Abstract
Vascular malformations and hemangiomas are common in children but remain a source of confusion during diagnosis, in part because of the lack of a uniform terminology. With the existing treatments for hemangiomas and vascular malformations, it is important to make the correct diagnosis initially to prevent adverse physical and emotional sequelae in not only the child but also the family. The diagnosis of vascular malformations is made primarily by the clinician and based on the physical exam. Imaging is carried out using predominantly ultrasound (US) and magnetic resonance imaging (MRI), which are complementary modalities. In most cases of vascular anomalies, US is the first line of imaging as it is readily available, less expensive, lacks ionizing radiation and does not require sedation. MRI is also of great help for further characterizing the lesions. Conventional arteriography is reserved for cases that require therapeutic intervention, more commonly for arteriovenous malformations. Radiographs usually play no role in diagnosing vascular anomalies in children. In this article, the author describes the terminology and types of hemangiomas and vascular malformations and their clinical, histological features, as well as the imaging approach and appearance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Confusion exists about vascular anomalies in children [1, 2]. In an effort to unify the terminology and to minimize confusion, the International Society for the Study of Vascular Malformations (ISSVA) adopted in 1996 the classification of vascular anomalies, which was first proposed in 1982 and revised in 1992 [3]. In this classification and the one used by the author, vascular anomalies are divided in two main categories: tumors and vascular malformations (Table 1). The suffix “oma” in the word hemangioma, which implies cellular proliferation and mitosis, indicates the neoplastic nature of the lesion. On the other hand, unless perturbed, vascular malformations exhibit a normal rate of endothelial cell turnover. Vascular malformations are not neoplasms but, rather, structural anomalies that result because of a localized error in vascular morphogenesis that can affect arteries, veins, capillaries and lymphatics. In this classification system, vascular malformations are further classified as simple, including venous, lymphatic and capillary malformations, and combined, which includes arteriovenous malformations and arteriovenous fistulas, or any other combination (Table 1) [4, 5]. Even though this classification system is still imperfect, it has helped to unify the terminology to a great extent for research purposes and when selecting the most suitable therapy. Currently, ultrasound (US), magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are the cornerstones of imaging vascular anomalies. In general and for most cases, US is the first imaging modality of choice, except in very extensive vascular anomalies, where the field of view is a limiting factor. MRI and MRA are complementary to US in further characterizing these images. However, most of these lesions occur in young children who require sedation, adding time and cost to the exam. MRA has virtually taken away the diagnostic role of conventional arteriography, which is reserved for cases that require therapeutic intervention, more commonly for arteriovenous malformations. Examining the patient when planning the MRI is very helpful to tailor the protocol (hemangioma vs. slow-flow vs. high-flow malformation). At the author’s institution, clinical photographs are taken routinely after consent is obtained and archived on PACS. Radiographs virtually play no role in the diagnosis of vascular anomalies and should not be obtained routinely as part of an MRI examination. Table 2 is a suggested general protocol used at the author’s institution for hemangiomas and/or vascular malformations.
Hemangiomas
Hemangiomas are true vascular neoplasms that have a specific pattern of evolution. Hemangiomas exist in two types: infantile (IH) and congenital (CH). The literature has referred to IHs by many names that are no longer used, including strawberry, capillary, juvenile and nascent hemangiomas.
Infantile hemangiomas
Infantile hemangiomas (IH) are much more common than the congenital type, occurring in approximately 3% to 10% of infants [6]. Risk factors include being Caucasian and female with low birth weight, prematurity and a history of chorionic villous sampling. Maternal risk factors include preeclampsia, placenta previa and multiple gestations [2, 6].
Histologically, IHs have a spectrum that changes according to the phase of evolution. During the proliferative phase, densely cellular lobules that replace existing tissue are seen, along with masses of capillaries lined by plump endothelial cells, pericytes, thin bands of fibrous tissue between lobules and abundant atypical mitotic figures. During the involuting phase, the capillary lumen dilates, the endothelial cells flatten, fibrous or adipose tissue replaces capillaries and mast cells increase without accompanying inflammation. In the fibrotic stage, a residual fibro-fatty mass with scant vascularity is always seen [7]. IH is the only tumor that is positive for Glucose Transporter Protein (GLUT1) independent of the stage. GLUT1 is therefore a highly specific marker for IH that may help elucidate the diagnosis in atypical cases [2, 8–10].
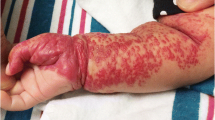
The diagnosis of IHs is usually based on a clinical exam. A critical aspect in the diagnosis is the fairly characteristic pattern of evolution. At birth, some IHs have a precursor lesion, seen as a localized area of skin discoloration or telangiectasia [4]. Most IHs start to grow between 2 weeks and 2 months of life, the proliferative phase, reaching a peak by 1 year of age. During this phase, the lesions are raised and reddish in color. This phase is followed by an involuting phase in which the lesion regresses spontaneously and decreases in size. The final stage of the process is the fibrotic stage characterized by a residual scar of varying size (Fig. 1). IHs can be focal, segmental or indeterminate lesions. Focal lesions are tumor-like and raised (Fig. 2), while segmental lesions are geographical and plaque-like (Fig. 1), and indeterminate lesions have combined features [4, 9]. Multiple lesions occur in up to 30% of patients, a clue to the diagnosis (Fig. 2) [2, 4, 9]. Some hemangiomas are localized predominantly or completely to the subcutaneous soft tissues, occasionally with intact, overlying skin making the clinical diagnosis challenging (Fig. 3) [4, 10].
Infantile hemangioma (IH), clinical appearance at different stages of evolution in a boy from birth to childhood. a Precursor lesion in the right cheek at birth extending into the neck displaying multiple telangiectasias. b IH in the proliferative phase, 12 weeks later with the fully developed hemangioma. The segmental plaque-like tumor has grown and the skin is raised, indurated and red. c After involution, at 7 years of age, there is a focal residual scar
IH, purely subcutaneous lesion in an 11-month-old boy. a Focal soft tissue prominence in the left supraclavicular region with intact overlying skin (arrows). b Coronal T1-W image shows the large mass confined to the subcutaneous soft tissues (asterisks) with overlying normal subcutaneous fat covering the lesion (white arrow)
The diagnosis of hemangiomas does not require imaging in most cases. Imaging is usually requested to assess the extension and depth of the lesion, to assess its relationship with adjacent structures, and in cases of atypical features or subcutaneous lesions. Additionally, imaging is performed in cases of multiple hemangiomas to evaluate internal organs due to the increased risk of associated visceral hemangiomas. In general, US and MRI are the preferred imaging modalities to evaluate hemangiomas [4, 11]. Computed tomography (CT) scans are an alternative in cases in which an MRI is not readily available or in cases of airway hemangiomas. A major disadvantage of CT scan is the use of ionizing radiation. The imaging appearance of IHs depends on the stage of evolution. During the proliferative phase, lesions appear on US as well-circumscribed masses of variable echogenicity and increased vascularity. On color Doppler, they contain high-flow vessels with low-resistance waveforms and draining veins but no arterialized veins to suggest arteriovenous shunting (Fig. 4). During the involution stage, the mass decreases in size, has less distinct borders and less vascularity, with high-resistance arteries. On MRI, the masses are well-defined, lobulated, hypointense or intermediate signal on T1-weighted images and hyperintense on fluid-sensitive sequences. On T1-weighted images, interspersed fat can be identified, and flow voids due to high-velocity arteries are usually present. IHs display a rapid and intense enhancement after contrast administration with variable washout. MRA can provide a map of the feeding arteries and draining veins and their relationship with adjacent vascular structures (Fig. 5). During involution, the degree of enhancement decreases, and the flow voids become less conspicuous and numerous. After involution, a scar is seen as hypointense tissue mixed with fat on T1 and T2-weighted images [4, 9, 11].
US appearance of an IH (same patient as in Fig. 2). a Gray-scale image shows a solid mass with well-defined margins in the subcutaneous soft tissues of the neck. The lesion is predominantly hyperechoic with scattered hypoechoic foci that correspond to vessels. b Color Doppler shows the vascular nature of IH with low-resistance arteries
MRI appearance of an IH (same patient as in Fig. 2). a Axial T1-W image of the neck lesion shows the lobulated, well-defined mass predominantly in the subcutaneous soft tissues (black arrows) with dermal involvement seen clinically (white arrow) containing hyperintense elements corresponding to fat. b Axial T2-W image of the neck lesion shows the large subcutaneous component of the lesion (asterisk), which is lobulated, well defined and hyperintense with flow voids (arrow). c Coronal T2-W image shows the second focal hyperintense lesion confined to the scalp at the vertex (arrows). d Sagittal T1-W image after contrast shows the marked, fairly homogeneous enhancement of the scalp and neck lesions (arrows). e Sagittal reconstructed MIP image of a head and neck MR angiogram shows arteries supplying both lesions with a rapid blush indicating the vascular nature of the lesion (arrows). The neck lesion has a very large arterial feeder corresponding to the flow void seen on (b) (arrowhead)
Congenital hemangiomas
Congenital hemangiomas (CH) are rare and much less common than IHs. Two subtypes exist: non-involuting congenital hemangioma (NICH) and rapidly involuting congenital hemangioma (RICH). These hemangiomas are fully developed at birth and, thus, can be seen in utero. The RICH type regresses much faster than the IH, usually during the first 2 years of life and even in utero; the NICH type has a static course, growing equal to the child’s growth, and never regresses. RICHs usually start to regress centrally first soon after birth, leaving a focal area of skin and subcutaneous fat atrophy [10]. In contrast to IH, CHs affect both sexes equally and lack precursor lesions [4, 9, 10, 12]. Different than IHs, these lesions are usually solitary; however, there are reported cases of patients with coexisting IH and CH [10, 13].
Histologically, both RICHs and NICHs have a lobular architecture and endothelial proliferation similar to IH. However, CHs are not reactive to the GLUT1 marker [7, 8]. The clinical appearance of CHs can be indistinguishable from that of IHs. NICHs tend to present as purple, nodular masses with superficial telangiectasias (Fig. 6), while RICHs have a peripheral pale halo and central ulceration, nodularity or scarring. These lesions are more common in the head and extremities near a joint [7, 9, 10, 12].
The imaging appearance of CHs is similar to IHs on US and MRI, so the history is crucial in the diagnosis (age of presentation, pattern of involution) (Fig. 7) [4, 10]. In CHs, larger, more visible vessels are present, with evidence of micro-shunting, sometimes simulating an AVM. Other features suggestive of a CH are less-distinct borders, associated fat stranding and occasionally calcifications [4, 9, 11, 12].
NICH, US appearance (same patient as in Fig. 6). a Gray-scale image shows a well-defined superficial solid mass with mixed but predominantly hypoechoic parenchyma. b Color Doppler image shows the marked increased vascularity inside the lesion with arterial waveform. The imaging features are indistinguishable from those of IH; however, the lesion was present at birth and has persisted unchanged through early adolescence
Vascular malformations
Vascular malformations are developmental anomalies that can be classified according to the type of vessel involved: arteries, veins, lymphatics or capillaries. Vascular malformations are generally distinct at birth, grow steadily in proportion to systemic growth until puberty, and never disappear or regress. However, vascular malformations can be discovered beyond infancy and even in adolescence or adulthood. Vascular malformations can be classified as low- or high-flow, according to the hemodynamics [3, 9, 11]. Low-flow vascular malformations have connections to the venous or capillary system and include capillary, venous and lymphatic malformations. High-flow vascular formations refer to malformations that have a connection with the arterial or capillary system and include arteriovenous malformations and fistulas. The most common vascular malformations are the lymphatic and the venous types, which affect most frequently the head and neck, followed by the extremities and trunk [11, 14]. Low-flow vascular malformations permeate all tissue layers, including skin, subcutaneous fat, internal organs, skeletal muscle and joints. Bone involvement can occur with the low-flow lesions in contrast to hemangiomas, which are not intraosseous as previously believed. The lesions formerly referred to as intraosseous hemangiomas are now known histologically as venous malformations [9, 15, 16].
Lymphatic malformations
Lymphatic malformations (LM) are low-flow vascular malformations further subdivided into macrocystic, microcystic and combined types, with therapeutic and prognostic implications. The macrocystic type, formerly cystic hygroma, is composed of locules larger than 2 cm. The microcystic type, formerly lymphangioma, is composed of smaller locules measuring between approximately 1 and 2 cm [11, 17, 18].
Histologically, LMs are composed of serpiginous dilated lymphatic channels and sacs separated by fibrous septae isolated from the normal lymphatic vessels. These vessels are lined by flat endothelial cells and surrounded by thickened smooth muscle. The endothelium of lymphatic vessels and LM express a specific receptor subtype, the vascular endothelial growth factor receptor 3 (VEGFR-3), which is essential for lymphangiogenesis. Potentially, this receptor can be used for histopathological diagnosis, keeping in mind that the LM can occur in combination with the other types [7, 14, 19].
Upon physical exam, LMs are soft, supple, non-pulsatile masses that transilluminate and have no associated bruit or increased temperature. LMs may change in size throughout the day or during episodes of infections such as common colds. The overlying skin is normal (Fig. 8) but can be erythematous in the case of superimposed infection or bluish in the case of hemorrhage. A clear or amber-colored lymph is usually aspirated from these lesions, or in cases of superimposed hemorrhage or infection, the lesion can contain blood similar to a VM or pus. Chyle can be occasionally aspirated from these lesions as well [11, 14, 17].
The imaging evaluation of LMs is usually done with US and MR imaging. When MR imaging is not available, CT is a suitable alternative. In general, on US, LMs in the natural state are seen as multiseptated cystic masses with no associated vascularity, soft tissue component or perilesional inflammatory changes (Fig. 9). The appearance of the contents can vary from anechoic to low-level echoes, fluid-fluid levels or solid components in cases of superimposed hemorrhage or infection [17]. When inflamed, perilesional and septal vascularity might be seen, but the internal elements should not have associated color flow. On MR imaging, LMs present as multiloculated cystic masses, hyperintense on fluid-sensitive sequences and hypointense on T1-W images. After gadolinium administration, there is minimal, if any, peripheral and septal enhancement. Likewise, the signal intensity of the contents varies if there is associated hemorrhage or infection; in these cases, they tend to be hyperintense to muscle on T1-W images due to the proteinaceous nature (Fig. 10). Superimposed inflammation can lead to significant septal and peripheral enhancement and, in cases of hemorrhage, to blooming on gradient sequences. LMs can be localized and focal or more diffuse and infiltrative. The latter tend to dissect and insinuate along structures, not respecting fascial planes, causing secondary mass effect upon adjacent structures [9, 11, 14, 18, 20]. In the diagnosis of LMs similar to VMs, the clinical exam plays an important role. As pediatric radiologists, we must be aware that imaging is obtained for additional reasons beyond the diagnosis or confirmation of a diagnosis, including the location (deep vs. superficial) of the lesion, its extent (i.e. intra-articular or intra-organ extension) and the relationship with adjacent structures (i.e. nerve or tendon involvement). Thus, a detailed description is important to guide surgeons or interventional radiologists toward the most suitable therapeutic option and to alert them of possible contraindications [20]. For the MR protocol of vascular malformations, refer to Table 2.
Lymphatic malformation (LM), US appearance (same patient as in Fig. 8). Color Doppler image of the cervical region shows the multiloculated cystic lesion with no color flow (arrows). The lesion is in contact with the internal carotid artery and internal jugular vein. The locules are slightly hyperechoic due to floating low-level echos, confirmed to be intralesional hemorrhage during sclerotherapy
LM, MRI appearance (same patient as in Fig. 8). a Axial T1-W image shows a well-defined round multiseptated mass (arrows) under the left sternocleidomastoid muscle. The lesion is hyperintense compared to muscle due to the presence of hemorrhage. b Axial T2-W image shows the round lesion with markedly hyperintense contents and fluid-fluid levels (arrows). c Axial T1-W fat-saturated postcontrast image depicts the septal enhancement by the mass (arrows)
Lymphangioma circumscriptum (LC) is a variant of LM thought to develop from abnormal subcutaneous lymphatic cisterns that lack communication with the normal lymphatic system and, instead, communicate with ectopic lymphatic vessels. Contractions of the smooth muscle lining these cisterns will cause the abnormal ectopic lymph vessels to dilate and protrude through the skin. Clinically, a LC is seen as a cluster of translucent cutaneous vesicles varying in size but usually small. The vesicles can change in color to pink, red or black after hemorrhage. The vesicles can rupture, draining blood, lymph or even chyle. LCs are best seen on MR images as localized, hyperintense thickening of the skin on fluid-sensitive sequences, with linear hyperintensities communicating with the underlying subcutaneous soft tissues (Fig. 11) [21, 22].
Lymphangioma circumscriptum (LC) in a 3-year-old girl. Axial T2-W image with fat saturation depicts the mixed, macro- and microcystic LM in the subcutaneous soft tissues of the right shoulder posteriorly. Note the dilated lymphatic channels in the superficial subcutaneous soft tissues and the dermal involvement (arrow)
Venous malformations
Venous malformations (VM) are also low-flow vascular malformations classified into sporadic VMs, dominantly inherited cutaneomucosal VMs, and dominantly inherited glomuvenous malformations (GVM), respectively accounting for 94%, 1% and 5% of VMs without sex preponderance [5, 7, 23, 24]. Some literature still refers to these as cavernous hemangiomas and venous angiomas, but usage of these terms adds to confusion about the topic and should be avoided [9].
Histologically, VMs are localized or diffuse anomalies of postcapillary vascular channels with walls deficient of smooth muscle, without valves or direct communication with the arterial system but communicating with normal veins. The veins display thickened walls lined by mature endothelium lacking internal elastic intima. GMVs are more infiltrative lesions composed of large, dilated, thin walled veins and subendothelial mural clusters of glomus cells [7, 23]. VMs (pure, combined or syndromic) have an elevated level of D-Dimer, a highly specific biomaker for VMs that can help differentiate them from glomuvenous malformations and lymphatic malformations that have normal D-Dimer levels. D-Dimer is responsible for the localized intravascular coagulopathy (LIC) observed in more than 40% of VMs [5, 23, 25].
On physical exam, sporadic VMs vary in size and shape. They can be localized and well-defined or diffuse and infiltrative, and they can involve the superficial and/or deep structures. These lesions are bluish or purple, show no focal increased temperature or bruit, are compressible and may enlarge during Valsalva maneuver, puberty or pregnancy (Fig. 12). VMs can be asymptomatic but may produce pain in the case of thrombosis related to postural venous ectasia or after physical activity [7, 9, 14, 18]. Dominantly inherited VMs are characterized clinically as small, less than 5 cm multifocal, round, bluish nodules or plaques with papules creating a pebbly surface. The lesions predominantly involve the skin, superficial subcutaneous soft tissues and mucosal membranes, and less frequently invade muscle and internal organs. GVMs present as tender, superficial, multifocal nodules or plaques, reddish or bluish in color. Compared with the sporadic VMs, GMVs tend to be more nodular, firmer and less compressible and do not swell with exercise or posture. The most common sites in children are the superficial soft tissues of the arms and legs [7, 26]. When physically examining the patient, it is important to carefully inquire for a family history because there is a large inter- and intrafamilial variability in the size, location and color of lesions [23].
The imaging evaluation of VM is done mainly with Doppler US and MRI, similar to LMs; however, additional mapping of the normal and abnormal venous system on MRA/MRV is important. On US, VMs have an extremely variable appearance ranging from predominantly solid to multicystic lesions. VMs can be localized and well-defined or infiltrative and, in many occasions, are misdiagnosed as a neoplasm due to the lack of discernible vascular spaces. Tubular or round anechoic structures that compress or fill in with low-level echoes upon distal augmentation can be seen [9, 11, 14, 18]. Although the vast majority demonstrates scant monophasic low-velocity flow, a minority demonstrates no detectable flow (Fig. 13) [26]. Echogenic foci corresponding to thrombus or shadowing phleboliths are highly specific for VMs and a valuable diagnostic clue [5, 9, 11, 27]. Arterial flow can be observed when the lesions are of the combined type or when the neighboring arteries crossing the lesion are interrogated [11]. MRI is an excellent modality to evaluate the extent of the lesion and the relationship with adjacent structures. On T1-weighted images, the lesions can be hypo- to hyperintense to muscle, and on fluid-sensitive sequences, markedly hyperintense, similar to fluid. The extension of the malformation and the relationship with critical structures are best assessed on fluid-sensitive sequences. On dynamic contrast-enhanced MRI, there is variable erratic and disorganized enhancement that progresses and eventually becomes more uniform. Calcifications (phleboliths) or hemosiderin can be demonstrated easily on gradient echo sequences. Phleboliths are hypointense on all sequences, and thrombi are hyperintense on T1-weighted images (Fig. 14). Adjacent draining veins can be identified. The more cavitary lesions present as more localized masses, and the more dysplastic lesions as multiple, tortuous, dilated varicose veins [11]. Fluid-fluid levels, although less common than in LMs, can be seen in VMs. On 2-D TOF images, due to the low flow, VMs frequently are not identified; however, the presence of a normal venous system can be evaluated and documented, which is useful when sclerotherapy is contemplated. On 3-D contrast-enhanced MRA, the lesions and their drainage into the systemic venous system can be mapped and should be documented on the report; in the absence of a deep venous system, the therapeutic options are limited [5, 9, 11, 18, 24]. Imaging of glomovenous malformations are similar to sporadic venous malformations, but the lesions are harder and non-compressible on US. A family history, the superficial confinement to the skin and subcutaneous tissues and the lack of pleboliths support their diagnosis [5, 24, 27].
VM, US appearance (same patient as in Fig. 12). a Gray-scale image shows multiple anechoic, dilated and tortuous vascular channels in the superficial subcutaneous soft tissues corresponding to varicosities (arrows). b Color Doppler shows no color inside the channels confirming the low-flow nature of the lesions. The scant color seen corresponds to neighboring native vascular structures (arrow)
VM, MRI appearance (same patient as in Fig. 12). a Coronal T1‐W image shows the VM as multiple serpiginous channels slightly hyperintense compared to muscle (arrows) involving the superficial subcutaneous soft tissues and the muscles of the thumb, index finger and thenar region. b On coronal STIR image, the VM is more conspicuous and the extension of the lesion can be evaluated (arrows). c Axial T2-W image with fat saturation clearly shows the VM encasing some of the flexor tendons (arrows) and infiltrating the muscle. d T1‐W image with fat with gadolinium shows enhancement by the lesion. At least two phleboliths (circles) are seen as hypointense foci inside the dilated veins
Extensive pure venous malformations of a limb, as the name implies, are extensive and diffuse involving a large segment of or the entire limb (Fig. 15), with potential extension into the trunk, perineum or both limbs. All the components of the limb can be affected, including the skin, subcutaneous soft tissues, muscles and joints (Fig. 16). With bone involvement, demineralization and hypoplasia are seen and the affected limb usually has a normal length or slight undergrowth. Intra-articular involvement may lead to hemarthrosis and joint destruction [24, 27–29]. With all sporadic VMs but more so with the extensive type, there is a specific associated localized intravascular coagulopathy (LIC) [5, 30]. LIC is a source of pain and thrombosis, leading to the development of phleboliths due to extremely elevated D-Dimer and low fibrinogen levels. These patients are at risk of developing disseminated intravascular coagulopathy (DIC) during surgical resection, sclerotherapy, elastic stocking removal, from a bone fracture, or pregnancy. Low molecular weight heparin should be given prophylactically before any intervention or in cases of DIC [5, 28, 30].
Diffuse pure extensive VM of the upper extremity (same patient as in Fig. 15). Coronal T2-W image with fat saturation of the upper extremity shows the extensive involvement of the muscles, subcutaneous soft tissues, dermis and elbow joint of the entire extremity by the hyperintense VM
VMs of the skeletal muscle or intramuscular VMs are often misdiagnosed as tumors, due in part to the use of wrong nomenclature and the sometimes later presentation. Two-thirds of the lesions are noted at birth and one-third during adolescence, probably due to the entire confinement to the muscle. Any muscle or group of muscles can be involved. Growth abnormalities or limb deformities are rare. Histologically, the lesions are composed of medium-sized, thin, walled vascular channels with flat endothelium, variable smooth muscle and dysplastic veins. Some lesions may have small vessels with capillary structure and proliferative activity admixed with large feeding and draining veins, which give rise to them being incorrectly called capillary hemangiomas. The cellular proliferation identified in these lesions is due to overactive endothelial proliferation inside a vein, the Masson phenomenon [18, 31, 32]. The imaging appearance of these lesions is similar to the other venous malformations; however, the lesions are confined to a muscle or group of muscles with the venous channels between muscle fibers often tubular or serpiginous and oriented in the long axis of the involved muscle (Fig. 17). It is important to recognize these lesions because the treatment is conservative if asymptomatic. If localized to a single muscle that can be sacrificed, complete excision is preferred, while sclerotherapy is preferred for more diffuse lesions involving non-expendable muscles [7, 31].
Intramuscular venous malformation of the left thigh in a 13-year-old girl. a Axial T2-W image with fat saturation shows the hyperintense VM confined to the left biceps femoris muscle (thick arrows). The lesion contains hypointense foci corresponding to phleboliths (thin arrows). b Coronal T2-W image with fat saturation demonstrates the left thigh VM oriented in the long axis of the muscle (arrows). The internal hypointense foci correspond to phleboliths
Capillary malformations
Another, less preferred term used to refer to capillary malformations (CM) is port wine stain because, clinically, these lesions display a dark red color resembling port wine.
Histologically, CMs are composed of mature ectatic capillary channels in normal numbers located in the superficial dermis surrounded by disorganized collagen. The density of pericapillary neurons is reduced, leading to decreased capillary tone and progressive ectasia [7, 18].
On physical exam, the lesions are geographic with a dark red color and associated papules [7]. CMs are associated with a variety of malformations such as Sturge-Weber and Klippel-Trenaunay syndromes (Fig. 18). Inherited CMs have been identified and are seen in the setting of CM-arteriovenous malformation (AVM) syndrome, an autosomal dominant disorder consisting of cutaneous capillary malformations associated with AVM or AVF due to a mutation in the RASA1 gene [7, 11, 23, 24]. Clinically, these CM are smaller and multifocal surrounded by a pale halo. In 30% of patients, there are associated fast-flow VMs: AVM or AVF (Parkes Weber syndrome) [23].
These lesions do not require routine imaging evaluation; however, they can occur in association with other vascular malformations and become a clue to the diagnosis. These lesions are usually confined to the dermis and can be seen as skin thickening on US. On MRI, these lesions are hyperintense on fluid-sensitive sequences and hypointense on T1-W images and display enhancement after contrast administration.
Arteriovenous malformations
Arteriovenous malformations (AVMs) are high-flow vascular malformations referred in the past as arteriovenous hemangiomas. Most AVMs are sporadic and solitary but can be multiple and in some cases associated with syndromes [9, 18].
The histology of AVMs is highly variable, with combinations of capillaries, venules and arterioles in a fibrous or fibromyxoid stroma. Arteries are larger and tortuous, and some have dissolution of their internal elastic lamina. Veins display muscular hypertrophy or fibrosis of the intima and no adventitia [7].
On physical exam, the diagnosis of AVMs is more challenging and can be delayed, not infrequently diagnosed in older children. These anomalies are seen as areas of cutaneous blush warm to the touch, with associated pulsations, thrills or murmurs (Fig. 19). AVMs may be asymptomatic in the first two decades of life, but the most common symptoms are pain, ulceration, bleeding, ischemia and congestive heart failure. The underlying soft tissues and osseous structures can be normal or hypertrophied [9, 11, 18, 20].
On US, AVMs are seen as ill-defined areas of mixed echogenicity without a discrete mass. Fat surrounding the lesion indicating atrophy can be seen. AVMs are well evaluated on color Doppler showing multiple vessels representing arteries with diastolic flow and arterialized draining veins with pulsatile flow, a distinguishing characteristic from hemangiomas (Fig. 20) [9, 11, 18]. MRI is useful in delineating the extent and possible bone involvement. Dilated feeding arteries and draining veins with signal voids are seen on T1 and fluid-sensitive sequences with no discernible soft tissue mass; thus, mass effect should not be present. The presence of a soft tissue mass should raise concern for a neoplasm [26]. Hypertrophy or atrophy of the adjacent soft tissues may be present (Fig. 21). There is heterogeneous enhancement after the administration of gadolinium. On gradient echo sequences, hyperintense vessels are seen, confirming the high-flow nature of AVMs. MRA provides a vascular map of the lesion depicting feeding arteries and draining veins as well as the nidus. Time-resolved MR angiography is extremely useful because it provides dynamic opacification of all components, including the nidus, due to the high temporal resolution but sacrifices spatial resolution (Fig. 21). The enhancement of a distinct mass is not expected, and if the contrast rise time or dynamic contrast MRA is performed, it should be done fairly quickly due to the high-flow nature of the lesions [9, 11, 18, 26, 33–35]. Conventional arteriogram plays an important role in not only the accurate characterization of AVMs because of selective injection of vessels but also the treatment, since most of these require embolization. Arteriograms have the advantage of selective catheterization of the feeding arteries before embolization and in depicting the nidus and early-draining veins (Fig. 22). Imaging features of AVMs on arteriograms include variable degrees of arterial dilatation and tortuosity, arteriovenous shunting and dilated early draining veins. In older patients, feeding artery aneurysms can be seen [18, 26, 36].
Arterio-venous malformation (AVM) of the hand, US appearance in a 10-year-old girl. a Gray-scale image reveals a focal prominence of the subcutaneous soft tissues of the hand (calipers) where a thrill is palpated without a discrete mass. b Color Doppler reveals a tangle of vessels distributed in a disorganized fashion. c Spectral analysis shows the arterialized waveform of a draining vein
Arterio-venous malformation (AVM) of the gluteal region, MRI appearance (same patient as in Fig. 19). a Coronal T2-W image shows marked asymmetrical hypertrophy of the muscles of the left gluteal region (arrows) with multiple flow voids but no discernible mass. b, c 4-D time-resolved MRA shows the large feeding vessel off the internal iliac artery (arrow) on the earlier phase with visualization of a nidus (asterisk) and an early draining vein (arrow) on a more delayed phase
AVM, arteriogram appearance (same patient as in Fig. 19). a Arterial phase image of an arteriogram after selective catheterization of a dilated branch of the left internal iliac artery (arrow) supplying the left gluteal AVM. b On a more delayed image, the nidus (asterisk) seen as a blush and the early draining vein (arrow) are seen. The child underwent embolization
Conclusion
Following a consistent classification system, such as the one proposed by the ISSVA, is of crucial importance when dealing with vascular anomalies in children. The successful efforts to unify the terminology have helped in reaching correct diagnoses and avoiding misdiagnoses that delay the available treatments and potentially could lead to erroneous treatment. In addition, the use of incorrect terminology can have a negative impact on the research and understanding of these lesions. As pediatric radiologists, it is important to directly examine patients with vascular anomalies because the first step in imaging vascular anomalies begins with the family history and direct clinical examination by the clinician and radiologist. Imaging is not used routinely to diagnose hemangiomas and vascular malformations because the diagnosis is mainly clinical. When imaging is requested, it should be targeted to address specific issues and answer questions required for the comprehensive interdisciplinary team to plan management. US and MRI have a complementary role in the evaluation of vascular anomalies, with US generally used as the first line of imaging and MRI when additional information is needed, in cases of very extensive lesions or when intervention is considered. Due to the higher cost, need for sedation and use of intravenous contrast, an US can obviate the need of an MRI particularly for younger patients and for fairly localized and superficial lesions.
References
Hassanein AH, Mulliken JB, Fishman SJ et al (2011) Evaluation of terminology for vascular anomalies in current literature. Plast Reconstr Surg 127:347–351
Boye E, Jinnin M, Olsen B (2009) Infantile hemangioma: challenges, new insights, and therapeutic options. J Craniofac Surg 20:678–684
Enjolras O, Mulliken JB (1997) Vascular tumors and vascular malformations (new issues). Adv Dermatol 13:375–423
Restrepo R, Palani R, Cervantes LF et al (2011) Hemangiomas revisited: the useful, the unusual and the new. Part 1: overview and clinical and imaging characteristics. Pediatr Radiol 41:895–904
Dompmartin A, Vikkula M, Boon L (2010) Venous malformation: update on etiopathogenesis and management. Phlebology 25:224–235
Haggstrom AN, Drolet BA, Baselga E et al (2007) Prospective study of infantile hemangiomas: demographic, prenatal and perinatal characteristics. J Pediatr 150:291–294
Bruder E, Alaggio R, Kozakewich HP et al (2012) Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Devel Pathol 15:26–61
North PE, Waner M, Mizeracki A et al (2000) GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol 31:11–22
Lowe LH, Marchant TC, Rivard D et al (2012) Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol 47:106–117
Mulliken JB, Enjolras O (2004) Congenital hemangiomas and infantile hemangiomas: missing links. J Am Acad Dermatol 50:875–882
Dubois J, Alison M (2010) Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 40:895–905
Gorincour G, Kokta V, Rypens F et al (2005) Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non involuting congenital hemangiomas. Pediatr Radiol 35:1178–1185
Walter JW, North PE, Warner M et al (2002) Somatic mutation of vascular endothelial growth factors receptors in juvenile hemangioma. Genes Chromosomes Canc 33:295–303
Puig S, Casati B, Staudenherz A et al (2005) Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol 53:35–45
Greene AK, Rogers GF, Mulliken JB (2007) Intrasosseous hemangiomas are malformations and not tumors. Plast Reconstr Surg 119:1949–1950, author reply 1950
Bruder E, Perez-Atayde AR, Jundt G et al (2009) Vascular lesions of bone in children, adolescents and young adults. A clinicopathological reappraisal and application of the ISSVA classification. Virchows Arch 454:161–179
Cahill AM, Nijs E, Ballah D et al (2011) Percutaneous sclerotherapy in neonatal and infant head and neck lymphatic malformations: a single center experience. J Pediatr Surg 46:2083–2095
Legiehn GM, Heran MKS (2006) Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am 37:435–474
Jeltsch M, Tammela T, Alitalo K et al (2003) Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res 314:69–84
Ernemann U, Kramer U, Miller S et al (2010) Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J Radiol 75:2–11
Narang T, Dipankar D, Dogra S (2011) Lymphangioma circumscriptum and Whimster’s hypothesis revisited. Skinmed 9:123–124
Patel G, Schwartz RA (2009) Cutaneous lymphangioma circumscriptum:frog spawn on the skin. Int J Dermatol 48:1290–1295
Boon LM, Baliieux F, Vikkula M (2011) Pathogenesis of vascular anomalies. Clin Plast Surg 38:7–19
Garzon MC, Huang JT, Enjolras O et al (2007) Vascular malformations. Part I. J Am Acad Dermatol 56:353–370
Dompmartin A, Ballieux F, Thibon P et al (2009) Elevated D-Dimer level in the differential diagnosis of venous malformations. Arch Dermatol 145:1239–1244
Leigiehn GM, Heran MK (2010) A step-by-step practical approach to imaging, diagnosis and interventional radiologic therapy in vascular malformations. Semin Intervent Radiol 27:209–231
Redondo P, Aguado L, Martinez-Cuesta A (2011) Diagnosis and management of extensive vascular malformation of the lower limb. Part I Clinical diagnosis. J Am Acad Dermatol 65:893–906
Enjolras O, Ciabrini D, Mazoyer E et al (1997) Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol 36:219–226
Kubiena HF, Liang MG, Mulliken JB (2006) Genuine diffuse phlebectasia of Bockenheimer: dissection of an eponym. Pediatr Dermatol 23:294–297
Dompmartin A, Acher A, Thibon P et al (2008) Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol 144:873–877
Hein KD, Mulliken JB, Kozakewich HPW et al (2002) Venous malformations of skeletal muscle. Plast Reconst Surg 110:1625–1635
Allen PW, Enzinger FM (1972) Hemangioma of skeletal muscle. Cancer 29:8–22
Ohgiya Y, Hashimoto T, Gokan T et al (2005) Dynamic MRI for distinguishing high-flow from low-flow peripheral vascular malformations. Am J Roentgenol 185:1131–1137
Kim JS, Chandler A, Borzykowksi R et al (2012) Maximizing time-time resolved MRA for differentiation of hemangiomas, vascular malformations and vascularized tumors. Pediatr Radiol 42:775–784
Mostardi PM, Young PM, McKusick MA et al (2012) High temporal and spatial resolution imaging of peripheral vascular malformations. J Magn Reson Imaging 36:933–942
Burrows PE, Laor T, Paltiel H et al (1998) Diagnostic imaging in the evaluation of vascular birthmarks. Pediatr Dermatol 16:455–488
Disclaimer
The author has no financial interests, investigational or off-label uses to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Restrepo, R. Multimodality imaging of vascular anomalies. Pediatr Radiol 43 (Suppl 1), 141–154 (2013). https://doi.org/10.1007/s00247-012-2584-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2584-y