Abstract
Contrast-enhanced ultrasonography (US) has become an important supplementary tool in many clinical applications in children. Contrast-enhanced voiding urosonography and intravenous US contrast agents have proved useful in routine clinical practice. Other applications of intracavitary contrast-enhanced US, particularly in children, have not been widely investigated but could serve as a practical and radiation-free problem-solver in several clinical settings. Intracavitary contrast-enhanced US is a real-time imaging modality similar to fluoroscopy with iodinated contrast agent. The US contrast agent solution is administered into physiological or non-physiological body cavities. There is no definitive list of established indications for intracavitary US contrast agent application. However, intracavitary contrast-enhanced US can be used for many clinical applications. It offers excellent real-time spatial resolution and allows for a more accurate delineation of the cavity anatomy, including the internal architecture of complex collections and possible communications within the cavity or with the surrounding structures through fistulous tracts. It can provide valuable information related to the insertion of catheters and tubes, and identify related complications such as confirming the position and patency of a catheter and identifying causes for drainage dysfunction or leakage. Patency of the ureter and biliary ducts can be evaluated, too. US contrast agent solution can be administered orally or a via nasogastric tube, or as an enema to evaluate the gastrointestinal tract. In this review we present potential clinical applications and procedural and dose recommendations regarding intracavitary contrast-enhanced ultrasonography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracavitary (nonvascular, intraluminal) contrast-enhanced ultrasonography (US) is a technique where the US contrast agent is administered into physiological or non-physiological cavities. It has been described as a novel problem-solving technique in numerous clinical applications in adults [1]. Little has been published on intracavitary contrast-enhanced US in children apart from a contrast-enhanced voiding urosonography, which is the only intracavitary contrast-enhanced US application officially approved in children.
Any fluid (e.g., urine, water or saline) can be used to fill and delineate a cavity or similar anatomical structure. This approach is used for quite a few US techniques such as sonographic enema or sonogenitography [2, 3]. But there are clinical situations where this is diagnostically insufficient, e.g., in cases of complex collections or when communications within a cavity or with surrounding compartments or organs (e.g., fistulas) need to be shown or detected. Manual injection of air-agitated saline or a shaken 5% glucose solution has been used as a substitute contrast agent in some intracavitary US applications to improve visualisation — e.g., to determine the location of the tip of a catheter, or to depict shunts in echocardiography. However, these approaches are often insufficient for an accurate and reliable diagnosis, e.g., the unequivocal visualisation of drainage or leakage. Heinzmann et al. [4] demonstrated the benefit of US contrast agent applications via drainage catheters in fluid collections and the biliary system, and even of orally administered US contrast agent compared to the results of sonographic examinations using just saline and fluoroscopy with iodinated radiopaque contrast agent in adults. These researchers found that intracavitary contrast-enhanced US provides important additional information about location and dimensions of drained fluid collections and their communication with surrounding structures [4]. Furthermore, it allows for better visualisation of the biliary tree compared to US with saline solution, and it might provide important additional information when administered orally.
Intracavitary contrast-enhanced US can also be used as a complementing tool for US-guided interventions [5, 6]. At present, there is no defined list for possible intracavitary contrast-enhanced US applications, and in most cases it is used as a problem-solving tool (Table 1). It can be carried out as a bedside exam. In such cases intracavitary contrast-enhanced US has great potential to replace fluoroscopy, according to the scarce but existing literature and personal experiences of individuals within the European Society of Paediatric Radiology (ESPR) abdominal imaging task force.
The purpose of this review is to describe intracavitary contrast-enhanced US techniques focusing on the most relevant possible clinical applications in children.
Intracavitary contrast-enhanced ultrasound technique
Intracavitary contrast-enhanced US is a multistep procedure. First, a standard and detailed conventional US examination should be performed to obtain baseline information about the area of interest. It is very important to determine the ability to access and to visualise the targeted cavity/lumen. When there is a question or doubt as to whether intracavitary contrast-enhanced US or fluoroscopy is the best technique to answer the clinical question, intracavitary contrast-enhanced US should be performed first, followed by fluoroscopy if needed. Prior studies have shown that iodine contrast agents are relatively heavier and denser than US contrast agents and that iodine remains in the dependent part within a cavity and might therefore provide false-negative results of the following intracavitary contrast-enhanced US study [7]. If a combined intracavitary and intravenous contrast-enhanced US is needed, the intravenous application should be performed first, followed by intracavitary contrast-enhanced US after the disappearance of the US contrast agent from the blood. Contrast-specific software with low mechanical index has to be activated and a real-time split dual image — the contrast-enhanced and the grey-scale images — should be displayed on the screen.
The dose and the application route are determined next. The US contrast agent has to be prepared according to manufacturer’s guidelines and diluted in 0.9% saline. The resulting US contrast agent solution is then injected into cavities or tubes. Gravity drip or direct contrast injection might be considered. There is no standard dose for intracavitary contrast-enhanced US and it varies according to the type, size and location of the examined cavity. In general, a considerably lower dose of US contrast agent is necessary for intracavitary use compared to intravenous applications because of the relatively small volume of fluid in a cavity and a longer stability of microbubbles. When US contrast agent is applied in a cavity, a balance must be found between a large amount of contrast solution, which is necessary to prevent pooling of contrast agent in the cavity (because there is no free movement or distribution of the agent within the cavity), and a smaller amount of contrast solution to prevent artefacts from excessive signal (the cavity borders can be obscured) [1]. The microbubbles might also accumulate at the upper part of a cavity and obscure dependent parts that are known from contrast-enhanced voiding urosonography (Fig. 1) [8]. In this case, it is recommended to remove some fluid and perform a normal saline flush to redistribute the microbubbles within the cavity. If the US contrast agent concentration is too high, attenuation might occur, which disturbs the visualisation of the cavity margins (particularly the posterior part) and a too-large volume might cause overflow. Too-low concentration decreases the diagnostic efficiency because there are not enough microbubbles to give a satisfactory signal. A higher dose of US contrast agent solution (1–2%) is recommended for evaluation of communicating or unobstructed cavities with drainage such as the renal collecting system, and a lower dose (0.2–0.5%) is required when the cavity is obstructed or the main purpose is to depict the tip of a catheter or a drain [9]. The recommended dose, based on adult literature and personal experience of members of the ESPR abdominal imaging task force, is between 0.1% and 3%, depending on the US equipment, the type and frequency of the transducer, the assessed structure, and the purpose of the examination. The most commonly reported US contrast agent concentration range is 0.1–1.0 mL of SonoVue (Bracco SpA, Milan, Italy), or a few drops diluted in ≥10 mL of a 0.9% saline [10], or 0.2–0.4% [1, 5, 11]. In practice, it is better to start with a low dose of US contrast agent solution, and if this is insufficient, additional US contrast agent can be added. Experience from contrast-enhanced voiding urosonography is of great help in dose determination for other intracavitary applications. During the examination, video-clips should be stored; if this is not possible, at least key still images must be documented.
Intravesical application of ultrasound contrast agent. Dual-screen mode display with contrast-specific (left) and grey-scale (right) images in a 6-month-old boy after febrile urinary tract infection. Accumulation of high-concentrated ultrasound contrast agent (arrows) in the non-dependent urinary bladder obscures the dependent part and the retrovesical space
The safety profile for different intracavitary contrast-enhanced US applications has not been investigated except for the intravesical application (i.e. contrast-enhanced voiding urosonography). All safety studies regarding contrast-enhanced voiding urosonography in children showed a high safety profile of intravesically applied US contrast agent without serious adverse effects and only up to 3.7% of the minor transient effects, which are most likely caused by bladder catheterization rather than the US contrast agent itself [12, 13]. There are no safety studies regarding other intracavitary US contrast agent applications in adults or children. On the other hand, there are no reports of any side effects, either. Moreover, intracavitary contrast-enhanced US is expected to have an even lower rate of adverse effects than intravenous applications because there is less likely contact with the systemic circulation [1].
Clinical applications of intracavitary contrast-enhanced ultrasound
Urogenital tract
US contrast agent application into the urogenital system is the most common reported intracavitary application in children. The most common and well-established intravesical application of US contrast agent solution, contrast-enhanced voiding urosonography, is not addressed this review.
In contrast-enhanced percutaneous nephrosonography the US contrast agent solution is administered through the percutaneous inserted nephrostomy catheter. It can be used to evaluate catheter-related complications, ureter patency (i.e. the level of obstruction) and the ureteric junctions, to depict ureteric rupture (e.g., urine leak), and to delineate a fistula (Figs. 2 and 3). The potential of contrast-enhanced percutaneous nephrosonography as an alternative to fluoroscopic imaging for evaluating ureter patency has been shown in adults [11, 14, 15]. During the baseline US examination, it is most important to place the child in a position that allows imagers to optimally follow the course of the ureter. One should consider changing the position of the child during the investigation if the examination is started in the prone position. One of the limitations of this method is that bowel gas can obscure visualisation of the entire ureter (graded compression might help), and it is more difficult to obtain a nice panoramic view. US contrast agent can occasionally be used as an alternative to fluoroscopy during percutaneous nephrostomy placement (contrast-enhanced US-guided percutaneous nephrostomy) [1, 16].
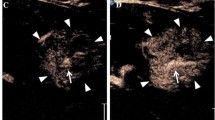
Bilateral nephrostomy in a 1.5-month-old girl who presented for evaluation of ureter patency by contrast-enhanced percutaneous nephrosonography. a, b Longitudinal sonograms at right renal level (a) and in the pelvis (b) show contrast agent in the ureter (arrowheads) and in the urinary bladder (star in b). c Anteroposterior fluoroscopic image with iodine contrast via the right-side nephrostomy provides the same information: both the pyeloureteric and vesicoureteric junctions are patent
Contrast-enhanced percutaneous nephrosonography in a 1-year-old boy with suspected ureterovesical stenosis. Dual-screen mode display with parametric microflow contrast (left) and grey-scale (right) images show ureterovesical stenosis with prevesical dilatation of the ureter (U) and a narrowed intramural part of the ureter (arrow). These were clearly depicted with only small jets of microbubbles (arrowheads) into the bladder lumen (star)
Evaluating the anatomy in congenital urogenital and anorectal anomalies is often challenging. Usually a combination of imaging techniques is required to delineate all cavities and abnormal connections among them. Contrast-enhanced voiding urosonography can improve the delineation of the urethra and demonstrate a possible urethro-vaginal fistula and urogenital sinus. In contrast-enhanced genito-urosonography the US contrast agent solution is applied into one of the abnormal perineal openings, such as into the urogenital sinus, or into the cloaca, or through a fistula canal (or a colonostomy) into the colon in contrast-enhanced colosonography (e.g., in anal atresia). The diagnostic accuracy can be increased by using 3-D/4-D US reconstructions and applying respective post-processing, e.g., an inversion technique [17]. The only case series that compared contrast-enhanced colosonography to fluoroscopic distal colonography in children with anorectal malformations showed that contrast-enhanced colosonography precisely depicted the complex anatomical relationships in all cases [18]. The advantages of contrast-enhanced US compared to fluoroscopy are that it is a radiation-free technique, provides good visualisation of structures in proximity to the genital tract, and has the potential ability of 3-D/4-D US reconstructions. Noteworthy, it is important to visualise the different cavities on the baseline US and to plan the imaging approach (e.g., anterior, posterior sagittal, trans-perineal views). In summary, considering all available knowledge and reports, the ESPR abdominal task force recommends intracavitary contrast-enhanced US as an option for evaluating anorectal and cloacal malformations [19]. In addition, intracavitary contrast-enhanced US could be helpful in the evaluation of uterine malformations (Müllerian fusion anomalies); the well-established adult application for tube patency assessment is irrelevant, particularly in early childhood.
The urethra is commonly evaluated as an intrinsic part of contrast-enhanced voiding urosonography during the voiding phase of the examination. If urethral pathology is not well seen during contrast-enhanced voiding urosonography, contrast-enhanced retrograde urethrosonography might offer an alternative to fluoroscopic retrograde urethrography [20]. As in fluoroscopic retrograde urethrography, a catheter is placed at the distal end of the anterior urethra (with or without fixation of a Foley catheter by inflating the balloon in the fossa navicularis) and the US contrast agent solution is slowly injected into the urethra. Alternatively, one can use the pull-back technique where the catheter is put close by the bladder neck and then slowly and stepwise withdrawn under constant US contrast agent solution infusion/injection, i.e. stopping every centimetre or so, adding contrast agent via the catheter. This technique is said to be particularly useful for detecting an ectopic ureter entering to the urethral lumen (Fig. 4). In addition, 3-D/4-D US reconstructions increase the depiction of urethral morphology [21].
Biliary system
US contrast agent solution can be administered into the biliary system through a percutaneously inserted biliary drainage catheter (contrast-enhanced cholangiosonography or by direct injection of US contrast agent solution into the gallbladder (contrast-enhanced percutaneous cholecysto-cholangiosonography) in children with suspected biliary atresia. Contrast-enhanced percutaneous cholecysto-cholangiosonography has proved to be an efficient method in many studies of biliary tree pathologies in adults, mainly related to the position, patency and efficiency of biliary catheter and postoperative complications [4, 22,23,24,25,26]. To our knowledge there are no publications on this technique in children, but from personal experience we have found that contrast-enhanced percutaneous cholecysto-cholangiosonography is very useful for evaluating biliary tree pathology after liver transplantation in children (Fig. 5). It should be considered when bile-drainage-related complications are suspected, or to define the level of bile drainage obstruction, bile leakage, and potential communications with surrounding structures. Zhou et al. [27] reported contrast-enhanced percutaneous cholecysto-cholangiosonography as a safe and effective tool in infants with suspected biliary atresia with equivocal US findings such as a gallbladder longer than 1.5 cm on conventional baseline US.
Contrast-enhanced cholangiosonography after liver transplantation in a 4-year-old boy. His biliary complication, leakage, was treated by percutaneous biliary drainage. Dual-screen mode display with contrast-specific (left) and grey-scale (right) US images shows a fluid collection in the liver. An injection of ultrasound contrast agent into the biliary catheter placed in Segment 2 was performed to search for recurrence of the bile leak. Opacification of the bile ducts (arrowhead) and the bowel (arrow, from bilio-enteric anastomosis) are present without any opacification of the fluid collection (circle). Biliary leakage could thereby be excluded
Again, some limitations of contrast-enhanced US of the biliary tract need to be considered. Visualisation of the biliary tract might be obscured by interfering bowel gas and by pooling of microbubbles within cystic duct remnants (e.g., after surgery), causing artefacts. In addition, the more subtle alterations of the bile ducts, e.g., in sclerosing cholangitis, are not always adequately depicted because of restricted detail resolution [4].
Pleural and peritoneal cavity
The assessment of the pleural space in the presence of an empyema is thought to be the most frequent intracavitary use of US contrast agent solution in children, according to a statement of the European Federation of Societies for Ultrasound in Medicine and Biology [28]. Deganello et al. [29] described the feasibility and diagnostic efficacy of combined intravenous and intrapleural contrast-enhanced US in children with complicated pneumonia. US contrast agent solution can be applied through a pleural drain and help assess drainage-related complications (drain position and patency, drainage effectiveness, non-communicating compartments) in cases where baseline US cannot reliably answer therapeutically relevant questions. US contrast agent might play a role in guiding and evaluating fibrinolytic therapy (i.e. confirming the adequacy of effective drainage after dissolution of the intrapleural septae that cause multiple loculations). Consequently, chest CT use might be reduced. Intravenous US contrast agent application is usually given before the intrapleural administration and, if necessary, can be used for assessing potentially necrotic lung areas, for example.
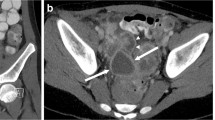
In contrast-enhanced sonographic peritoneography, US contrast agent is administered via a peritoneal catheter, e.g., using an existing peritoneal dialysis catheter. It might help to confirm catheter patency and to demonstrate the contrast agent distribution within the peritoneal cavity (Fig. 6). However, the exact localisation of the peritoneal catheter tip might be more challenging, and retroperitoneal leakage can be obscured by overlying bowel.
Contrast-enhanced ultrasound peritoneography in a 5-year-old girl with peritoneal catheter problems. a–c Transverse contrast-enhanced sonograms show free peritoneal distribution of the ultrasound contrast agent in the contrast-specific images (left) around the liver (a), between intestinal loops (b) and around the urinary bladder (c). Corresponding grey-scale US images are on the right
Gastrointestinal tract
Intracavitary contrast-enhanced US of the gastrointestinal tract is less frequent, probably because water or any other drinks or fluid (e.g., polyethylene glycol solution) is often sufficient to achieve good visualisation of the respective digestive tract. This approach has been described for exploration of the upper gastrointestinal tract (oesphagus, stomach, duodenal loop); small bowel (small intestine contrast-enhanced ultrasound using polyethylene glycol); and the colon and rectum (saline enema or hydrocolon) [2, 3, 30,31,32,33]. Intracavitary contrast-enhanced US of the gastrointestinal tract can provide additional information in cases where the details needed regarding the endoluminal morphology are not provided by conventional approaches, for example better delineation of the mucosa surface to depict contour irregularities, a better image of the lumen (e.g., in cases of stenosis), and more conspicuous delineation of the bowel’s relation to surrounding structures [4, 34]. The clinical scenarios in children where enteric US contrast agent application could be useful are in evaluation of the duodenal loop position to exclude malrotation in vomiting children, or in achieving a better demonstration of the site and length of duodenal stenosis (Fig. 7). Additionally, intracavitary contrast-enhanced US can be useful to confirm the correct position of a percutaneous gastrostomy tube or a potential leak. Orally administered US contrast agent solution has been described for screening children with suspected gastro-oesophageal reflux and for monitoring children undergoing medical or surgical treatment of gastro-oesophageal reflux. However, it has no potential to replace a well-standardized 24-h pH-metry, and usually other filling techniques using tea or water, even a formula meal, are sufficient [35].
Imaging in a 13-year-old boy with recurrent vomiting. a Anteroposterior fluoroscopic image shows dilation of a proximal part (arrows) of the duodenum — potentially indicating a stenosis. The ligament of Treitz was positioned a little lower than normal. Most of the small-bowel loops were situated in the right abdomen with the cecum on the left side, indicative of malrotation. b Contrast-enhanced ultrasound of the duodenal loop (dual-screen mode display with contrast-specific [left] and grey-scale [right] images) was performed to exclude external obstruction because of a marked dilation of the proximal duodenum. The proximal part of the duodenal loop (arrowheads) was dilated and filled with ultrasound contrast agent for a while. c Subsequently, the contrast agent advanced without signs of outside compression, thus no evidence for annular pancreas or superior mesenteric artery syndrome was identified. Ultrasound contrast agent was seen within the small-bowel loops situated on the right side of the abdomen (not shown). At surgery an almost normal position of duodenal loop was confirmed
Abscesses and fistulas
Ultrasound contrast agent solution can be administered into abscesses or collections to outline the whole cavity and identify morphological details such as the presence of septations, loculations and fistulous communications with nearby structures. Intracavitary contrast-enhanced US of an abscess might provide more accurate or conspicuous information compared to the information obtained by only injecting saline. It might also be a helpful approach to guide the insertion of a drainage catheter into the larger loculation by monitoring the drainage efficiency or checking for potential leakage [1, 5].
Fistulas can be the result of congenital malformations (described in urogenital part) or an acquired disease and are usually treated by surgery. Intracavitary contrast-enhanced US has been found useful in the detection of post-surgical gastrointestinal fistulas and fistulas associated with extraluminal Crohn disease [36, 37].
Other intracavitary contrast-enhanced ultrasound applications
In theory, most indications for fluoroscopy with iodinated contrast agents could become indications for intracavitary contrast-enhanced US — provided there are sufficient sonographic access and a treatment-relevant clinical question. Contrast-enhanced US-guided interventions are reported as an adjunctive tool that improves the success rate and helps to avoid complications during interventional procedures [5, 6].
Discussion
Intracavitary contrast-enhanced US has great potential to complement or even replace fluoroscopy in many of the examinations described in this review (Table 1). It has been shown to be useful as an adjunct tool in contrast-enhanced US-guided interventions. It is important to be familiar with the advantages and limitations of its use.
There are many advantages of intracavitary contrast-enhanced US compared to fluoroscopy. Contrast-enhanced US is a radiation-free method. It is a real-time dynamic examination in which the distribution of microbubbles within and outside a cavity can be assessed during a long observation time. It can be performed at the bedside or in an intraoperative setting, particularly if it is hazardous to transfer a child to the radiology suite. Ultrasound machines are widely available in every hospital, and most modern devices are equipped with dedicated contrast-specific software.
However, the limitations of the method have to be acknowledged. First, the overall experience in children is quite limited. Therefore, there cannot be a single standardized approach, but rather an individual case-by-case approach must be applied. Additionally, all known limitations regarding the use of ultrasound have to be taken into account (e.g., the impact of body habitus, disturbing bowel gas, insufficient access to some anatomical regions), and it is more difficult or even impossible to obtain a panoramic display. It can also be challenging to display the pathology to the clinicians and surgeons who might think it is an inferior “road map” for them compared to the more familiar conventional fluoroscopy. The cost of contrast-enhanced US might be higher than classic fluoroscopic iodinated radiopaque contrast agents, with yet unsolved reimbursement issues. And finally, at present all nonvascular contrast-enhanced US applications, particularly in children, are off-label except for contrast-enhanced voiding urosonography. Nevertheless, off-label use is a common situation in paediatrics, where many drugs have to be used without being tested (no randomized studies) or approved for children; almost 40% of the drug prescriptions given to children in the general population are off-label, reaching 67% in hospitals [38, 39]. Thus the parents or legal representatives have to be informed about the use of off-label US contrast agents and written informed consent is needed as well as a justifying indication. Despite all restrictions regarding off-label use some contrast-enhanced US examinations have already been included in the recommendations of the ESPR abdominal task force as an alternative option, not only for contrast-enhanced voiding urosonography [40, 41] but also for other intracavitary applications, such as evaluation of the urethra with retrograde contrast-enhanced urethrosonography [17, 38] or imaging of cloacal and urogenital malformations [19, 42]. The ESPR abdominal task force recommends using the same US contrast agent concentration (0.1–3% saline solution) as for contrast-enhanced voiding urosonography for all other non-vascular intracavitary and intraluminal applications, with higher concentrations (0.5–1%, up to 3%) for high-frequency transducers and non-obstructive communicating cavities (e.g., collecting system) and the lower end of the US contrast agent concentration range (0.1–1%) for lower frequencies in obstructed non-communicating cavities (such as an abscess). Because of the lack of evidence, no strict recommendations for indications can be issued; however, the task force encourages consideration of this novel approach as a non-irradiating imaging option for various applications. This is particularly true for applications via drainage catheters/tubes, regardless of the type of cavity/lumen (most common in the urinary tract, the biliary tree, and pleural cavity), for contrast-enhanced genito-urosonography and colosonography, for fistula detection, and for various US-guided interventional procedures where some promising experience has been accumulated. Intracavitary contrast-enhanced US is also listed as an alternative method in various situations in adults and children by the European Federation of Societies for Ultrasound in Medicine and Biology [8, 26].
Currently, there is a growing trend in paediatric radiology towards noninvasive, radiation-free and child-friendly methods — in accordance with the Image Gently and ALARA (as low as reasonably achievable) dose principles. Intracavitary contrast-enhanced US has not been extensively investigated in children (especially in infants and neonates), but studies in adults and published case series or case reports in children as well as personal experiences and communications in the field of paediatric radiology are encouraging. However, knowledge, education and experience are important, and even if these US contrast agent applications are currently off-label, it does not mean that they are banned.
Conclusion
Various intracavitary contrast-enhanced US examinations have been recognized as safe problem-solving examinations. Paediatric radiologists have to advocate for positively impacting new methods and make them available for children — even if these applications are currently off-label.
References
Yusuf GT, Fang C, Huang DY et al (2018) Endocavitary contrast enhanced ultrasound (CEUS): a novel problem solving technique. Insights Imaging 9:303–311
Riccabona M, Coley B (2014) US-guided interventions. In: Riccabona M (ed) Pediatric ultrasound: requisites and applications, 1st edn. Springer, Berlin, pp 60–75
Nagy E, Riccabona M (2019) Sonographic enema (“hydrocolon”) for the assessment of colonic pathology: case series to demonstrate the feasibility in neonates and infants. Pediatr Radiol 49:S285–S286
Heinzmann A, Müller T, Leitlein J et al (2012) Endocavitary contrast enhanced ultrasound (CEUS) — work in progress. Ultraschall Med 33:76–84
Huang DY, Yusuf GT, Daneshi M et al (2017) Contrast-enhanced US-guided interventions: improving success rate and avoiding complications using US contrast agents. Radiographics 37:652–664
Menendez-Castro C, Zapke M, Fahlbusch F et al (2017) Microbubbles in macrocysts — contrast-enhanced ultrasound assisted sclerosant therapy of a congenital macrocystic lymphangioma: a case report. BMC Med Imaging 17:39
Colleran GC, Paltiel HJ, Barnewolt CE, Chow JS (2016) Residual intravesical iodinated contrast: a potential cause of false-negative reflux study at contrast-enhanced voiding urosonography. Pediatr Radiol 46:1614–1617
Ključevšek D, Pečanac O, Tomažič M, Glušič M (2019) Potential causes of insufficient bladder contrast opacification and premature microbubble destruction during contrast-enhanced voiding urosonography in children. J Clin Ultrasound 47:36–41
Zheng R, Xu E (2013) Intracavitary contrast-enhanced ultrasound. In: Weskott HP (ed) Contrast-enhanced ultrasound, 2nd edn. UNI-MED Verlag, Bremen, pp 214–221
Sidhu PS, Cantisani V, Dietrich CF et al (2018) The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (short version). Ultraschall Med 39:154–180
Daneshi M, Yusuf GT, Fang C et al (2019) Contrast-enhanced ultrasound (CEUS) nephrostogram: utility and accuracy as an alternative to fluoroscopic imaging of the urinary tract. Clin Radiol 74:167.e9–167.e16
Papadopoulou F, Ntoulia A, Siomou E, Darge K (2014) Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol 44:719–728
Darge K, Papadopoulou F, Ntoulia A et al (2013) Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr Radiol 43:1063–1073
Chi T, Usawachintachit M, Weinstein S et al (2017) Contrast enhanced ultrasound as a radiation-free alternative to fluoroscopic nephrostogram for evaluating ureteral patency. J Urol 198:1367–1373
Chi T, Usawachintachit M, Mongan J et al (2017) Feasibility of antegrade contrast-enhanced US nephrostograms to evaluate ureteral patency. Radiology 283:273–279
Liu BX, Huang GL, Xie XH et al (2017) Contrast-enhanced US-assisted percutaneous nephrostomy: a technique to increase success rate for patients with nondilated renal collecting system. Radiology 285:293–301
Seranio N, Darge K, Canning DA, Back SJ (2018) Contrast enhanced genitosonography (CEGS) of urogenital sinus: a case of improved conspicuity with image inversion. Radiol Case Rep 13:652–654
Chow JS, Paltiel HJ, Padua HM et al (2019) Contrast-enhanced colosonography for the evaluation of children with an imperforate anus. J Ultrasound Med 38:2777–2783
Riccabona M, Lobo ML, Ording-Müller LS et al (2017) European Society of Paediatric Radiology abdominal imaging task force recommendations in paediatric uroradiology, Part IX: imaging in anorectal and cloacal malformation, imaging in childhood ovarian torsion, and efforts in standardising paediatric uroradiology terminology. Pediatr Radiol 47:1369–1380
Riccabona M, Darge K, Lobo ML et al (2015) ESPR uroradiology taskforce — imaging recommendations in paediatric uroradiology, Part VIII: retrograde urethrography, imaging disorder of sexual development and imaging childhood testicular torsion. Pediatr Radiol 45:2023–2028
Woźniak MM, Pawelec A, Wieczorek AP et al (2013) 2D/3D/4D contrast-enhanced voiding urosonography in the diagnosis and monitoring of treatment of vesicoureteral reflux in children — can it replace voiding cystourethrography? J Ultrason 13:394–407
Luyao Z, Xiaoyan X, Huixiong X et al (2012) Percutaneous ultrasound-guided cholangiography using microbubbles to evaluate the dilated biliary tract: initial experience. Eur Radiol 22:371–378
Spârchez Z, Radu P, Spârchez M et al (2013) Intracavitary applications of ultrasound contrast agents in hepatogastroenterology. J Gastrointestin Liver Dis 22:349–353
Xu EJ, Zheng RQ, Su ZZ et al (2012) Intra-biliary contrast-enhanced ultrasound for evaluating biliary obstruction during percutaneous transhepatic biliary drainage: a preliminary study. Eur J Radiol 81:3846–3850
Ignee A, Cui X, Schuessler G, Dietrich CF (2015) Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z Gastroenterol 53:385–390
Ignee A, Baum U, Schuessler G, Dietrich CF (2009) Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD). Endoscopy 41:725–726
Zhou LY, Chen SL, Chen HD et al (2018) Percutaneous US-guided cholecystocholangiography with microbubbles for assessment of infants with US findings equivocal for biliary atresia and gallbladder longer than 1.5 cm: a pilot study. Radiology 286:1033–1039
Sidhu PS, Cantisani V, Deganello A et al (2017) Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement. Ultraschall Med 38:33–43
Deganello A, Rafailidis V, Sellars ME et al (2017) Intravenous and intracavitary use of contrast-enhanced ultrasound in the evaluation and management of complicated pediatric pneumonia. J Ultrasound Med 36:1943–1954
Pallotta N, Civitelli F, Di Nardo G et al (2013) Small intestine contrast ultrasonography in pediatric Crohn's disease. J Pediatr 163:778–784
Flaum V, Schneider A, Gomes Ferreira C et al (2016) Twenty years' experience for reduction of ileocolic intussusceptions by saline enema under sonography control. J Pediatr Surg 51:179–182
Cho HH, Cheon JE, Choi YH et al (2015) Ultrasound-guided contrast enema for meconium obstruction in very low birth weight infants: factors that affect treatment success. Eur J Radiol 84:2024–2031
Nakaoka T, Nishimoto S, Tsukazaki Y et al (2017) Ultrasound-guided hydrostatic enema for meconium obstruction in extremely low birth weight infants: a preliminary report. Pediatr Surg Int 33:1019–1022
Badea R, Socaciu M, Ciobanu L et al (2010) Contrast-enhanced ultrasonography (CEUS) for the evaluation of the inflammation of the digestive tract wall. J Gastrointestin Liver Dis 19:439–444
Farina R, Pennisi F, La Rosa M et al (2008) Contrast-enhanced colour-Doppler sonography versus pH-metry in the diagnosis of gastro-oesophageal reflux in children. Radiol Med 113:591–598
Xu EJ, Zhang M, Li K et al (2018) Intracavitary contrast-enhanced ultrasound in the management of post-surgical gastrointestinal fistulas. Ultrasound Med Biol 44:502–507
Mao R, Chen YJ, Chen BL et al (2019) Intra-cavitary contrast-enhanced ultrasound: a novel radiation-free method to detect abscess associated penetrating disease in Crohn's disease. J Crohns Colitis 13:593–599
Conroy S, McIntyre J, Choonara I, Stephenson T (2000) Drug trials in children: problems and the way forward. Br J Clin Pharmacol 49:93–97
Schreiber-Dietrich DG, Cui XW, Piscaglia F et al (2014) Contrast enhanced ultrasound in pediatric patients: a real challenge. Z Gastroenterol 52:1178–1184
Riccabona M, Vivier PH, Ntoulia A et al (2014) ESPR uroradiology task force imaging recommendations in paediatric uroradiology, Part VII: standardised terminology, impact of existing recommendations, and update on contrast-enhanced ultrasound of the paediatric urogenital tract. Pediatr Radiol 44:1478–1484
Riccabona M, Lobo ML, Augdal TA et al (2018) European Society of Paediatric Radiology abdominal imaging task force recommendations in paediatric uroradiology, Part X: how to perform paediatric gastrointestinal ultrasonography, use gadolinium as a contrast agent in children, follow up paediatric testicular microlithiasis, and an update on paediatric contrast-enhanced ultrasound. Pediatr Radiol 48:1528–1536
Avni FE, Lerisson H, Lobo ML et al (2019) Plea for a standardized imaging approach to disorders of sex development in neonates: consensus proposal from European Society of Paediatric Radiology task force. Pediatr Radiol 49:1240–1247
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ključevšek, D., Riccabona, M., Ording Müller, LS. et al. Intracavitary contrast-enhanced ultrasonography in children: review with procedural recommendations and clinical applications from the European Society of Paediatric Radiology abdominal imaging task force. Pediatr Radiol 50, 596–606 (2020). https://doi.org/10.1007/s00247-019-04611-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04611-1