Abstract
Vascular malformations are a heterogeneous group of entities, many of which present in the pediatric age group. Sonography plays a major role in the management of children with these vascular anomalies by providing information that helps in diagnosing them, in assessing lesion extent and complications, and in monitoring response to therapy. The interpretation of sonographic findings requires correlation with clinical findings, some of which can be easily obtained at the time of scanning. This has to be combined with the use of appropriate nomenclature and the most updated classification in order to categorize these patients into the appropriate management pathway. Some vascular malformations are part of combined vascular anomalies or are associated with syndromes that include other disorders, frequently limb overgrowth, and these are now being reclassified based on their underlying genetic mutation. Sonography has limitations in the evaluation of some vascular malformations and in these cases MR imaging might be considered the imaging modality of choice, particularly for lesions that are large, that involve multiple compartments or are associated with other soft-tissue and bone abnormalities. In this article, which is part 2 of a two-part series, the authors review the most relevant clinical and sonographic features of arteriovenous, capillary, venous and lymphatic malformations as well as vascular malformations that are part of more complex conditions or associated with syndromes, including Parkes–Weber syndrome, phosphatase and tensin homologue (PTEN) hamartoma tumor syndromes, Klippel–Trénaunay syndrome, CLOVES (congenital lipomatous overgrowth, vascular malformations, epidermal nevi and skeletal anomalies) syndrome, fibro-adipose vascular anomaly and Proteus syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As mentioned in the part 1 of this series, vascular malformation is one of the two broad categories in which vascular anomalies are divided in the classification proposed by the International Society for the Study of Vascular Anomalies (ISSVA) [1] (Table 1).
Sonography plays a major role in the evaluation of vascular malformations by providing valuable information that helps in diagnosing, in assessing lesion extent and complications and in monitoring response to therapy. One must reiterate that the interpretation of the sonographic findings has to be correlated with the clinical findings. When the clinical information has not been provided by the referring clinician, the sonographer or the radiologist has the opportunity to get valuable clues by asking a few basic questions and observing the lesion just before starting the US examination (see part 1 of this series). The final interpretation should not only discuss the sonographic findings but should also make use of appropriate nomenclature referring to the most updated classification because this information is used to categorize these patients into the appropriate management pathway.
It is important to remember that sonography has limitations and might not be useful in accurately assessing the extent of larger and deeper lesions, in detecting flow in low-flow lesions and in assessing associated soft-tissue and bone abnormalities. In these situations MR imaging is a better option.
In part 2 of this series, we review the most relevant clinical and sonographic features of simple vascular malformations. These are summarized in Table 2. We also review some complex combined vascular malformations, which are often associated with syndromes, and we have categorized them based on their genetic origin.
Vascular malformations
In the 2014 revision of the ISSVA classification, vascular malformations were subdivided into four categories: simple, combined, of a major vessel, and associated with other anomalies [1] (Table 1). The simple vascular malformations were further categorized based on the type of vessel affected (arteriovenous, capillary, venous or lymphatic malformations). Combined malformations are those with two or more types of vascular malformation forming a lesion. Malformation of a major vessel includes anomalies of origin, course, number, length, diameter, valves, communication (arteriovenous fistula) and persistence of an embryonic vessel. Vascular malformations can be associated with multiple syndromes, and in these conditions they are one of several presenting anomalies.
Vascular malformations are composed of abnormal vessels and show cellular turnover without proliferation or cellular hyperplasia [2, 3]. They are congenital but many vascular malformations are not evident at birth and manifest in infancy or even at a later age. They grow commensurately with the child although the growth can be stimulated by hormonal influence during puberty or pregnancy, trauma, clotting or can be idiopathic [2]. Regression is not expected to occur.
Arteriovenous malformation
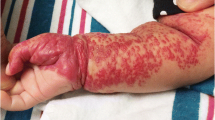
Arteriovenous malformations represent anomalous connections between arteries and veins formed by a complex network of primitive vessels and with partial or complete absence of normal intervening capillaries [4]. Arteriovenous malformations are frequently in the differential diagnosis of high-flow lesions, although they are overall less common than many high-flow tumors. The clinical progression of arteriovenous malformations along with their severity is graded using the Schobinger classification, in which stage I represents a quiescent lesion, more often seen in young children. In stage II, the lesion has expanded and become warmer, with a palpable thrill. In stage III, there are signs of destruction with ulcers, hemorrhage or bone lytic lesions. Stage IV reflects the development of heart failure in a stage III patient [4]. One of the characteristic presentations at the time of imaging is growth and pain in a longstanding lesion, which is owed to its upstaging in the Schobinger classification, most commonly near times of puberty or pregnancy, and it is thought to be secondary to hormonal influences [5]. These patients commonly present with ischemic symptoms from arteriovenous shunting, bleeding and ulceration (Fig. 1). Precautions should be taken when scanning these lesions because they can be extremely fragile and a large amount of blood can be quickly lost. A US division plan for this, including a hemostasis kit, can be extremely beneficial. A hemostasis kit should at a minimum include one of the many commercially available hemostasis pads, 4″ × 4″ cotton gauze, a large occlusive adherent dressing, gloves and an elastic-type tape or self-adherent wrap for creating a pressure dressing if needed. A combination of endovascular embolization and surgical resection is the mainstay of treatment for arteriovenous malformations today.
Arteriovenous malformation with cutaneous ulceration (Schobinger stage III) in a 13-year-old girl who presented with rapid growth of longstanding pulsatile lesion in the face and upper neck requiring multiple transfusions. The malformation was successfully treated with endovascular embolization and surgical resection. (Parental consent was obtained for publication of this clinical photograph)
On sonography, arteriovenous malformations are characterized by the presence of a conglomerate of tortuous vessels, which are often visible on gray-scale imaging, typically without a discrete soft-tissue mass [6,7,8,9] (Fig. 2). Sonography can be used to grossly estimate the size of the lesion and determine its complexity by assessing the number of inflow and outflow vessels and its association with adjacent structures [9]. Arteriovenous malformations can involve multiple soft-tissue planes and adjacent bone [10]. Color Doppler interrogation reveals the characteristic high flow of these lesions by showing high vascular density with multidirectional flow. Spectral Doppler analysis shows high velocities, low resistance and spectral broadening in the arteries, which is more evident when compared with normal arteries in the adjacent soft tissues not involved by the malformation or in the contralateral side. Spectral Doppler analysis of the venous component shows pulsatile flow with relatively high velocities [6,7,8,9, 11,12,13] (Fig. 2). Spectral Doppler sonography can also be used to monitor response to interventional radiologic treatment [9].
Arteriovenous malformation (Schobinger stage II) in a 7-year-old girl who presented with persistent swelling in the left ankle for several months. a Transverse sonogram shows a subcutaneous mass composed of a tangle of prominent vessels (arrows). b Transverse color Doppler sonogram confirms the high vascular density of the mass. c Spectral Doppler analysis shows typical high-velocity arterial flow with a low-resistance pattern reflecting the abnormal arteriovenous communication
In the series of Paltiel et al. [6], 43% of arteriovenous malformations had no gray-scale imaging abnormality and were only recognized using color Doppler sonography. Although this could be in part from lower resolution of older equipment, it still reinforces the value of using color Doppler sonography in the evaluation of suspected vascular anomalies.
Dubois et al. [11] reported three cases of arteriovenous malformation that presented on sonography with a small soft-tissue mass, in contrast to the classic description of lack of a discrete mass in this type of vascular anomaly. These cases can be difficult to differentiate from hemangiomas. This unusual sonographic pattern might be related to recent descriptions of a relatively high frequency of atypical findings of soft-tissue arteriovenous malformations on MR imaging, including 43% presenting with a discrete soft-tissue mass [14].
Fatty tissue is frequently seen surrounding arteriovenous malformations because of fibro-fatty proliferation [12]. However, this should be differentiated from the intramuscular fast-flow vascular anomalies seen in children with phosphatase and tensin homologue (PTEN) gene mutations, which are now referred to as PTEN hamartoma of soft tissue. In these hamartomas there is substantial amount of intralesional fat, which appears as hyperechoic tissue admixed with large vessels [15] (see section on PTEN hamartoma tumor syndromes).
Capillary malformation
Several types of lesions are listed in the category of capillary malformations, including the port wine stain, a relatively common cutaneous or mucosal lesion seen in 0.3% of newborns [2]. This often presents as a well-demarcated red or pink macule in any area of the body, usually with a segmental distribution. Capillary malformations are not usually clinically significant in and of themselves, with the exception of significant cosmetic concerns, but these can be important because of their association with a host of other disorders, such as Sturge-Weber syndrome, the newly described diffuse capillary malformation with overgrowth [16] and several other entities that are discussed separately in this article. The treatment approach for capillary malformation typically involves pulsed-dye laser in addition to the specific treatment of the associated underlying vascular anomalies.
With the usual transducers, capillary malformations are often not visible or only manifest as focal thickening of the skin and subcutaneous soft tissues, which is easier to recognize when comparing with the normal contralateral side [6]. With dedicated sonographic equipment to evaluate the skin, including the use of 20-MHz transducers, capillary malformations can appear as superficial hypoechoic areas that vary in depth from 0.2 mm to 3.7 mm with a mean maximum depth of 1.0 mm [17]. However even with the use of this type of equipment, 18% of capillary malformations are not recognized [17]. Color Doppler sonography might reveal slightly increased vascular density when compared with the adjacent normal dermis [18], likely reflecting progressive dilatation of ectatic capillary channels [8].
Venous malformation
Venous malformations are the most common vascular malformation, with a prevalence of 1% in the general population [19]. They present clinically with dysplastic ectatic superficial veins or, more frequently, with a soft-tissue mass that can be associated with a bluish discoloration of the overlying skin if superficial in location (Fig. 3). They are usually very soft and compressible, and the typical history includes fluctuating size with activity, and gravity-dependent position. They can also be clinically suspected when the superficial component shows purple venous blebs (Fig. 4). The white pebbles of phleboliths are occasionally seen within a dilated vein in venous malformations, or felt by the sonographer’s fingers during scanning. Venous malformations are episodically tender, usually from internal clot formation and sometimes stretching and distension. Venous malformations can also become more symptomatic during puberty and pregnancy, and this is secondary to increased intralesional clotting. Treatment typically includes image-guided sclerotherapy or surgical resection [20].
Clinical photograph of venous malformations in a 15-year-old boy who presented with soft compressible masses (arrows) and diffuse purple skin discoloration in the upper extremity. The masses engorged when the arm was placed down and decreased in size when the arm was elevated. There was also skin discoloration in the right chest wall. These findings are all consistent with venous malformation
Venous malformation in a 5-year-old girl who presented with growing purple blebs of the tongue and left face (arrows) and pain. These venous blebs are consistent with venous malformation. The girl was successfully treated with image-guided sodium tetradecyl sulfate sclerotherapy. (Parental consent was obtained for publication of this clinical photograph)
On sonography, venous malformations often present as compressible, well-marginated masses with a spongiform appearance from the presence of ectatic venous spaces separated by echogenic septa [21] (Fig. 5). Sometimes they appear as a multicystic mass resembling a lymphatic malformation (see next section; Fig. 6) and infrequently they show anechoic, tubular structures that can be easily recognized as vascular channels [21] (Fig. 7). They are generally heterogeneous and mostly hypoechoic when compared with the adjacent subcutaneous soft tissues, although occasionally they appear isoechoic or hyperechoic [21] (Fig. 8). The detection of intralesional calcifications representing phleboliths is very helpful in the diagnosis of venous malformations (Fig. 5) because these are rare in other pediatric soft-tissue masses. However this sign’s practical utility is often overstated because phleboliths are not common in pediatric venous malformations. Trop et al. [21] recognized phleboliths in only 16% of cases in their series and Paltiel et al. [6] in only 9%.
Venous malformation in a 2-year-old girl who presented with a 6-month history of right paraspinal mass. a Longitudinal sonogram shows a well-defined intramuscular mass (arrows) that is heterogeneous from the presence of hypoechoic venous spaces separated by hyperechoic septa. This is the typical spongy appearance of venous malformations. b Transverse sonogram shows the presence of an intralesional calcification in keeping with a phlebolith (arrow)
Venous malformation in a 3-year-old boy who presented with a mass in the left posterior chest wall. Color Doppler sonogram shows a subcutaneous lesion composed of multiple small irregular anechoic spaces (arrows), some slightly tubular and others more round in shape, without internal flow. Flow only appears in the soft tissues adjacent to the presumed vascular spaces. The sonographic appearance of the lesion can be interpreted as a lymphatic malformation, but subsequent MR imaging (not shown) revealed enhancement of the anechoic spaces in keeping with a venous malformation
Venous malformation in a 10-year-old girl who presented with intermittent swelling in the dorsum of the foot. Longitudinal sonogram shows anechoic, tortuous, tubular structures (arrows) and others with a more round shape (arrowheads), in keeping with large dysplastic veins as part of a venous malformation. Subcutaneous fat (F) is noted between the dysplastic veins
Venous malformation in a 16-year-old girl who presented with a mass in the right upper arm. Color Doppler sonogram shows an irregular mass in the subcutaneous tissues (arrows) that is predominantly hyperechoic but contains a few hypoechoic areas. There is no significant vascularity in the lesion. This is an atypical presentation of venous malformation that could be confused with a microcystic lymphatic malformation or with a fat-containing mass. The final diagnosis was made with MR imaging
Doppler interrogation shows low vascular density, although it not infrequently has difficulty demonstrating intralesional vascularity (Figs. 6 and 8). The detection of flow can be enhanced by applying compression or performing the Valsalva maneuver [12]. Spectral Doppler analysis typically shows venous flow, although Trop et al. [21] reported absence of flow in 16% of their cases, even when optimizing the scanning technique. This apparent lack of vascularity is attributed either to very slow flow that is not detectable with conventional equipment or to extensive thrombosis of the venous spaces. On occasion arterial flow can be detected, and this is thought to indicate the presence of a capillary malformation component (combined vascular malformation) [12, 21], but it could also just represent normal arteries coursing through the lesion or rarely a sign of intravascular papillary endothelial hyperplasia, also known as Masson tumor, which appears to be an abnormal response to thrombosis [22, 23].
Some venous malformations are intra-articular or juxta-articular with involvement of a joint, particularly the knee (Fig. 9). These used to be incorrectly referred to as synovial hemangiomas. The importance of making the diagnosis of these lesions is that they can cause repeated episodes of hemarthrosis, leading to synovial hypertrophy and damage of the articular cartilage and resulting in early development of osteoarthritis [24]. This involvement must be identified for treatment planning so that inadvertent passage of sclerosing agent into the joint during sclerotherapy does not occur because this can result in joint damage. Surgical excision of these lesions is usually recommended.
Venous malformation in a 4-year-old girl who presented with repeated episodes of swelling of the right knee. a Longitudinal and (b) transverse sonograms of the right distal thigh and right knee show heterogeneous soft-tissue thickening in the suprapatellar recess. There are a hypoechoic component containing thin echogenic lines anterior to the distal femur (F) caused by venous malformation (arrows) and a more hyperechoic and lobulated component representing a combination of involvement of the synovium by venous malformation and secondary synovial hypertrophy (arrowheads). There is an associated knee joint effusion (E), likely representing hemarthrosis
There is therefore a wide spectrum of sonographic appearances of venous malformations which, along with atypical clinical presentations, can sometimes result in a diagnostic challenge. Further evaluation with MR imaging might be indicated in these situations.
Lymphatic malformation
Lymphatic malformations are subclassified into macrocystic, microcystic and mixed types. Some authors have proposed a size criterion to differentiate macrocystic from microcystic lesions, using as a cut-off value 1 cm or 2 cm, whereas other authors use as a criterion for macrocystic type the fact that the lesion can be successfully reduced in size with aspiration or sclerosis as opposed to the microcystic lesions, which cannot [25]. As with other disorders, the correct categorization of these lesions has a significant impact on which treatment options are offered. Lymphangioma is an old term used for lymphatic malformations that should be avoided because the suffix “-oma” should be reserved for proliferating tumors.
The typical clinical history of lymphatic malformation is of a lesion growing with the child, with episodic pain that is many times concurrent with viral illnesses or minor trauma. Some helpful clinical signs aid in the diagnosis of lymphatic malformation. The most helpful for microcystic lymphatic malformations is the presence of lymphatic vesicles, which can be found on the skin (Fig. 10) and on the mucosal surfaces of the oral cavity, where they are easily identifiable. Additionally, large exophytic, ecchymotic lesions are suggestive of macrocystic lymphatic malformations complicated with intralesional hemorrhage (Fig. 11). The treatment of microcystic lymphatic malformations has undergone a recent evolution with the introduction of mechanistic target of rapamycin (mTOR) inhibitors such as sirolimus, which have been found to be effective [26]. Image-guided bleomycin sclerotherapy and surgical resection can also be effectively used in select cases [27]. Surface lesions of the oral cavity and skin can be safely and effectively treated with carbon dioxide laser photoevaporation procedures, although the recurrence rate without concurrent medical therapy is high [20]. Image-guided sclerotherapy is usually the front-line treatment for macrocystic lymphatic malformations [28]. Mechanistic targets of rapamycin inhibitors are also used in certain clinical scenarios of widespread involvement, either primarily or as adjunctive therapy, whereas surgical resection is usually reserved as a second-line therapy.
Clinical photograph of microcystic lymphatic malformation in a 6-year-old girl who presented with painful swelling of the abdominal wall that had been intermittent for years and grew as she grew. Clinical exam shows numerous fluid-filled vesicles of the skin, some with internal hemorrhage and hematocrit levels (arrows), representing stasis and layering of heavier cellular elements of blood dependent to liquid supernatant. This is consistent with microcystic lymphatic malformation
Clinical photograph of macrocystic lymphatic malformation in a 3-day-old girl who presented with rapidly growing chest wall masses and decreased hemoglobin requiring transfer to the neonatal intensive care unit. There is a right-side ecchymotic mass consistent with macrocystic lymphatic malformations, complicated by intralesional hemorrhage that required transfusion. The girl was successfully treated with image-guided doxycycline sclerotherapy
Lymphatic malformations are more frequently found in the subcutaneous tissue, although involvement of multiple planes is not rare. Macrocystic lymphatic malformations appear as a lesion containing multiple cystic spaces that are separated from one another by thin septa. Compression with the transducer can deform the cystic spaces but they do not collapse, and this can be a helpful hint in the differentiation from venous malformations [29]. The cystic spaces can be anechoic (Fig. 12) or have varying degrees of echogenicity, sometimes with fluid-fluid levels, the latter more often seen when complicated with hemorrhage [7, 30] (Fig. 13). Fluid-fluid levels in vascular anomalies are not pathognomonic of lymphatic malformations because they can also be seen in venous malformations because of very slow flow with blood stagnation and formation of hematocrit levels [20]. Hematocrit levels represent stasis and layering of heavier cellular elements of blood dependent to liquid supernatant. Doppler interrogation of lymphatic malformations often reveals absence of flow or low vascular density with arterial or venous flow confined to the septa (Fig. 12). The flow should not be present within the cystic spaces, which would be more characteristic of a venous malformation. In case of superimposed infection, increased echogenicity and hyperemia of the adjacent soft tissues becomes evident and the fluid in the cystic spaces tends to be more echogenic.
Macrocystic lymphatic malformation in an 18-day-old boy who presented with a mass in the right shoulder. a Transverse sonogram shows a subcutaneous multicystic mass (arrows). The cystic spaces are anechoic and separated from one another by thin septa. b Longitudinal color Doppler sonogram shows flow that is confined to the septa, without flow in the cystic spaces
Macrocystic lymphatic malformation in a newborn boy with antenatal diagnosis of neck mass. Transverse sonogram of the right and anterior aspects of the neck shows a large complex mass composed of cystic spaces of different shapes and sizes, some anechoic and others hyperechoic from hemorrhage. A fluid-debris level is seen within a large cyst posteriorly (arrows). The fluid-debris level is vertically oriented because of the transducer position on the right side of the neck
Microcystic lymphatic malformations often appear as ill-defined hyperechoic lesions that contain scattered cystic spaces measuring less than 1–2 cm, although sometimes no cysts are visible (Fig. 14). The increased echogenicity is attributed to the multiple interfaces caused by tiny cysts that are beyond the resolution of the US equipment [7, 12]. Doppler interrogation shows absent flow or low vascular density.
Microcystic lymphatic malformation in a 10-year-old girl who presented with a 2-year history of slowly progressive swelling in the right axilla associated with cutaneous vesicles. Transverse sonogram shows an ill-defined subcutaneous lesion (arrows) that is predominantly hyperechoic and contains tiny cysts (arrowheads) measuring less than 1 cm
Combined macrocystic and microcystic lymphatic malformations are not uncommon and show sonographic features of both types of lesions.
Sonographic mimickers of vascular malformations
We have described the main sonographic features that help in distinguishing the types of vascular malformations that are most frequently seen in children. However there are instances in which the differential diagnosis includes non-vascular tumors. Unfortunately the literature has mainly focused on this issue with regard to MR imaging, with very little information about sonographic appearances [23, 30].
As mentioned, venous malformations can sometimes have unusual nonspecific sonographic appearances, including the presence of intralesional arterial flow, making it difficult to exclude neoplastic lesions. Furthermore the clinical scenario might not be helpful, particularly in deep intramuscular lesions, which often present at a later age and are associated with pain — both red flags for neoplastic lesions [23]. In this situation, further investigation with MR imaging is the usual recommendation. In our experience sarcomas can mimic the gray-scale appearance of venous malformations because areas of necrosis and prominent vascular spaces can result in a hypoechoic spongy appearance, particularly with intramuscular lesions. However in this situation, Doppler sonography is usually extremely useful by showing a pattern of vascularity that is not typical for venous malformation, therefore alerting to the possibility of a neoplasm that requires further investigation with MR imaging.
Other neoplastic lesions can mimic the sonographic appearance of lymphatic malformations, particularly the macrocystic and combined types. These lesions include synovial sarcoma, epithelioid sarcoma and angiomatoid fibrous histiocytoma [30, 31]. To make the correct diagnosis, it is important to have a good clinical history and pay attention to the presence of thick septa and solid vascularized components, which raise suspicion for a neoplastic tumor because these are not usually seen in lymphatic malformations [30].
Complex and syndromic vascular anomalies
A long and heterogeneous list of entities is associated with vascular malformations, and many correspond to overgrowth syndromes. These are often better evaluated with MR imaging because of their commonly extensive involvement of the extremities, sometimes with extension into the trunk, and also because of their frequent association with other soft-tissue and bone anomalies including limb overgrowth [3, 32,33,34].
RASA1 gene mutation-related vascular anomalies
Mutations in the RASA1 gene are found in entities that can present with high-flow vascular malformations. These include capillary malformation–arteriovenous malformation (CM-AVM) disorders, which are characterized by the presence of multiple cutaneous capillary malformations associated with underlying arteriovenous malformations [35]. Parkes–Weber syndrome is a specific type of CM-AVM that presents with limb overgrowth, more commonly affecting one of the lower extremities [33,34,35]. On clinical exam, the affected limb is very warm, showing a temperature discrepancy when compared with the unaffected limb. Typically pulsatility of the involved extremity is not appreciated. Common symptoms are exercise intolerance and arterial claudication from arteriovenous shunting and relative hypoxemia to the affected limb. Visually this manifests as non-healing ulcers of the involved extremity in advanced cases (Fig. 15).
Clinical photograph of Parkes–Weber syndrome in a 14-year-old girl initially diagnosed with Klippel–Trénaunay syndrome referred for an ultrasound exam after unsuccessful skin graft of the anterior thigh. Findings of capillary malformation, overgrowth and non-healing ulcers are noted (arrows), the latter consistent with Parkes–Weber syndrome, resulting in a change in the diagnosis and treatment. Endovascular embolization of arteriovenous fistulas was performed, with subsequent clinical improvement
Sonography can be helpful in assessing the increased flow to the affected extremity and in evaluating the more superficial arteriovenous malformations [35]. Sonography can also aid in diagnosis by showing arteriovenous malformations that lie deep to the clinically evident capillary malformations [36]. The arteriovenous shunting shows on Doppler interrogation with characteristic low-resistance arterial waveforms and pulsatile venous flow.
PTEN hamartoma tumor syndromes
Mutations in the PTEN tumor suppressor gene are now known to be the common underlying factor in both Bannayan–Riley–Ruvalcaba syndrome and Cowden syndrome and therefore these two conditions are now better referred to as PTEN hamartoma tumor syndromes [33]. These syndromes present with high-flow vascular anomalies that were traditionally regarded as arteriovenous malformations. However more recent observations have documented that these high-flow lesions correspond at histology with an overgrowth of an admixture of adipose tissue, fibrous tissue and abnormal blood vessels of various types [37, 38]. Kurek et al. [38] coined the term PTEN hamartoma of soft tissue (PHOST) for these lesions, although it is not clear whether they are true hamartomas or benign neoplasms. Associated clinical findings that help in the diagnosis include macrocephaly and developmental delay as well as a history of intestinal hamartomas. In boys, characteristic penile freckles might be seen. Recognition of a PHOST can be very important because it can be the first manifestation of PTEN hamartoma tumor syndromes, which are associated with a higher risk of developing certain malignant tumors, particularly of breast, thyroid, endometrium and kidney [38]. Treatment has traditionally been surgical for symptomatic lesions, although there are early reports of a possible role for mTOR inhibitors [39].
PHOST is usually multiple and the bulk of the lesion is in an intramuscular location although involvement of the adjacent fascial plane, subcutaneous tissue and dermis is common and occasionally might even cause disruption of underlying cortical bone [38]. There is typically disproportionate dilatation of the proximal veins draining the lesion. The sonographic appearance of PHOST has rarely been documented [15]. These lesions tend to be ill-defined, causing expansion and disruption of the affected muscle, and show heterogeneous echogenicity, being predominantly hyperechoic from the ectopic adipocytic tissue, and with visible large vessels on gray-scale images (Fig. 16). Color Doppler interrogation shows a high vascular density and spectral Doppler analysis shows features suggestive of arteriovenous malformation.
PTEN hamartoma of soft tissue (PHOST) in a 7-year-old boy who presented with swelling of the right lower thigh and with a suprapatellar bruit on exam. a Transverse sonogram shows a large, ill-defined hyperechoic mass (arrows) within the quadriceps femoris muscle. The mass is heterogeneous and predominantly hyperechoic from fatty tissue, but it also contains hypoechoic areas representing large vessels (arrowheads). F femur. b Transverse sonogram shows a cluster of large vessels (arrows) within the mass, representing the typical large veins draining this type of lesion. c Transverse color Doppler sonogram and spectral Doppler analysis show multiple large vessels within the mass, some with pulsatile and turbulent venous flow from the anomalous arteriovenous communication. At the initial presentation, the diagnosis of atypical arteriovenous malformation was made. Subsequent genetic testing confirmed PTEN mutation, which is in conjunction with the imaging findings prompted the diagnosis of PHOST
PIK3CA gene mutation-related syndromes
Somatic mosaic mutations of the PIK3CA (phosphatidylinositol-4, 5-biphospate 3-kinase catalytic subunit alpha) gene, now grouped as PIK3CA-related overgrowth spectrum, can manifest phenotypically as different entities presenting with vascular anomalies, including Klippel–Trénaunay syndrome, CLOVES (congenital lipomatous overgrowth, vascular malformations, epidermal nevi and skeletal anomalies) and fibro-adipose vascular anomaly (FAVA) [33]. Clinically these lesions can be suspected when marked overgrowth is identified and combined low-flow vascular anomalies are clinically evident [33, 40, 41]. When scanning these plaques of lymphatic vesicles and underlying phlebectasia with embryonic veins, one must be prepared for bleeding and have a hemostasis plan in the US division because these lesions can be quite friable and the authors have encountered this situation on multiple occasions.
Klippel–Trénaunay syndrome
Klippel–Trénaunay syndrome presents with capillary, venous and lymphatic malformations without high-flow lesions, commonly affecting one lower extremity, although bilateral cases, involvement of an upper extremity and extension into the trunk are not rare [32]. The classic triad includes capillary malformation, venous malformation/varicosities and limb overgrowth involving the bone and soft tissues (Fig. 17). The typical venous anomaly is the presence of a dilated vein in the lateral aspect of the thigh and lower leg, known as marginal vein of Servelle, seen in approximately 55% of the patients; this has been associated with a hypoplastic or interrupted lower limb deep venous system [32]. Sonography can be useful in evaluating localized venous or lymphatic malformations, demonstrating the venous anatomy and helping to evaluate patients with acute localized pain by showing thrombosis of the anomalous veins (Fig. 18), an increasingly recognized cause of mortality and morbidity in this patient population [33].
Clinical photograph of Klippel–Trénaunay syndrome in a 12-year-old girl who presented with bleeding from the lateral left thigh and history of pulmonary emboli. Note the soft-tissue overgrowth, associated plaques of lymphatic vesicles (arrow) and capillary malformation (arrowheads) along the entire leg, extending to the lateral foot. Embryonic veins were noted on sonography
CLOVES syndrome
CLOVES syndrome typically presents with a congenital truncal lipomatous mass that sometimes contains a lymphatic malformation (Fig. 19). In addition, venous malformations (phlebectasia) and less commonly arteriovenous malformations, frequently in paraspinal location, might also be present in addition to cutaneous and musculoskeletal anomalies such as epidermal nevus, hand and feet overgrowth, macrodactyly and sandal gap toe deformity [33, 40] (Figs. 19 and 20). There is no specific information regarding the role of sonography in CLOVES syndrome but it may be able to help in the evaluation of the aforementioned vascular anomalies.
Clinical photograph shows typical findings in CLOVES syndrome, here in a 2-month-old boy with left upper extremity and chest wall lipomatous overgrowth. There is ecchymosis (arrow) from internal hemorrhage within a macrocystic lymphatic malformation. There is also a capillary malformation involving the right lower leg and foot as well as macrodactyly (curved arrow). CLOVES congenital lipomatous overgrowth, vascular malformations, epidermal nevi and skeletal anomalies
Clinical photograph shows CLOVES syndrome in an 11-year-old girl with right lower limb asymmetrical overgrowth, capillary malformation on the lateral right thigh and sandal gap toe deformity in both feet. In addition, sonography showed an underlying macrocystic lymphatic malformation, which was causing pain and was successfully treated with image-guided doxycycline sclerotherapy. CLOVES congenital lipomatous overgrowth, vascular malformations, epidermal nevi and skeletal anomalies
Fibro-adipose vascular anomaly (FAVA)
FAVA is a complex mesenchymal malformation characterized by focal or diffuse intramuscular fibro-fatty replacement associated with low-flow vascular malformations — predominantly phlebectasia and less commonly lymphatic malformations — that can also affect the adjacent subcutaneous tissues but usually without dermal involvement [42]. FAVA is more frequently seen as an isolated intramuscular lesion that can be very large, although occasionally it represents a complex combined vascular anomaly component of an overgrowth syndrome. The muscles most commonly affected are those in the calf, particularly the gastrocnemius, and in the forearm. Clinically these can present at any age with constant and severe pain.
Sonography shows an ill-defined hyperechoic intramuscular solid mass representing the fibro-fatty replacement [15, 42] (Fig. 21). Large veins are noted within the mass, which can extend into the overlying subcutaneous tissues and is sometimes complicated by thrombi.
Fibro-adipose vascular anomaly (FAVA) in a 14-year-old girl who presented with swelling and pain in the right calf. a Longitudinal sonogram using extended field-of-view technique shows a heterogeneous mass (arrows) within the gastrocnemius muscle. The mass is predominantly hyperechoic because of the presence of fibro-fatty tissue but also contains large vessels. b Transverse color Doppler sonogram and spectral Doppler analysis show that the vessels on gray-scale images are large veins, in keeping with the typical phlebectasia seen in FAVA. The sonographic findings were subsequently confirmed with MR imaging
AKT1 somatic gene mutation-related Proteus syndrome
Proteus syndrome is a rare condition characterized by progressive, segmental and disproportionate overgrowth that affects the dermis, fat, bones and central nervous system [41]. Mosaic somatic mutation of the AKT1 gene has been found in more than 90% of patients with Proteus syndrome. These patients might have low-flow vascular malformations, including capillary, venous, lymphatic and combined types. Some of the clinical manifestations overlap with those seen in CLOVES syndrome, although in Proteus syndrome there are no high-flow vascular malformations and the lipomatous mass is postnatal instead of congenital and can affect the extremities in addition to the trunk [40, 41]. Cerebriform nevus is the most characteristic skin lesion in children with Proteus syndrome [41] (Fig. 22).
There is limited information about the role of sonography in Proteus syndrome and because of its associated anomalies, MR imaging is the first-line modality in these children. However there are reports of sonography use in the detection of thrombi in enlarged peripheral veins in patients with Proteus syndrome [43].
Conclusion
Sonography is a useful tool in the evaluation of pediatric soft-tissue vascular malformations, providing valuable information for diagnosis and in some cases for monitoring treatment and complications. This requires close correlation with the clinical findings and use of updated classification. However it is important to recognize that some entities, particularly venous malformations, can have a broad spectrum of appearances and that the diagnosis using sonography alone is sometimes limited. It is also noteworthy that sonography has a limited role in complex and syndromic vascular anomalies, mainly because of lesion extent, inherent characteristics of the lesion, and associated anomalies. MR imaging might be indicated in such cases.
References
International Society for the Study of Vascular Anomalies (2014) ISSVA classification of vascular anomalies. http://www.issva.org/UserFiles/file/Classifications-2014-Final.pdf. Accessed 12 April 2017
Frieden I, Enjolras O, Esterly N (2003) Vascular birthmarks and other abnormalities of blood vessels and lymphatics. In: Schachner LA, Hansen RC (eds) Pediatric dermatology, 3rd edn. Mosby, New York, pp 833–862
Merrow AC, Gupta A, Patel MN et al (2016) 2014 revised classification of vascular lesions from the International Society for the Study of Vascular Anomalies: radiologic–pathologic update. Radiographics 36:1494–1516
Uller W, Alomari AI, Richter GT (2014) Arteriovenous malformations. Semin Pediatr Surg 23:203–207
Enjolras O, Wassef M, Chapot R (2007) Color atlas of vascular tumors and vascular malformations, 1st edn. Cambridge University Press, New York
Paltiel HJ, Burrows PE, Kozakewich HP et al (2000) Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 214:747–754
Dubois J, Garel L (1999) Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol 29:879–893
Legiehn GM, Heran MK (2006) Classification, diagnosis and interventional radiologic management of vascular malformations. Orthop Clin N Am 37:435–474
Dunham GM, Ingraham CR, Maki JH et al (2016) Finding the nidus: detection and workup of non-central nervous system arteriovenous malformations. Radiographics 36:891–903
Lowe L, Marchant TC, Rivard DC et al (2012) Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol 47:106–117
Dubois J, Patriquin HB, Garel L et al (1998) Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR Am J Roentgenol 171:247–252
Dubois J, Alison M (2010) Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 40:895–905
Ballah D, Cahill AM, Fontalvo L et al (2011) Vascular anomalies: what they are, how to diagnose them, and how to treat them. Curr Probl Diagn Radiol 40:233–247
Patel AS, Schulman JM, Ruben BS et al (2015) Atypical MRI features in soft-tissue arteriovenous malformation: a novel imaging appearance with radiologic–pathologic correlation. Pediatr Radiol 45:1515–1521
Sheybani EF, Eutsler EP, Navarro OM (2016) Fat-containing soft-tissue masses in children. Pediatr Radiol 46:1760–1773
Lee MS, Liang MG, Mulliken JB (2013) Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol 69:589–594
Troilius A, Svendsen G, Ljunggren B (2000) Ultrasound investigation of port wine stains. Acta Derm Venereol 80:196–199
Alfageme Roldán F, Salgüero Fernández I, Muñoz Garza Z (2016) Update on the use of ultrasound in vascular anomalies. Actas Dermosifiliogr 107:284–293
Legiehn GM, Heran MK (2008) Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin N Am 46:545–597
Behr GG, Johnson CM (2013) Vascular anomalies: hemangiomas and beyond — part 2, slow-flow lesions. AJR Am J Roentgenol 200:423–436
Trop I, Dubois J, Guibaud L et al (1999) Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 212:841–845
Restrepo R (2013) Multimodality imaging of vascular anomalies. Pediatr Radiol 43:S141–S154
Olivieri B, White CL, Restrepo R et al (2016) Low-flow vascular malformation pitfalls: from clinical examination to practical imaging evaluation — part 2, venous malformation mimickers. AJR Am J Roentgenol 206:952–962
Dalmonte P, Granata C, Fulcheri E et al (2012) Intra-articular venous malformations of the knee. J Pediatr Orthop 32:394–398
Wassef M, Blei F, Adams D et al (2015) Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 136:e203–e214
Hammill AM, Wentzel M, Gupta A et al (2011) Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer 57:1018–1024
Acevedo JL, Shah RK, Brietzke SE (2008) Nonsurgical therapies for lymphangiomas: a systematic review. Otolaryngol Head Neck Surg 138:418–424
Cahill AM, Nijs EL (2011) Pediatric vascular malformations: pathophysiology, diagnosis, and the role of interventional radiology. Cardiovasc Intervent Radiol 34:691–704
Morrow MS, Oliveira AM (2014) Imaging of lumps and bumps in pediatric patients: an algorithm for appropriate imaging and pictorial review. Semin Ultrasound CT MR 35:415–429
White CL, Olivieri B, Restrepo R et al (2016) Low-flow vascular malformation pitfalls: from clinical examination to practical imaging evaluation — part 1, lymphatic malformation mimickers. AJR Am J Roentgenol 206:940–951
Yikilmaz A, Ngan BY, Navarro OM (2015) Imaging of childhood angiomatoid fibrous histiocytoma with pathological correlation. Pediatr Radiol 45:1796–1802
Lobo-Mueller E, Amaral JG, Babyn PS et al (2009) Complex combined vascular malformations and vascular malformation syndromes affecting the extremities in children. Semin Musculoskelet Radiol 13:255–276
Uller W, Fishman SJ, Alomari AI (2014) Overgrowth syndromes with complex vascular anomalies. Semin Pediatr Surg 23:208–215
Navarro OM (2016) Magnetic resonance imaging of pediatric soft-tissue vascular anomalies. Pediatr Radiol 46:891–901
Clemens RK, Pfammatter T, Meier TO et al (2015) Combined and complex vascular malformations. Vasa 44:92–105
Kim C, Ko CJ, Baker KE et al (2015) Histopathologic and ultrasound characteristics of cutaneous capillary malformations in a patient with capillary malformation-arteriovenous malformation syndrome. Pediatr Dermatol 32:128–131
Tan WH, Baris HN, Burrows PE et al (2007) The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and management. J Med Genet 44:594–602
Kurek KC, Howard E, Tenant L (2012) PTEN hamartoma of soft tissue: a distinctive lesion in PTEN syndromes. Am J Surg Pathol 36:671–687
Iacobas I, Burrows PE, Adams DM et al (2011) Oral rapamycin in the treatment of patients with hamartoma syndromes and PTEN mutation. Pediatr Blood Cancer 57:321–323
Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I et al (2017) CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS). Clin Genet 91:14–21
Hagen SL, Hook KP (2016) Overgrowth syndromes with vascular malformations. Semin Cutan Med Surg 35:161–169
Alomari AI, Spencer SA, Arnold RW et al (2014) Fibro-adipose vascular anomaly: clinical–radiologic–pathologic features of a newly delineated disorder of the extremity. J Pediatr Orthop 34:109–117
Kaduthodil MJ, Prasad DS, Lowe AS et al (2012) Imaging manifestations in Proteus syndrome — an unusual multisystem developmental disorder. Br J Radiol 85:e793–e799
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no financial interests, investigational or off-label uses to disclose.
Rights and permissions
About this article
Cite this article
Johnson, C.M., Navarro, O.M. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 2: vascular malformations. Pediatr Radiol 47, 1196–1208 (2017). https://doi.org/10.1007/s00247-017-3906-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3906-x