.
Tubing and lines
If a peripheral IV cannula is needed for CEUS, it should ideally be placed in the left arm so that the person administering the contrast agent can stand on the child’s left side while the US examiner scans from the child’s right side. However, if a right-side line is placed, the child’s right arm can be placed above his or her head and the person administering the contrast agent can stand at the head of the bed.
So that there is no delay administering the normal saline flush, a 3-way stopcock is connected to the IV or intracavitary catheter, enabling simultaneous connection of the UCA and normal saline to the line (Fig. 7). The UCA is connected at the 180° (in-line) stopcock port, relative to the patient, to minimize shearing forces that can disrupt the microbubbles, and the normal saline is connected at the 90° port (side port) [29]. If UCA needs to be administered in a steady concentration over the course of the examination, it can be diluted into normal saline. In this case, the UCA/normal saline solution is also administered through the 180° port of the 3-way stopcock apparatus. Additionally, a syringe filled with normal saline is connected to the 90° port of the stopcock for the purpose of re-mixing the solution or diluting the solution (e.g., in intracavitary applications), if needed.
Contrast-enhanced US practice guidelines recommend using a catheter gauge (G) of at least 20 G, including central lines and implantable port systems, unless the filter system requires high injection pressure [30]. Larger bore catheters and needles are often too large for young or small children. However, using smaller ones requires a slower UCA administration rate. The catheter lumen size and injection rate should be considered when optimizing image quality. A flow phantom study found that the enhancement of two of the contrast agents tested was reduced when administered through a 24-G catheter, although microbubble concentration did not significantly decline [31]. In addition, injecting the UCA too slowly can compromise the qualitative and quantitative interpretation of contrast inflow and outflow as well as peak enhancement analysis. Meanwhile, previous in vitro work that used non-commercial contrast agents to test variations in the flow rate from 0.01 mL/s to 0.5 mL/s with catheter sizes ranging 23–30 G showed that microbubble destruction increased with smaller catheter caliber and a faster flow rate [32]. Both of these studies were done in vitro because testing these aspects of contrast administration in vivo would be technically difficult because of catheter placement as well as physiological differences among subjects.
Post-contrast imaging
Intravenous contrast-enhanced ultrasound
Contrast-enhanced US is ideally performed by two people working together: an examiner who maintains the US probe position over the desired anatomy and obtains the US images, and another person who administers and manages the contrast agent. For intravenous CEUS examination, the examiner begins the US timer after the contrast bolus is injected and before the IV cannula is flushed with normal saline to push forward any residual volume of contrast agent. After IV injection of UCA, the microbubbles remain strictly intravascular, so the CEUS evaluates their distribution in real time within the vascular bed of the examined organ or lesion.
Diagnostic intravenous CEUS evaluates not only the vascular pattern of the lesion or organ, but also the relative vascularity compared to adjacent normal tissue or other reference organs, such as when lung perfusion is assessed in relation to the liver or spleen. To accurately quantify and compare vascular perfusion parameters, it is important that the US scan settings and contrast dose remain unchanged during the image acquisition as well as between examinations if treatment-related changes are being assessed.
Contrast-enhanced US imaging involves acquiring a continuous cine clip starting with the IV injection of contrast agent until about 60 s. After that, intermittent images or short loops can be acquired to assess the washout of contrast agent in the tissue, avoiding excessive bubble destruction from continuous imaging. However, if quantitative CEUS is being performed to assess tissue perfusion, data acquisition must be continuous, so scanning must be carried on from contrast injection all the way through until microbubbles are completely dissipated. At the conclusion of the study, characteristic still frames can be saved from the cine loop to depict key vascular phases of contrast wash-in and washout [33]. If additional doses are warranted, it is important to be sure that the UCA is appropriately reconstituted prior to the next injection. Furthermore, whenever a subsequent injection of contrast agent is needed, adequate time should be allowed for the contrast agent to clear naturally from the body. Alternatively, flash mode (instant, momentary increase of MI) can be used to destroy microbubbles so that residual contrast circulation does not influence the interpretation. However, it is important to note that when using flash mode, only contrast microbubbles within the imaged field of view are disrupted at the time of higher MI imaging; following flash mode, a new volume of circulating microbubbles will come into the lesion or region of interest. Practically, the flash technique is preferred during contrast infusion-based rather than injection-based administration of UCA, where the transient increase in MI destroys the microbubbles in the imaging plane and the new volume of circulating microbubbles replenishes the contrast agent flow to the lesion or region.
Quantitative intravenous contrast-enhanced ultrasonography
In addition to a qualitative description of how contrast agent appears in a lesion or a parenchymal region relative to normal adjacent tissue or organs, quantitative parameters of contrast kinetics can also be measured. Images obtained during IV injection or infusion can be analyzed for contrast flow within tissues, which is useful for cardiac and post-treatment lesion assessment [33, 34]. Proprietary software has been established for quantifying the perfusion parameters and can be used on US units or via offline applications; these include VueBox (Bracco Suisse, Milan, Italy) [34].
By manually placing a region of interest within the examined area, the wash-in and washout characteristics of a tissue or lesion can be studied. The regions of interest should be sized appropriately to cover the examined area to achieve reliable measurements and avoid surrounding tissue. A time-intensity curve is generated by stationary, continuous scanning and recording in the region of interest throughout all phases of contrast enhancement. Various imaging parameters can be calculated, including peak enhancement, time to peak enhancement, area under the curve, regional blood volume, regional blood flow velocity, slope of the ascending curve, and slope of the descending curve [33, 34]. These terms are defined in the following ways:
-
Peak enhancement is the maximum enhancement that is achieved within the examined region of interest after UCA injection.
-
Time-to-peak enhancement is the time from the start of contrast injection until peak enhancement is reached.
-
Area under the curve corresponds to the overall enhancement of the given region of interest during the entire examination period.
-
Regional blood volume is generated from the area under the curve and corresponds to the volume of microbubbles that is measured in the examined region of interest during the examination time.
-
Regional blood flow velocity is measured from the division of the area under the curve and corresponds to the volume of microbubbles that is measured in the examined region of interest during the examination time.
-
Slope of the ascending curve is the maximum rate at which microbubbles of contrast agent enter or wash in the region of interest and corresponds to the wash-in rate.
-
Slope of the descending curve is the maximum rate at which microbubbles of contrast agent exit or wash out from the region of interest and corresponds to the washout rate.
Contrast-enhanced voiding urosonography
During contrast-enhanced voiding urosonography (ceVUS) the examiner evaluates the urinary bladder and kidneys during bladder filling and voiding phases. Additional urethral imaging is carried out during voiding. Still images or short cine clips of the kidneys and bladder can be saved throughout the study. Cyclical bladder filling is performed when age- or clinically appropriate.
Other intracavitary applications
Depending on the indication, UCAs can be administered into physiological or non-physiological body cavities. Physiological cavities refer to spaces that are normally present in the body, such as the genitourinary tract, biliary tract, bowel and pleural cavities. Examples of non-physiological cavities are abscesses, fistulous tracts, and cysts [35]. Intracavitary CEUS imaging is usually performed with direct UCA administration through an existing catheter or tube to ascertain its correct location, to demonstrate abnormal fistulous communications, and to delineate the morphology of complicated collections or abscesses.
Ultrasound contrast agent dosing
General principles for ultrasound contrast agent dosing
The optimal UCA dose depends on the sensitivity of the US scanner software, the US transducers used, the organ that is being examined, and the indication for the examination [22].
The degree of tissue enhancement is determined by the concentration of the microbubbles, with higher microbubble concentrations resulting in brighter tissue signals. However, similar to B-mode US imaging of highly echogenic structures (e.g., calcifications, cortical bone) that produce brighter signals and impair visualization of more distal structures, increased microbubble concentration in the near field results in signal loss in the far field. This phenomenon has been characteristically described in cases of intracavitary UCA administration, where high accumulation of microbubbles in the near field is associated with strong posterior acoustic shadow that obscures visualization of the far field (Fig. 8) [13, 36]. In the case of IV administration, a higher concentration of UCA within the examined tissue is associated with strong enhancement in the near field and weaker resonation of microbubbles in the deeper structures, and therefore reduced signal in the far field [19, 22]. If a lesion is located deeper within an organ, such as in the liver, it is ideal to avoid imaging through the larger vessels to visualize the lesion because the higher concentration of flow within the vessel relative to the parenchyma can result in a falsely reduced signal within the lesion [13]. Insufficient contrast agent results in reduced signal imaging, making it difficult to characterize the degree of washout during the delayed phase. Characterizing this degree of washout is crucial to determining malignancy, especially for liver lesions.
Contrast-enhanced voiding urosonography (ceVUS) with too-high contrast concentration. Transverse image of the bladder with dual-screen display of the B-mode (left) and contrast (right) images during ceVUS in a 7-year-old girl show that the concentration of the contrast agent in the bladder is too high, resulting in a strong posterior acoustic shadow caused by the contrast agent. The bladder base is obscured. This can be improved by diluting the contrast agent with saline, draining the bladder and refilling with a lower concentration, or by transiently increasing the mechanical index to disrupt microbubbles and decrease the contrast concentration in the bladder
Intravenous ultrasound contrast agent dose
The FDA-approved dose for pediatric liver and echocardiography is 0.03 mL/kg up to 2.4 mL per injection for liver applications and up to 2 mL per injection for echocardiography applications, which can be repeated once during the examination [8]. Prior to this package labeling, several dose recommendations of SonoVue/Lumason were made based on child weight, age, or practitioner experience, with single-dose injections ranging 0.1–4.8 mL [37]. Although recommended doses are available for the labeled indications, an incremental increase or decrease in the dose might be needed to account for equipment, technique and patient-related factors. Being able to recognize the appearance of suboptimal contrast concentration, either too much or too little, is an important part of study optimization. Factors that might require an increase in UCA dose include (1) using smaller-caliber IV catheters, (2) using higher-frequency transducers, (3) needing to image tissues or organs with relatively less blood flow, (4) imaging deeper structures and (5) needing to combat microbubble destruction to optimize image quality.
Optison is used off-label in children. In one safety and efficacy study including children with abdominopelvic malignancies, escalating doses of Optison were used on the basis of body surface area, beginning at a very low dose of 0.125 mL/m2 and escalating in 0.075 mL/m2 increments to 0.350 mL/m2 [38]. In this study the actual doses of Optison ranged 0.07–0.73 mL. Subsequent studies have used weight-based doses, with children weighing less than 20 kg receiving 0.3 mL and children weighing 20 kg or more receiving 0.5–0.6 mL [39,40,41,42].
There is no recommended pediatric IV dosing for Definity. Few publications exist regarding its use in children. A study of UCA safety at one center described its use for eight examinations in pediatric oncology patients but did not explicitly state the dose [40]. Three primarily adult studies that included a few children used Definity for liver, cardiac and neuroimaging, but again these studies did not state a specific pediatric dose [43,44,45]. The adult dose for IV indications ranges 0.2–0.3 mL [12].
Intravesical ultrasound contrast agent dose
SonoVue/Lumason is approved for intravesical administration in children to detect and grade vesicoureteral reflux. A dose of 1 mL is the current package label dosing [8]. Direct instillation of SonoVue/Lumason at doses of 0.5–2.5 mL have been reported, with most of authors considering 0.5 mL or 1 mL sufficient for optimal imaging [46,47,48,49,50,51,52,53,54,55]. Smaller volumes of Optison are described, ranging 0.15–0.5 mL for bladder filling [37, 56, 57]. The UCA can also be diluted in saline and infused into the bladder. The concentration of the UCA/normal saline solution ranges from 0.2% to 1% of bladder filling using SonoVue/Lumason and 0.06–0.5% for Optison [37, 57, 58]. There are no reports of Definity being used for ceVUS.
Optimizing the UCA concentration for intravesical administration is important. If the concentration of microbubbles is too high, a strong posterior acoustic shadow obscures visualization of the bladder base and distal ureters. If the concentration is too low, then it limits visualization of vesicoureteral reflux. While waiting for the child to void, continuous scanning of the bladder should be avoided to minimize microbubble destruction.
Intracavitary ultrasound contrast agent dose
Other cavities into which UCA can be instilled are the vagina and colon to define congenital genitourinary and anorectal anomalies. For these applications, dosing of 0.25–0.30 mL of Lumason diluted in 250 mL saline (0.1–0.12% concentration of Lumason/normal saline solution) has been reported [59, 60].
Contrast dose for interventional intracavitary techniques is reported to range 0.1–1.0 mL diluted in 50 mL or more of saline, with higher concentrations needed to evaluate unobstructed cavities and delineate fistulous tracts and lower concentrations in obstructed cavities [35].
Ultrasound contrast agent preparation
Each contrast agent has specific storage and reconstitution instructions that can be found in their respective package inserts. Commercial agents are packaged as single-use vials.
SonoVue/Lumason
The kit is stored at room temperature, 25 °C (77 °F) with a permitted range of 15–30 °C (59–86 °F) [8]. All components needed for reconstitution are within the kit. The plunger is screwed onto the prefilled 5-mL saline syringe, which is then connected to the mini-spike. The mini-spike is inserted in the vial septum and the saline is injected into the vial. The vial is then shaken vigorously by hand for 20 s until it forms a milky white solution, after which the UCA is ready to be withdrawn into a syringe for immediate use. In the event that there is a delay in the administration, the contrast components will separate into layers. If this occurs, prior to use, the agent should be reagitated until it has a milky white appearance. A vial may be used for up to 6 h from the time of reconstitution [61]. The adherence to these small steps in the preparation of the UCA helps to avoid microbubble destruction and unnecessary repeat examinations (Fig. 9).
Lumason reconstitution. a The Lumason kit contains all necessary parts for reconstitution, including directions attached to the lid, one glass vial, mini-spike, prefilled syringe with diluent and the syringe plunger rod. The plunger rod is attached by twisting it clockwise into the open end of the syringe. b Snap off the syringe cap to reveal the luer lock end and connect the mini-spike to the syringe. c Flip open the protective glass vial cap and remove protective cap from the mini-spike. Line up the spike with the center circle of the rubber stopper on the vial. d Insert the spike using firm pressure and inject the diluent into the vial. e Remove the syringe and close the green cap of the mini-spike. Shake vigorously by hand for a minimum of 20 s. When appropriately agitated, Lumason will have a milky white appearance and is ready for use. f Withdraw intended dose for immediate use. In this case a 3 mL syringe was used. The Lumason syringe can also be used for intravenous injection
Optison
The 3-mL vial is stored in a refrigerator at 2–8 °C (36–46 °F), and brought to room temperature before use [9]. The vial is inverted and gently rotated until it is a milky white suspension, then vented with either a vent spike or 18-G needle before being withdrawn into the injection syringe. It should be used within 1 min of resuspension. If it is not used within this time frame, the syringe should be gently rotated and inverted to reconstitute the agent prior to administration (Fig. 10).
Optison reconstitution. a Optison comes in a box of five 3-mL vials. The vial is removed from refrigeration and comes to room temperature. A spike or 18-G needle and a syringe are needed to draw up the contrast agent. b Gently agitate the vial by rolling between hands and inverting to resuspend the agent and bring it to room temperature. Inspect to be sure it is milky white and completely resuspended. c Flip protective cap off of the vial and remove the protective cover from the spike. Line the tip of the spike in the center of the rubber stopper and insert with firm pressure. An 18-G needle could be used as an alternative to the spike. d Remove the spike cap and attach syringe to withdraw intended dose for immediate use
Definity
The 2-mL vial needs refrigeration and is maintained at 2–8 °C (36–46 °F) [10]. The vial is brought to room temperature prior to activation. It is activated by shaking the vial in a proprietary shaker, Vialmix (Lantheus), for 45 s. Once properly mixed, a milky white suspension forms that should be used within 5 min. If there is a delay, Definity can be shaken by hand for 10 s to reconstitute. The vial can be used for up to 12 h from the time of Vialmix. If it is unused, it can be returned to refrigeration for up to 24 h. When it is time to withdraw the UCA from the vial, use the Intellipin (dispensing pin), PINSYNC (vented vial adaptor; Lantheus), or an 18-G or 20-G syringe needle. Once the contrast agent is withdrawn into the syringe, it must be used immediately (Fig. 11).
Definity reconstitution. a Definity comes as box of 4 or 16 2-mL vials. The vial is removed from refrigeration and comes to room temperature. It is then placed in the Vialmix shaker for 45 s. b At the end of 45 s, Definity has a milky white appearance. An 18-G needle, dispensing pin, or vial adapter is used to withdraw the contrast agent. c Remove the protective cap from the vial. Invert the vial and withdraw the intended dose for immediate use
Ultrasound contrast agent administration techniques
Both IV and intracavitary CEUS applications are used in children. Neither blood nor urine tests are required prior to UCA administration.
Intravenous administration
There are two main IV administration techniques: bolus-based injection and infusion-based administration.
Bolus injection
The bolus-based technique is widely used in diagnostic imaging to evaluate the wash-in and washout characteristics of a tissue or lesion [62]. Wash-in is described as the progressive enhancement within an area of interest from the arrival of microbubbles until maximum enhancement (peak enhancement), and washout is described as the subsequent reduction in enhancement following the peak.
The UCA injection is immediately followed by the saline flush. It has been suggested that a bolus rate of 1–2 mL/s is appropriate for minimizing microbubble destruction during administration and achieving optimal in vivo perfusion [9, 12]. The administration rate might need to be lower for smaller-caliber catheters.
Infusion
The infusion-based technique is mostly used for cardiac imaging or any other indication that requires prolonged imaging under a steady contrast concentration. Infusion-based technique consists of continuous slow administration of contrast agent. The contrast agent is usually diluted with saline solution, although undiluted contrast infusion has also been described, administered using an infusion pump to keep the infusion rate steady. Alternatively, drip infusion can be used.
Because contrast agent is continuously circulating, the washout phase of a lesion cannot be studied. Because lesion washout is an important feature that occurs most commonly in malignant lesions, this method cannot adequately assess focal lesions, particularly in the liver [22]. However, if evaluation of contrast kinetics during an infusion is needed, the “destruction-replenishment” technique can be applied where microbubbles in the plane of imaging are intentionally burst by a transient increase of the MI. Then the tissue reperfusion is evaluated by immediate visualization of in-flow of new bubbles within the region of interest. Most infusion studies have used SonoVue. There are no published reports of Optison infusion imaging in children. Definity has been used in echocardiography for children using an infusion scheme of a 3% dilution (4–6 mL/min) [63].
Intracavitary administration
Contrast-enhanced voiding urosonography
For ceVUS performance, the UCA is prepared according to the manufacturer’s recommendations. It can be either directly instilled into the bladder followed by a gravity infusion of saline, or infused as a UCA/normal saline solution in a similar manner to voiding cystourethrogram contrast agent. The solution can either be hung from an IV pole above the patient or pressurized with a sphygmomanometer cuff [36, 64].
Interventional radiology applications
Intracavitary administration of UCA is typically done via a direct injection for both physiological and non-physiological cavities through tubes or catheters. CEUS not only avoids the radiation associated with fluoroscopic intracavitary examinations, but it also has the capability to disrupt the microbubble agent, whereas the radiographic contrast agent persists unless it is drained, flushed, or is resorbed over time.
Conclusion
Performing diagnostic-quality examinations for qualitative and quantitative analysis is an integral part of introducing CEUS in a pediatric practice. Examiners need to have an understanding of how image quality is affected by scanner settings, examination techniques, and US contrast administration and dose. With expanding use and applications in children, these principles can be applied and used to optimize both IV and intracavitary CEUS examinations.
References
Greis C (2009) Ultrasound contrast agents as markers of vascularity and microcirculation. Clin Hemorheol Microcirc 43:1–9
Correas JM, Bridal L, Lesavre A et al (2001) Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol 11:1316–1328
Burns PN, Wilson SR (2006) Microbubble contrast for radiological imaging: 1. Principles. Ultrasound Q 22:5–13
Wilson SR, Burns PN (2006) Microbubble contrast for radiological imaging: 2. Applications. Ultrasound Q 22:15–18
Kessner R, Nakamoto DA, Kondray V et al (2019) Contrast-enhanced ultrasound guidance for interventional procedures. J Ultrasound Med 38:2541–2557
Yusuf GT, Fang C, Huang DY et al (2018) Endocavitary contrast enhanced ultrasound (CEUS): a novel problem solving technique. Insights Imaging 9:303–311
Huang DY, Yusuf GT, Daneshi M et al (2018) Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol 43:960–976
Bracco Diagnostics (2019) Lumason: highlights of prescribing information. https://imaging.bracco.com/sites/braccoimaging.com/files/technica_sheet_pdf/us-en-2020-01-15-spc-lumason.pdf. Accessed 26 July 2020
GE Healthcare (2016) Optison: highlights of prescribing information. http://www3.gehealthcare.com/~/media/documents/MarketoPDFsnogating/OPT-1H-OSLO_Optison_BK. Accessed 26 July 2020
Lantheus Medical Imaging (2001) Definity: highlights of prescribing information. https://www.lantheus.com//assets/Definity-PI-8.5x11_mktg-515987-0318.pdf. Accessed 26 July 2020
Bracco International (2001) SonoVue: summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/sonovue-epar-product-information_en.pdf. Accessed 26 July 2020
Dietrich CF, Averkiou M, Nielsen MB et al (2018) How to perform contrast-enhanced ultrasound (CEUS). Ultrasound Int Open 4:E2–E15
Greis C (2014) Technical aspects of contrast-enhanced ultrasound (CEUS) examinations: tips and tricks. Clin Hemorheol Microcirc 58:89–95
Quaia E (2007) Contrast-specific ultrasound techniques. Radiol Med 112:473–490
Azmin M, Harfield C, Ahmad Z et al (2012) How do microbubbles and ultrasound interact? Basic physical, dynamic and engineering principles. Curr Pharm Des 18:2118–2134
Sen T, Tufekcioglu O, Koza Y (2015) Mechanical index. Anatol J Cardiol 15:334–336
Nelson TR, Fowlkes JB, Abramowicz JS et al (2009) Ultrasound biosafety considerations for the practicing sonographer and sonologist. J Ultrasound Med 28:139–150
American Institute of Ultrasound in Medicine (2020) AIUM practice parameter for the performance of contrast-enhanced ultrasound examinations. J Ultrasound Med 39(3):421–429. https://doi.org/10.1002/jum.15204
Fetzer DT, Rafailidis V, Peterson C et al (2018) Artifacts in contrast-enhanced ultrasound: a pictorial essay. Abdom Radiol 43:977–997
Postema M, Schmitz G (2005) Ultrasonic fragmentation of microbubbles: a theoretical approach of the flash in flash-echo. Conf Proc IEEE Eng Med Biol Soc 4:4023–4026
Averkiou MA, Bruce MF, Powers JE et al (2020) Imaging methods for ultrasound contrast agents. Ultrasound Med Biol 46:498–517
Dietrich CF, Ignee A, Hocke M et al (2011) Pitfalls and artefacts using contrast enhanced ultrasound. Z Gastroenterol 49:350–356
Averkiou M, Lampaskis M, Kyriakopoulou K et al (2010) Quantification of tumor microvascularity with respiratory gated contrast enhanced ultrasound for monitoring therapy. Ultrasound Med Biol 36:68–77
Lassau N, Chapotot L, Benatsou B et al (2012) Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: the French multicenter support for innovative and expensive techniques study. Investig Radiol 47:711–716
McDicken WN, Anderson T (2011) Chapter 1: basic physics of medical ultrasound. In: Allan PLP, Baxter GM, Weston M (eds) Clinical ultrasound, 3rd edn. Churchill Livingstone, Edinburgh, pp 3–15
Ng AW, Swanevelder J (2011) Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain 11:186–192
Piscaglia F, Nolsoe C, Dietrich CF et al (2012) The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 33:33–59
Helfield B (2019) A review of phospholipid encapsulated ultrasound contrast agent microbubble physics. Ultrasound Med Biol 45:282–300
Kramer MR, Bhagat N, Back SJ et al (2018) Influence of contrast-enhanced ultrasound administration setups on microbubble enhancement: a focus on pediatric applications. Pediatr Radiol 48:101–108
Barr RG (2017) How to develop a contrast-enhanced ultrasound program. J Ultrasound Med 36:1225–1240
Eisenbrey JR, Daecher A, Kramer MR et al (2015) Effects of needle and catheter size on commercially available ultrasound contrast agents. J Ultrasound Med 34:1961–1968
Talu E, Powell RL, Longo ML et al (2008) Needle size and injection rate impact microbubble contrast agent population. Ultrasound Med Biol 34:1182–1185
Greis C (2011) Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc 49:137–149
Tranquart F, Mercier L, Frinking P et al (2012) Perfusion quantification in contrast-enhanced ultrasound (CEUS) — ready for research projects and routine clinical use. Ultraschall Med 33:S31–S38
Kljucevsek D, Riccabona M, Ording Muller LS et al (2020) Intracavitary contrast-enhanced ultrasonography in children: review with procedural recommendations and clinical applications from the European Society of Paediatric Radiology abdominal imaging task force. Pediatr Radiol 50:596–606
Darge K (2008) Voiding urosonography with ultrasound contrast agents for the diagnosis of vesicoureteric reflux in children. I. Procedure. Pediatr Radiol 38:40–53
Ntoulia A, Back SJ, Shellikeri S et al (2018) Contrast-enhanced voiding urosonography (ceVUS) with the intravesical administration of the ultrasound contrast agent Optison for vesicoureteral reflux detection in children: a prospective clinical trial. Pediatr Radiol 48:216–226
McCarville MB, Kaste SC, Hoffer FA et al (2012) Contrast-enhanced sonography of malignant pediatric abdominal and pelvic solid tumors: preliminary safety and feasibility data. Pediatr Radiol 42:824–833
Armstrong LB, Mooney DP, Paltiel H et al (2018) Contrast enhanced ultrasound for the evaluation of blunt pediatric abdominal trauma. J Pediatr Surg 53:548–552
Coleman JL, Navid F, Furman WL et al (2014) Safety of ultrasound contrast agents in the pediatric oncologic population: a single-institution experience. AJR Am J Roentgenol 202:966–970
McCarville MB, Coleman JL, Guo J et al (2016) Use of quantitative dynamic contrast-enhanced ultrasound to assess response to antiangiogenic therapy in children and adolescents with solid malignancies: a pilot study. AJR Am J Roentgenol 206:933–939
McMahon CJ, Ayres NA, Bezold LI et al (2005) Safety and efficacy of intravenous contrast imaging in pediatric echocardiography. Pediatr Cardiol 26:413–417
Bhayana D, Kim TK, Jang HJ et al (2010) Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol 194:977–983
Heppner P, Ellegala DB, Durieux M et al (2006) Contrast ultrasonographic assessment of cerebral perfusion in patients undergoing decompressive craniectomy for traumatic brain injury. J Neurosurg 104:738–745
Lanka B, Jang HJ, Kim TK et al (2007) Impact of contrast-enhanced ultrasonography in a tertiary clinical practice. J Ultrasound Med 26:1703–1714
Ascenti G, Zimbaro G, Mazziotti S et al (2004) Harmonic US imaging of vesicoureteric reflux in children: usefulness of a second generation US contrast agent. Pediatr Radiol 34:481–487
Duran C, del Riego J, Riera L et al (2012) Voiding urosonography including urethrosonography: high-quality examinations with an optimised procedure using a second-generation US contrast agent. Pediatr Radiol 42:660–667
Faizah MZ, Hamzaini AH, Kanaheswari Y et al (2015) Contrast enhanced voiding urosonography (ce-VUS) as a radiation-free technique in the diagnosis of vesicoureteric reflux: our early experience. Med J Malaysia 70:269–272
Kis E, Nyitrai A, Varkonyi I et al (2010) Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol 25:2289–2293
Kljucevsek D, Battelino N, Tomazic M et al (2012) A comparison of echo-enhanced voiding urosonography with X-ray voiding cystourethrography in the first year of life. Acta Paediatr 101:e235–e239
Papadopoulou F, Anthopoulou A, Siomou E et al (2009) Harmonic voiding urosonography with a second-generation contrast agent for the diagnosis of vesicoureteral reflux. Pediatr Radiol 39:239–244
Papadopoulou F, Ntoulia A, Siomou E et al (2014) Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol 44:719–728
Wong LS, Tse KS, Fan TW et al (2014) Voiding urosonography with second-generation ultrasound contrast versus micturating cystourethrography in the diagnosis of vesicoureteric reflux. Eur J Pediatr 173:1095–1101
Babu R, Gopinath V, Sai V (2015) Voiding urosonography: contrast-enhanced ultrasound cystography to diagnose vesico-ureteric reflux: a pilot study. J Indian Assoc Pediatr Surg 20:40–41
Wozniak MM, Osemlak P, Pawelec A et al (2014) Intraoperative contrast-enhanced urosonography during endoscopic treatment of vesicoureteral reflux in children. Pediatr Radiol 44:1093–1100
Colleran GC, Barnewolt CE, Chow JS et al (2016) Intrarenal reflux: diagnosis at contrast-enhanced voiding urosonography. J Ultrasound Med 35:1811–1819
Colleran GC, Paltiel HJ, Barnewolt CE et al (2016) Residual intravesical iodinated contrast: a potential cause of false-negative reflux study at contrast-enhanced voiding urosonography. Pediatr Radiol 46:1614–1617
Riccabona M, Vivier PH, Ntoulia A et al (2014) ESPR uroradiology task force imaging recommendations in paediatric uroradiology, Part VII: standardised terminology, impact of existing recommendations, and update on contrast-enhanced ultrasound of the paediatric urogenital tract. Pediatr Radiol 44:1478–1484
Chow JS, Paltiel HJ, Padua HM et al (2019) Contrast-enhanced colosonography for the evaluation of children with an imperforate anus. J Ultrasound Med 38:2777–2783
Seranio N, Darge K, Canning DA et al (2018) Contrast enhanced genitosonography (CEGS) of urogenital sinus: a case of improved conspicuity with image inversion. Radiol Case Rep 13:652–654
Robrecht J, Darge K (2007) In-vitro comparison of a 1st- and a 2nd-generation US contrast agent for reflux diagnosis. Rofo 179:818–825
Tang MX, Mulvana H, Gauthier T et al (2011) Quantitative contrast-enhanced ultrasound imaging: a review of sources of variability. Interface Focus 1:520–539
Kutty S, Xiao Y, Olson J et al (2016) Safety and efficacy of cardiac ultrasound contrast in children and adolescents for resting and stress echocardiography. J Am Soc Echocardiogr 29:655–662
Duran C, Beltran VP, Gonzalez A et al (2017) Contrast-enhanced voiding urosonography for vesicoureteral reflux diagnosis in children. Radiographics 37:1854–1869
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, M., Back, S.J., Didier, R.A. et al. Pediatric contrast-enhanced ultrasound: optimization of techniques and dosing. Pediatr Radiol 51, 2147–2160 (2021). https://doi.org/10.1007/s00247-020-04812-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04812-z