Abstract
Most haemangiomas and vascular malformations are identified according to clinical criteria. A good knowledge of the classification and clinical characteristics of the vascular anomalies is necessary when managing these patients. However, some cases are challenging either because of an atypical presentation (e.g., soft-tissue mass with normal overlying skin) or because of classification difficulties. Doppler US and MRI are the two main imaging modalities that allow classification of the vascular anomalies and are useful in those clinically uncertain cases to establish the correct diagnosis. This aids the choice of the most appropriate treatment and to inform the parents of the prognosis. High-resolution grey-scale and Doppler US allow excellent visualization of most superficial masses. Doppler US is the easiest way to assess the haemodynamics of a vascular lesion and to clarify a doubtful diagnosis between a haemangioma and vascular malformation. MRI is the best technique for evaluating the extent of the lesions and their relationship to adjacent structures. While newly developed drugs from angiogenesis research labs are awaited, radiologists have an important role in the treatment of haemangiomas and vascular malformations. Intervention remains crucial in cases of alarming haemangiomas and venous malformations (VM), lymphatic malformations (LM) and arteriovenous malformations (AVM). A multidisciplinary team, including paediatricians, haematologists, surgeons and radiologists, must manage the problem cases both in terms of diagnostic work-up and therapeutic options. This paper will briefly discuss the imaging findings and treatment of vascular anomalies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
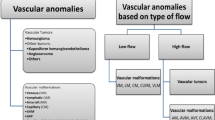
Vascular anomalies comprise a wide spectrum of lesions involving all parts of the body. In the past, diagnosis and treatment of vascular anomalies was hampered by a considerable confusion due to the use of improper terminology. A biological classification has helped resolve the confusion regarding terminology in the field of vascular anomalies. In 1982, on the basis of cellular kinetics and clinical behaviour, Mulliken and Glowacki [1] proposed the most helpful classification for vascular anomalies [2]. In this classification, vascular anomalies are divided into vascular tumours (cellular proliferation and hyperplasia) and vascular malformations (lesions that arise by dysmorphogenesis and exhibit normal endothelial turnover) (Table 1). In 1996, this classification was adopted by the International Society for the Study of Vascular Anomalies (ISSVA).
The most frequent vascular tumours in infancy are infantile haemangiomas. Congenital haemangiomas (NICH: non-involuting congenital haemangiomas, or RICH: rapidly involuting congenital haemangiomas), haemangioendotheliomas, tufted angiomas and sarcomas are other vascular tumours seen in children.
Vascular malformations are classified into slow-flow malformations including capillary malformations (CM), venous malformations (VM), lymphatic malformations (LM), capillary and venous malformations (CVM), capillary lymphatic and venous malformations (CLVM), and high-flow malformations including arteriovenous fistula (AVF) and arteriovenous malformations (AVM).
Complex-combined malformations are found in some syndromes: Klippel-Trenaunay, Parkes-Weber, Blue Rubber Bleb, Proteus and Maffucci.
Most haemangiomas and vascular malformations are recognized on clinical grounds. Clinical evaluation and genetics are extensively discussed in the literature. We will focus on the implications for the radiologist in assessing vascular anomalies. The radiologist has to establish the diagnosis using colour Doppler US and MR imaging and should be aware of associated syndromes or the possibility of multiple organ involvement to be able to recommend appropriate additional imaging investigations. Lastly, interventional radiologists play a major role in the treatment of vascular malformations with the increasing efficacy of sclerotherapy and embolization therapies.
Vascular tumours
Infantile haemangiomas (in proliferative phase)
The best diagnostic clue for infantile hemangioma is the presence of high flow soft tissue mass.
US findings
Hyperechoic and/or hypoechoic lesions can be seen. A single or a few vessels may be visible at Grey-scale US that most often correspond to arteries. The lesion displays increased colour flow due to numerous arteries and veins. The spectral analysis displays low resistance. Direct arteriovenous shunting is rare but may be seen and is frequently misinterpreted as an AVM (Figs. 1 and 2) [3].
MR findings
MRI typically shows a well-defined, non-infiltrating lesion, with an intermediate signal intensity on T1-weighted sequences and increased signal intensity on T2-weighted sequences. Fast-flow vessels are identified by the presence of flow voids within and around the soft-tissue mass on spin-echo (SE) sequences and as high signal intensity on gradient-recalled echo (GRE) sequences. Perilesional oedema should not be seen. Vessels with fast-flowing blood are often at the periphery of the mass [4]. After gadolinium injection, strong enhancement is observed (Fig. 3).
Three-month-old girl. a Axial T1-W scan of the head shows a subcutaneous hypointense mass located in the parotid. Fast flow vessels are also identified by the presence of flow voids within the mass. b On axial T2-W FS scan the mass is hyperintense. Flow voids are still detected. No perilesional oedema was identified. c One month after propanolol treatment significant regression was noticed. Axial T1-W contrast-enhanced FS scan shows strong enhancement of the haemangioma
Associations with infantile hemangiomas
Multiple haemangiomas of the skin have traditionally been linked with potential visceral haemangiomas [5]. However, segmental haemangiomas are more frequently associated with anomalies like PHACE syndrome (posterior fossa anomalies, haemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects and eye anomalies) [6]. Most of these segmental haemangiomas are telangiectatic or reticular and do not present a high-flow pattern on Doppler US. Nevertheless, in our experience, internal organ involvement can be seen including the liver, gastrointestinal tract, pancreas and lung with imaging findings similar to soft tissue infantile haemangiomas.
Take home point
If the soft-tissue mass shows few arteries or veins, the spectral analysis shows a high resistance index and, if you notice perilesional oedema on T2-W MR imaging, rule out another tumoral lesion, e.g., sarcoma, neuroblastoma, myofibromatosis, tufted angioma, haemangiopericytoma, infantile myofibromatosis, fibrosarcoma, rhabdomyosarcoma, metastatic neuroblastoma or other tumours (Fig. 4).
Treatment
In the majority of cases, no treatment is required because of spontaneous involution in cases of infantile haemangiomas and haemangioendotheliomas. Approximately 10–20% of all haemangiomas need to be treated. The major indications for treatment are periocular location with vision compromise, high-output cardiac failure, ulceration, compression of the airway, facial haemangiomas with rapid growth and distortion (presumed to result in important cosmetic sequelae) and symptomatic muscular haemangiomas. Medical treatment is usually attempted first with most of the vascular anomaly groups using propanolol as a first-line therapy with excellent results [7]. Other treatments include steroids, interferon, vincristine and laser. Embolization and/or surgery are required when medical alternatives are ineffective, mostly in cases of liver haemangiomas with cardiac failure that does not respond to pharmacologic treatment.
Congenital haemangiomas
The notion of congenital haemangiomas was first introduced in 1996 by Boon et al. [8]. Among this group, two sub-sets were subsequently identified: NICH that undergo a proportional growth with the child but no regression, and RICH in which regression is complete within 14 months after birth. It is noteworthy to mention that sometimes it may be clinically difficult to distinguish a common infantile haemangioma (CIH) from a RICH when the CIH is present and prominent at birth and undergoes quick but moderate proliferative phase. The GLUT1 marker is negative compared to infantile haemangiomas. However, in segmental haemangiomas the GLUT1 is positive [9].
US findings
RICH and NICH share the same imaging findings. Most of these imaging findings are similar to those of infantile haemangiomas with some differences like various-sized vascular aneurysms, intravascular thrombi (never seen in infantile haemangiomas), increased venous component and arteriovenous shunting. It is important to be aware that some malignant tumours and AVMs have similar features (Fig. 5).
Treatment
For NICH, since embolization is less effective, surgical resection is the best option.
Haemangioendotheliomas
US findings
They are seen as ill-defined soft-tissue masses with variable echogenicity.
Calcifications can be present (never seen in infantile haemangiomas). Doppler US shows a high, moderate or low vessel density with high and low resistance index [10].
MR imaging
T1-W MRI sequences show a heterogeneous soft-tissue mass that is isointense or hypointense compared to the muscle. T2-W sequences show a hyperintense lesion with subcutaneous stranding. Signal voids can be seen on GRE and represent haemosiderin or other blood products. Contrast-enhanced imaging displays diffuse, heterogeneous enhancement in the soft-tissue mass. Unlike infantile haemangiomas, destruction of the adjacent bones can be seen in haemangioendotheliomas (Fig. 6) [11].
Three-year-old boy with red/purple-coloured soft-tissue mass in the anterior thigh. a Pulsed-wave colour Doppler US shows a few vessels with high resistance index. b Axial T2-W FS image shows an ill-defined hyperintense subcutaneous and muscular lesion. Subcutaneous stranding is clearly seen. c Axial contrast-enhanced FS T1-W scan shows heterogeneous diffuse enhancement. Biopsy confirmed the diagnosis of haemangioendothelioma
Key facts
Often associated with Kasabach-Merritt phenomenon, ill-defined lesion, calcifications.
Treatment
Medical treatment is recommended. Steroids are the first-choice treatment. Interferon, vincristine and embolization are also useful in refractory steroids treatment. Embolization is always associated with medical treatment. More studies are necessary in order to evaluate the effectiveness of propanolol in the treatment of haemangioendotheliomas.
Vascular malformations
Doppler US is the first examination to perform when a vascular malformation is suspected. This inexpensive and noninvasive examination allows differentiation between low-flow and fast-flow lesions.
Low flow vascular malformations
Venous malformations (VM)
Best diagnostic clue
The presence of phleboliths.
US findings
VMs can be classified in two types: cavitary and dysplastic. The cavitary VM is more common. Grey-scale US shows a compressible hypoechoic and heterogeneous infiltrative lesion [12, 13]. After applying compression, US shows the movement of blood into the cavities (Fig. 7) and phleboliths can be identified [12]. Doppler US typically shows no flow or monophasic low-velocity flow. Most of the time Doppler flow is difficult to obtain because of below-threshold flow or thrombosis. Dynamic manoeuvres, such as Valsalva or manual compression, are sometimes necessary to induce visible Doppler flow. Arterial flow can be observed in lesions with a capillary component. This is especially found in cases of CVM. Vessel density and Doppler shift are typically low. Sometimes, a high resistive index is present indicating a normal arterial vessel in the mass. Dysplastic VMs consist of multiple varicose veins. On B-mode imaging multiple anechoic tubular, tortuous channels infiltrating the subcutaneous fat, muscles, tendons or other tissues are observed. Doppler interrogation reveals slow venous flow.
MR findings
MRI is an excellent modality to define the extension of the lesions and their relationship to adjacent structures. The examination protocol should begin with SE or fast SE T1-W sequence for basic anatomic evaluation. The extension of the malformation should be assessed with a T2-W sequence with fat suppression (FS). Fat suppression with short TI (inversion time) inversion recovery (STIR) T2-W sequence using a 512 matrix are well suited for this purpose. T2-W GRE sequences can also be used to demonstrate calcification or haemosiderin. On GRE sequences, the absence of signal in the blood vessel in the vicinity of the malformation suggests a slow-flow malformation [14]. FSE T1-W sequence with FS should be performed after gadolinium injection to evaluate the perfusion of the malformation. In our institution, we perform a dynamic perfusion study at 1, 2, 5 and 10 min after contrast infusion using a volumetric interpolated breath-hold examination (VIBE) sequence (Fig. 8). These contrast-enhanced 3-D acquisitions are also useful to appreciate the drainage of the malformation in the venous system [15]. Usually, VMs are hypo- or isointense on T1-W sequences. In cases of haemorrhage or thrombosis, a heterogeneous signal can be observed on T1-sequences. Abnormal veins can be observed in the area of the malformation. On T2-W sequences, VMs are of high signal. Areas of low signal can be observed related to thrombosis, septation inside the malformation or phleboliths. On T2-W sequences, the extension of the malformation into adjacent structures is usually clearly delineated.
Eight-year-old girl with a soft-tissue lesion of the cheek. a Grey-scale US shows a compressible soft-tissue hypoechoic lesion with phleboliths. b Axial T2-W FS scan shows the extension of the hyperintense VM containing phleboliths. c, d Axial contrast-enhanced T1-W FS images shows heterogeneous enhancement of the VM at 2 min (c) and 5 min (d) later
Treatment
Most VMs are managed conservatively with compression bandage to the extremity. The indications for treatment are pain, articular involvement, disfigurement and gastrointestinal bleeding. The first-line treatment is sclerotherapy and can be followed by resection, laser and photodynamic therapy. Many sclerosing agents are used like dehydrated ethanol, sodium tetradecyl sulfate, polidocanol and bleomycin.
Lymphatic malformations (LM)
Best diagnostic clue
Cutaneous angiokeratosis for microcystic LM and cystic lesions for macrocystic LM.
US findings
Grey-scale US imaging is instrumental in characterizing the type of LM. Macrocystic LMs consist of multiloculated cystic lesions (Fig. 9) [13]. Pure microcystic lesions are ill-defined and hyperechoic due to numerous wall interfaces. Mixed lesions consist of cystic and solid lesion related to the size of the cyst. Colour Doppler reveals vascular channels in the septa, including veins and arteries as confirmed by spectral analysis.
MR findings
On MRI the characteristic findings are the presence of a heterogeneous fluid-filled mass with an iso- to hyposignal on T1-W sequences and hypersignal on T2-W sequences (Fig. 10). Sometimes high signal on T1-W sequences or a fluid level can be observed in cases of a cyst with a high content of protein or haemorrhagic contents [14]. Pure LMs have absent or minimal enhancement of septa whereas combined lymphatic VMs show enhancement of the lymphatic space [14].
Treatment
Macrocystic LMs can be treated either by surgery or sclerotherapy. Usually macrocystic LMs respond well to sclerotherapy. Numerous sclerosing agents are used: ethanol, sodium tetradecyl sulfate and doxycycline in North America; alcoholic solution of zein (Ethibloc, Ethicon, Somerville, NJ) in Europe and Canada [16, 17]; OKT3 [(Centocor Ortho Biotech Inc, Horsham, PA) picibanil, that is a killed strain of group A Streptococcus pyogenes] in Japan [18]. More recently, bleomycin has been used in macrocystic and mixed malformations with a success rate varying between 70 and 95% [19–24]. Microcystic LMs do not respond well to sclerotherapy, although a good response using bleomycin and OKT3 has been reported by several authors [22, 25]. Microcystic lymphangiomas should be managed conservatively but, if a treatment is required, the surgical approach should be favoured. Recurrence rates of 40% after incomplete excision and of 17% after macroscopically complete excision have been reported [26]. The role of bleomycin sclerotherapy combined with surgery is not yet certain.
Syndromes with vascular skin lesions and slow-flow malformations
Sturge-Weber syndrome
is a nonheritable cutaneous disorder that consists of a unilateral facial port-wine stain in the trigeminal area; an ipsilateral leptomeningeal malformation and malformation of the choroid of the eye; atrophy and calcifications in the subjacent cerebral cortex; seizures; hemiparesis and visual field defects contralateral to the brain lesion; mental retardation of variable degree and sometimes buphthalmos or glaucoma [27].
Klippel-Trenaunay syndrome
is a capillary venolymphatic malformation (CLVM) with limb overgrowth [28, 29]. Clinical presentation is variable and depends on the predominance of abnormal lymphatic or venous vessels. This syndrome includes a dermal capillary stain associated with venous varicosities. These anomalous veins have deformed, insufficient or absent valves. The pathognomonic marginal vein of Servelle is often identified in the subcutaneous fat of the lateral calf and thigh and can communicate with the deep venous system at various levels. Lymphoedema can be associated with malformations and hypoplasia of the lymphatic vessels. The lower limb is more frequently involved with possible extension of the malformation into the perineum or sometimes in the abdomen. The upper limb, the trunk or the neck are rarely involved [30]. Contrast venography is useful in selected patients to depict the route of drainage and the feasibility of resecting or sclerosing varicosities [2].
Blue rubber bleb nevus syndrome
is characterized by multiple VMs of the skin with multiple gastrointestinal VMs. The gastrointestinal lesions can result in haemorrhage, intussuception and volvulus. It is a sporadic disease, but familial cases have been reported [31, 32].
Maffucci syndrome
is a nonheritable syndrome that consists of diffuse enchondromatosis involving the metacarpal phalanges of the hands and feet associated with multiple venous or LMs [33].
Proteus syndrome
consists of multiple subcutaneous hamartomatous tumours, hemihypertrophy, pigmented nevi, gigantism involving the extremities (hand, foot), intra-abdominal lipomatosis, pachydermia, macrocephaly, bony exostosis and lymphatic venous malformations (LVM) [34–37].
Bannayan-Riley-Ruvalcaba syndrome
is an autosomal-dominant condition with a variable clinical phenotype. The disorder is associated with phosphatase and tensin homolog (PTEN) gene mutation on chromosome 10q. Clinical features include macrocephaly, pseudopapilloedema, pigmented maculas on the penis, gastrointestinal polyposis, visceral lipoma, thyroiditis, capillary and combined malformations.
Glomovenous malformation
is an autosomal-dominant condition, also known as glomangioma. It is characterized by multiple, often tender, blue nodular dermal lesions in the skin. Glomovenous malformations are multifocal and painful to palpation. US imaging features are similar to those of VM with the exception that the lesion cannot be completely emptied by compression. The lesions are VMs with the presence of glomus cells.
High-flow vascular malformations
Arteriovenous malformations (AVM)
Best diagnostic clue
Numerous visible arterial and venous vessels and high diastolic flow.
US findings
AVMs are poorly defined with no or little tissue mass visible. Most of the time, fat tissue can be seen around the AVM. The lesion is made of multiple feeding arteries with increased diastolic flow and increased venous return with systolic/diastolic flow. Power or colour Doppler examination is helpful to delineate the network of the malformation (Fig. 11). Unlike haemangiomas, there is always arterialisation of all the draining veins (i.e. pulsatile flow) in AVMs.
MR findings
MRI examination allows evaluation of the extension into adjacent structures, especially bone involvement. MR imaging findings include dilated feeding arteries and draining veins with little tissue matrix and no venous lakes [39]. Signal voids are typically observed in these vessels on both T1- and T2-W SE sequences, whereas hypersignal is observed on gradient-echo and angiographic sequence indicating a high-flow lesion [14]. Gadolinium-enhanced MR angiography is helpful to evaluate feeding arteries and draining veins. The presence of early venous filling is typically seen in AVMs. Using time-resolved MR angiography sequence it is now possible to evaluate the dynamic opacification of AVM (Fig. 12) [40–44]. Since these sequences have a high temporal resolution there is a compromise on spatial resolution that is lower than conventional 3-D MR.
MR imaging (same patient as Fig. 11). a Coronal T2-W FS scan of the hand shows flow voids. b Coronal gradient-echo T2-W image shows numerous tortuous bright vessels. c Contrast-enhanced 3-D acquisition illustrates the arterial feeding of the arteriovenous malformation
Treatment
AVMs are an important challenge for interventional angiographers. Some AVMs respond well to embolization while others progress despite embolotherapy. For this reason we recommend conservative management in quiescent AVMs. However, treatment is required in cases with severe cosmetic consequences, ulceration, pain associated with distal steal phenomenon and/or gangrene. Less commonly bleeding, compartment syndrome, severe overgrowth, congestive heart failure and failure to thrive can lead to intervention. Embolization is the first-choice treatment for AVMs. The procedure should be performed by well-trained angiographers and under general anaesthesia. To destroy the AVM and reduce the risk of recurrence, super-selective catheterization is necessary combined with a percutaneous direct puncture of the nidus when feasible. The best agent to destroy the nidus is dehydrated alcohol. Other agents can be used like onyx and histoacryl (N-butyl cyanoacrylate, not approved by the FDA).
Syndromes with high-flow malformations
Parkes-Weber syndrome
involves a combination of AVFs, congenital varicose veins and a cutaneous capillary malformation associated with limb hypertrophy [45].
Rendu-Osler-Weber syndrome
(hereditary haemorrhagic telangiectasia) manifests as diffuse mucosal telangiectasia involving the nasopharynx, gastrointestinal tract and sometimes the urinary and genital mucosa. AVFs and arterial aneurysms involving the pulmonary, hepatic and digestive arteries are typically observed [46].
Capillary malformation–AVM syndrome
is a hereditary disorder characterized by cutaneous capillary malformation associated with AVM or AVF. Mutations in the RASA1 gene are reported in this condition [38].
Cobb syndrome
is a rare nonhereditary syndrome where a cutaneous capillary malformation is associated with AVM of the spinal cord [38, 47].
Conclusion
Diagnostic and interventional radiologists play a major role within a team dealing with vascular anomalies. They must be aware of the clinical history and findings to be able to make the right diagnosis and propose the best therapeutic options.
References
Mulliken JB, Glowacki J (1982) Classification of pediatric vascular lesions. Plast Reconstr Surg 70:120–121
Mulliken JB, Fishman SJ, Burrows PE (2000) Vascular anomalies. Curr Probl Surg 37:517–584
Dubois J, Patriquin HB, Garel L et al (1998) Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR 171:247–252
Konez O, Burrows PE (2002) Magnetic resonance of vascular anomalies. Magn Reson Imaging Clin N Am 10:363–388
Metry DW, Hawrot A, Altman C et al (2004) Association of solitary, segmental hemangiomas of the skin with visceral hemangiomatosis. Arch Dermatol 140:591–596
Metry DW, Dowd CF, Barkovich AJ et al (2001) The many faces of PHACE syndrome. J Pediatr 139:117–123
Léauté-Labrèze C, Taïeb A (2008) Efficacy of beta-blockers in infantile capillary haemangiomas: the physiopathological significance and therapeutic consequences. Ann Dermatol Venereol 135:860–862
Boon LM, Enjolras O, Mulliken JB (1996) Congenital hemangioma: evidence of accelerated involution. J Pediatr 128:329–335
North PE, Waner M, James CA et al (2001) Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol 137:1607–1620
Dubois J, Garel L, David M et al (2002) Vascular soft-tissue tumors in infancy: distinguishing features on Doppler sonography. AJR 178:1541–1545
Robertson RL, Robson CD, Barnes PD et al (1999) Head and neck vascular anomalies of childhood. Neuroimaging Clin N Am 9:115–132
Trop I, Dubois J, Guibaud L et al (1999) Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 212:841–845
Paltiel HJ, Burrows PE, Kozakewich HP et al (2000) Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 214:747–754
Siegel MJ (2001) Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am 39:701–720
Li W, David V, Kaplan R et al (1998) Three-dimensional low dose gadolinium-enhanced peripheral MR venography. J Magn Reson Imaging 8:630–633
Dubois J, Garel L, Abela A et al (1997) Lymphangiomas in children: percutaneous sclerotherapy with an alcoholic solution of zein. Radiology 204:651–654
Molitch HI, Unger EC, Witte CL et al (1995) Percutaneous sclerotherapy of lymphangiomas. Radiology 194:343–347
Ogita S, Tsuto T, Nakamura K et al (1994) OK-432 therapy in 64 patients with lymphangioma. J Pediatr Surg 29:784–785
Baskin D, Tander B, Bankaoğlu M (2005) Local bleomycin injection in the treatment of lymphangioma. Eur J Pediatr Surg 15:383–386
Muir T, Kirsten M, Fourie P et al (2004) Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int 19:766–773
Orford J, Barker A, Thonell S et al (1995) Bleomycin therapy for cystic hygroma. J Pediatr Surg 30:1282–1287
Zhong PQ, Zhi FX, Li R et al (1998) Long-term results of intratumorous bleomycin-A5 injection for head and neck lymphangioma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:139–144
Zulfiqar MA, Zaleha AM, Zakaria Z et al (1999) The treatment of neck lymphangioma with intralesional injection of bleomycin. Med J Malaysia 54:478–481
Sanlialp I, Karnak I, Tanyel FC et al (2003) Sclerotherapy for lymphangioma in children. Int J Pediatr Otorhinolaryngol 67:795–800
Sung MW, Lee DW, Kim DY et al (2001) Sclerotherapy with picibanil (OK-432) for congenital lymphatic malformation in the head and neck. Laryngoscope 111:1430–1433
Alqahtani A, Nguyen LT, Flageole H et al (1999) 25 years’ experience with lymphangiomas in children. J Pediatr Surg 34:1164–1168
Enjolras O, Riche MC, Merland JJ (1985) Facial port-wine stains and Sturge-Weber syndrome. Pediatrics 76:48–51
Jacob AG, Driscoll DJ, Shaughnessy WJ et al (1998) Klippel-Trénaunay syndrome: spectrum and management. Mayo Clin Proc 73:28–36
Gloviczki P, Driscoll DJ (2007) Klippel-Trenaunay syndrome: current management. Phlebology 22:291–298
Cohen MM Jr (2000) Klippel-Trenaunay syndrome. Am J Med Genet 93:171–175
Moodley M, Ramdial P (1993) Blue rubber bleb nevus syndrome: case report and review of the literature. Pediatrics 92:160–162
Fretzin DF, Potter B (1965) Blue rubber bleb nevus. Arch Intern Med 116:924–929
Esterly NB (1996) Cutaneous hemangiomas, vascular stains and malformations, and associated syndromes. Curr Probl Pediatr 26:3–39
Wiedemann HR, Burgio GR (1986) Encephalocraniocutaneous lipomatosis and Proteus syndrome. Am J Med Genet 25:403–404
Clark RD, Donnai D, Rogers J et al (1987) Proteus syndrome: an expanded phenotype. Am J Med Genet 27:99–117
Samlaska CP, Levin SW, James WD et al (1989) Proteus syndrome. Arch Dermatol 125:1109–1114
Darmstadt GL, Lane AT (1994) Proteus syndrome. Pediatr Dermatol 11:222–226
Garzon MC, Huang JT, Enjolras O et al (2007) Vascular malformations. Part II: associated syndromes. J Am Acad Dermatol 56:541–564
Hovius SE, Borg DH, Paans PR et al (1996) The diagnostic value of magnetic resonance imaging in combination with angiography in patients with vascular malformations: a prospective study. Ann Plast Surg 37:278–285
Taschner CA, Gieseke J, Le Thuc V et al (2008) Intracranial arteriovenous malformation: time-resolved contrast-enhanced MR angiography with combination of parallel imaging, keyhole acquisition, and k-space sampling techniques at 1.5 T. Radiology 246:871–879
Reinacher PC, Stracke P, Reinges MH et al (2007) Contrast-enhanced time-resolved 3-D MRA: applications in neurosurgery and interventional neuroradiology. Neuroradiology 49:S3–S13
Saleh RS, Lohan DG, Villablanca JP et al (2008) Assessment of craniospinal arteriovenous malformations at 3 T with highly temporally and highly spatially resolved contrast-enhanced MR angiography. AJNR 29:1024–1031
Wu Y, Kim N, Korosec FR et al (2007) 3D time-resolved contrast-enhanced cerebrovascular MR angiography with subsecond frame update times using radial k-space trajectories and highly constrained projection reconstruction. AJNR 28:2001–2004
Ziyeh S, Strecker R, Berlis A et al (2005) Dynamic 3D MR angiography of intra- and extracranial vascular malformations at 3 T: a technical note. AJNR 26:630–634
Enjolras O (1997) Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol 24:701–710
Guttmacher AE, Marchuk DA, White RI Jr (1995) Hereditary hemorrhagic telangiectasia. N Engl J Med 333:918–924
Jessen RT, Thompson S, Smith EB (1977) Cobb syndrome. Arch Dermatol 113:1587–1590
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubois, J., Alison, M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 40, 895–905 (2010). https://doi.org/10.1007/s00247-010-1621-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-010-1621-y