Abstract
Purpose
To investigate whether there is an increased signal intensity (SI) of dentate nucleus (DN) and globus pallidus (GP) on unenhanced T1-weighted magnetic resonance imaging (MRI), in patients who had undergone multiple administrations of gadoxetate disodium.
Materials and methods
We retrospectevely included stage III melanoma patients, who had been previously enrolled in a trial of adjuvant therapy and who had undergone whole-body contrast-enhanced MRIs with gadoxetate disodium every three months for their follow-up. The SI ratios of DN-to-pons and GP-to-thalamus on unenhanced T1-weighted images were calculated. The difference in SI ratios between the first and the last MRI examinations was assessed and a linear mixed model was performed to detect how SI ratios varied with the number of administrations.
Results
Eighteen patients were included in our study. The number of gadoxetate disodium administrations ranged from 2 to 18. Paired t-test did not show any significant difference in DN-to-pons (p=0.21) and GP-to-thalamus (p=0.09) SI ratios by the end of the study. DN-to-pons SI ratio and GP-to-thalamus SI ratio did not significantly increase with increasing the number of administrations (p=0.14 and p=0.06, respectively).
Conclusion
Multiple administrations of gadoxetate disodium are not associated with increased SI in DN and GP in the brain.
Key Points
• Gadolinium may deposit in the human brain after multiple GBCA administrations.
• Gadolinium deposition is associated with increased T1W signal intensity
• Increase in signal intensity is most apparent within the DN and GP
• Multiple administrations of gadoxetate disodium do not increase T1W signal
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased signal intensity (SI) of dentate nucleus (DN) and globus pallidus (GP) has been reported on unenhanced T1-weighted magnetic resonance imaging (MRI) in patients with normal renal function, who received multiple administrations of gadolinium-based contrast agents (GBCAs) for contrast-enhanced magnetic resonance imaging (CE-MRI) [1–13]. The majority of these studies suggested a correlation between the administration of GBCAs and hyperintensity of DN and GP on unenhanced T1-weighted MRI, without investigating the presence of gadolinium deposits in post-mortem brain specimens [1–5, 7, 9–13]. Some retrospective studies, using inductively coupled plasma mass spectrometry, have demonstrated gadolinium deposits in post-mortem brain specimens of patients who received GBCAs for CE-MRI [6, 8, 14]. Further prospective animal studies have confirmed the presence of gadolinium brain deposits in rats subjected to multiple GBCAs administrations [15–18].
Different types of GBCAs have been investigated, trying to understand whether the molecular structures of GBCAs could influence the SI of the brain nuclei (DN and GP) on T1-weighted MRI and the gadolinium brain deposition: linear ionic (gadopenetate dimeglumine, gadobenate dimeglumine), linear non-ionic GBCAs (gadodiamide) and macrocyclic (gadoteridol, gadoterate meglumine and gadobutrol) [19]. Linear GBCAs have been reported to be more associated with high SI of brain nuclei on unenhanced T1-weighted MRI and with gadolinium brain deposition compared to macrocyclic GBCAs [19]. In addition linear non-ionic GBCAs have been reported to have a greater effect on MRI SI than the linear ionic ones [9].
Gadoxetate disodium is a liver-specific GBCA, with a linear ionic molecule, that has up to 50% hepatobiliary excretion in the normal liver [20]. For this reason, gadoxetate disodium is indicated for liver CE-MRI, thereby making it difficult to investigate the correlation between multiple administrations of gadoxetate disodium and the SI of DN and GP using intraindividual comparisons. A recent study has investigated this correlation by comparing a group of subjects who received multiple administrations of gadoxetate disodium with a control group of subjects who did not receive prior GBCA administrations [21].
In the present study we investigated the correlation between multiple administrations of gadoxetate disodium and SI of DN and GP on unenhanced T1-weighted MRI, by performing an intraindividual comparative analysis in a cohort of patients with stage III melanoma at high risk of developing distant metastases, who had undergone multiple whole-body CE-MRIs (WB CE-MRI) using gadoxetate disodium for disease surveillance.
Materials and methods
Patients
From February 2009 to August 2013, at the European Institute of Oncology, 23 melanoma patients were enrolled in a prospective randomized trial evaluating the efficacy of ipilimumab as adjuvant therapy. Inclusion criteria of the trial were: 1) completely excised melanoma, based on hystological confirmation; 2) a diagnosis of stage III melanoma according to AJCC criteria (i.e., absence of distant metastases) [22]; and 3) no in transit metastasis. Exclusion criteria of the trial included unknown primary, ocular, or mucosal melanoma, Eastern Cooperative Oncology Group performance status greater than 1, autoimmune disease, uncontrolled infections, cardio-vascular disease, white blood cell count lower than 2·5 × 109 cells per L, absolute neutrophil count lower than 1·0 × 109 cells per L, platelets lower than 75 × 109 cells per L, haemoglobin concentration less than 9 g/dL, creatinine higher than 2.5 times the upper normal limit, hepatic enzymes or lactate dehydrogenase higher than two times the upper normal limit, use of systemic corticosteroids, and previous systemic therapy for melanoma. Patients were randomly assigned to receive ipilimumab or placebo every 3 weeks for four doses, then every 3 months for up to a maximum of 3 years, or until disease recurrence, unacceptable toxicity, or treatment refusal. The primary endpoint was recurrence-free survival by intention-to treat analysis [23]. All patients underwent WB CE-MRI for follow-up every three months. Radiological findings have been partially published in a previous paper [24]. Institutional Review Board approval was obtained and all patients signed an informed consent.
In this study we retrospectevely reassessed the brain CE-MRI examinations of the 18 out of 23 patients who had undergone at least two gadoxetate disodium administrations and had not previously undergone other GBCAs administration. Previous GBCAs administrations were excluded relying on the patient’s response to the specific question (i.e., “Have you ever received an MRI contrast-agent?”) contained in the questionnaire filled out before the first MRI scan. The demographic and clinical characteristics of all 18 patients are shown in Table 1.
Imaging and data analysis
As part of the WB CE-MRI protocol, brain CE-MRI was performed at a 1.5T scanner (Magnetom Avanto scanner, Siemens Healthcare, Erlangen, Germany) with axial unenhanced T1- and T2-weighted images, axial and coronal contrast-enhanced T1-weighted images. Axial unenhanced T1-weighted spin echo images were obtained using the following parameters: repetition time (msec)/echo time (msec), 500/10; section thickness, 5 mm; distant factor, 0; number of signals acquired, 2; and matrix size, 256 x 320; and acquisition time 4.53 min. Gadoxetate disodium (Primovist@, Bayer) was administered at the recommended dose of 0.1 ml/kg (0.025 millimoles/kg) of body weight.
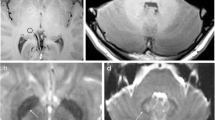
Two radiologists conducted the analysis blinded to the data. The scans were firstly assessed for intracranial abnormalities and patients with signal abrnormalities (i.e., infarcts, parivascular spaces, hemorrhage, etc.) of the DN, GP, Thalamus and Pons on T2-weighted images were excluded from further analysis. Oval ROIs were placed around the right DN, central pons, right GP , and right thalamus on the unenhanced T1-weighted images (Fig. 1) according to a previously described method [13]. In cases where the assessment of the right side of the brain could not be performed due to metal or pulsation artefact, the left side was used. Agreement on the correct ROI placement was reached by consensus and all ROI measurements were conducted within a single session. The mean SI within the DN was divided by the mean SI within the central pons in order to calculate the DN-to-pons SI ratio. The mean SI within the GP was divided by the mean SI within the thalamus in order to calculate the GP-to-thalamus SI ratio.
Statistical analysis
Qualitative data were expressed as absolute frequencies while quantitative data were expressed as mean ± standard deviation (SD) and range. The normality of quantitative data was tested by the Kolgomorov-Smirnov test. The comparison of means between the first and the last brain CE-MRI examinations was performed with a paired t-test for normal data (DN-to-pons and GP-to-thalamus SI ratios, AST, ALT and total bilirubin) and Wilcoxon signed-rank test for non-normal data (eGFR).
To estimate the statistical power, DN-to-pons SI ratio was considered as the target variable. Since there are no intraindividual comparative studies which have investigated the effect of the administration of gadoxetate disodium on the DN-to-pons SI ratio, we considered data of the study by Cao et al. who analyzed a similar type of GBCA (ionic, linear), with a similar number of administrations (11.11 ± 5.59 in our study versus 12.1 ± 5.2 in the study by Cao et al.) and similar method of image analysis. The authors reported a DN-to-pons mean SI ratio of 0.98 ± 0.045 at first CE-MRI examination and 1.063 ± 0.105 at the last CE-MRI examination in 25 paired subjects, which correspond to a large effect size (Cohen’s d = 1.028). Assuming this effect size and using a two-tailed paired t-test with α= 0.05, a sample of 18 subjects was allowed to reach a statistical power of 83%.
A linear mixed model for repeated measures analysis was applied to all the collected measures to detect how the relative signal change varied on the basis of the number of administrations. Administrations of gadoxetate disodium were considered as fixed effect, and patients were considered as random effect.
The strength of association of the examined variables was determined by the value of the Spearman’s rank correlation coefficient (ρ). Statistical analysis was performed using the SPSS 20.0 for Windows software package (SPSS Inc., USA) and SAS software (SAS Institute Inc., Cary,IN, USA).
Results
A total of 216 brain CE-MRI examinations were performed in 18 patients. The number of gadoxetate disodium administration for all 18 patients ranged from 2 to 18 (median: 13; average, 11.11±5.59). The average total dose of contrast agent administered was 85.17±44.38 ml (range of 14–140 ml). Renal and liver function parameters at the beginning and end of the study are outlined in Table 2. A significant decrease in eGFR was noted at the last CE-MRI examination (p<0.05). All parameters of liver function remained normal.
In four out of 18 patients small focal infarcts were detected in the subcortical and periventricular cerebral white matter, which did not show any modification during the study period. In the other patients no intracranial abnormalities were detected. In all patients no signal abnormalities were detected within the DN, GP, Thalamus and Pons.
Paired t-test did not show a significant difference in GP-to-thalamus (p=0.09; Fig. 2) and DN-to-pons (p=0.21; Fig. 3) SI ratios at the end of the study. For both DN-to-pons SI ratio and GP-to-thalamus SI ratio, the linear mixed model showed that SI did not significantly increase with increasing the number of gadoxetate disodium administration (slope= -0.00084, p=0.14 and slope= 0.000746, p=0.06, respectively; Fig. 4).
Using Spearman’s correlation coefficient, the correlations between GP-to-thalamus and DN-to-pons SI ratios and number of administrations, total amount of contrast administered, eGFR, AST and ALT are shown in Table 3.
The GP-to-thalamus and DN-to-pons SI in the last CE-MRI examination did not show any statistically significant correlation with the examined parameters (Table 3).
Discussion
Our study did not show a significant increase in SI within both DN and GP on unenhanced T1-weighted MRI in patients with stage III melanoma after multiple administrations of gadoxetate disodium. Although there was a trend towards the significance for an increase of GP-to-thalamus SI ratio with increasing the number of administrations, we noted that this result was mostly influenced by the last measure of the patient who received 18 administrations. If we removed this measure, slope was 0.000595 and p-value was 0.13. Our results suggest that the effect of the administration of gadoxetate disodium on the SI of the indicator regions could be weak. Future intraindividual comparative studies should confirm our results using a smaller effect size to calculate the sample size.
To the best of our knowledge, this is the first intraindividual comparative study which investigated the correlation between the gadoxetate disodium administration and SI of DN and GP. A recent study by Kahn et al. showed an increased SI in the DN of patients who received from ten to 37 administrations of gadoxetate disodium (subgroup A3), but not in those who received less than ten administrations (subgroups A1 and A2) [21]. In addition, the authors showed a positive correlation between the numbers of administrations and the SI in DN, but they failed to detect the same correlation for the SI in GP. Our results are partially in agreement with those by Kahn et al. In our cohort the number of administrations was more similar to that of the subgroups A1 (<5 administrations) and A2 (<10 administrations) of the study by Kahn et al., in which the authors failed to detect an increased SI in both the DN and GP. However some limitations of the study by Kahn et al. should be acknowledged: 1) the authors did not perform intraindividual comparative analysis; 2) they included patients with up to two non–liver-specific GBCAs in the past; and 3) CE-MRIs were performed with different MR scanners and protocols.
A recent pathological study on cadavers has revealed low levels of gadolinium deposition in the brain tissue of a patient with hepatocellular carcinoma who had undergone ten administrations of gadoxetate disodium, for a total dose of 100 ml [14]. However, in this study gadoxetate disodium showed a relatively low normalized gadolinium deposition ratios (gadolinium deposited in 1 g of tissue per millimole of GBCA administered), corroborating the absence of increased SI in our patients.
Gadoxetate disodium is a hepatospecific GBCA, with a linear ionic molecular structure. Our results are not in agreement with previous retrospective studies demonstrating an increase of SI in the brain tissue after administration of various linear GBCAs: gadopenetate dimeglumine [1, 3, 5, 8, 11, 13], gadodiamide [1, 2, 4, 8, 9, 15, 16], and gadobenate dimeglumine [10, 11]. The discrepancy may be in part explained by the different stability of linear GBCAs: gadoxetate disodium has been reported to release 1.1% of its gadolinium in human serum at 37 °C after 15 days versus 1.9% of gadopenetate dimeglumine and gadobenate dimeglumine. This could be because of the slightly higher excess of free ligand (0.5% vs. 0.1% or no excess in the other formulations), which may attenuate the release of gadolinium [25]. Furthermore, the release of gadolinium from the ionic linear GBCAs (gadopenetate dimeglumine, gadobenate dimeglumine, gadoxetate disodium) has been reported to be about ten times lower than that from the non-ionic GBCAs (gadodiamide, gadoversetamide) [25]. This data may support the reported differences in brain SI between linear ionic (gadobenate dimeglumine) and linear non-ionic (gadodiamide) GBCAs, showing that the linear non-ionic GBCA has a greater effect than the linear ionic one [9]. Finally the reccomended dosage of gadoxetate disodium is lower (0.025 mmol/kg) compared to other GBCAs (typically 0.1 mmol/kg), thus allowing a reduction of the cumulative dose which has been demostrated to correlate with the signal changes and the gadolinium deposition in the brain of deceased patients [6].
The strength of our study consists in the fact that we analyzed patients of a clinical trial of adiuvant therapy, who had locally advanced melanoma (stage III) and underwent multiple WB CE-MRI examinations for surveillance, because they had an high risk of developing metastases. Thanks to the prospective design of the trial, we used the same T1-weighted spin-echo sequence to calculate the SI ratios at baseline and final examinations, as suggested by a recent study [26]. In addition, we reduced the number of pre-existing clinical factors that could affect hyperintensity on unenhanced T1-weighted MRI in the brain nuclei. The first of these was poor renal and/or liver function since gadoxetate disodium is cleared from the body through the liver (50%) and the kidneys (50%). A recent study conducted in haemodialysis patients has confirmed that renal function may affect the rate of gadolinium deposition in the brain after CE-MRI with linear GBCAs administrations [27]. We included patients without brain disease and history of previous brain treatments (surgery and/or radiotherapy). The majority of previous studies investigated linear GBCAs in patients with intracranial disease [1–5, 8, 9], and only one of them included multiple sclerosis patients [2]. Multiple sclerosis represents the major confounder as hyperintensity in the dentate nucleus has previously been reported in patients with secondary progressive multiple sclerosis [28].
The foremost limitation of this study is the small number of patients; however,the number of administrations for each patient (median = 13) and the cumulative dose of gadoxetate disodium (mean: 85.17 ml) is considerable. We acknowledge that there are no published reports on the use of gadoxetate disodium in evaluating the brain. Gadoxetate disodium was used for the whole-body surveillance of our patients in order to have the highest sensitivity in liver metastases detection, which is one of the most frequent sites. In addition, melanoma brain metastases are intrinsically bright and also contain blood products leading to brighter enhancement in brain images. Finally, we note that the T1 relaxivity of this contrast agent is almost twice in comparison to other GBCAs [29]. Furthermore, we highlight that CE-MRI was able to detect brain metastases with a diameter less than 10 millimeters in three out of 23 patients participating in the surveillance protocol. Finally, some of the enrolled patients could be treated with Ipilimumab that might represent a confounder.
In conclusion, our results demonstrated that multiple administrations of gadoxetate disodium are not associated with increased SI in DN and GP in melanoma patients with normal renal and liver functions.
Abbreviations
- DN:
-
Dentate nucleus
- GBCA:
-
Gadolinium-based contrast agent
- GP:
-
Globus pallidus
- CE-MRI:
-
Contrast-enhanced magnetic resonance imaging
- SI:
-
Signal intensity
- WB:
-
Whole-body
References
Kanda T, Ishii K, Kawaguchi H et al (2014) High SI intensity in the GP and GP on unenhanced T1- weightedMR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 SI intensity of the GP on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Kanda T, Osawa M, Oba H et al (2015) High SI intensity in GP on unenhanced T1-weighted images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:800–809
Quattrocchi CC, Malio CA, Errante Y et al (2015) Gadodiamide and GP T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Investig Radiol 50:470–472
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the GP and GP is dependent on the class of contrast agent. Radiology 275:783–791
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Inracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Jubisavljevic S (2016) Increasing SI intensity within the GP and GP on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26:807–815
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruvama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, Semelka RC (2015) High SI intensity in GP and GP on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276:836–844
Weberling L, Kieslich P, Kickingereder P et al (2015) Increased SI intensity in the GP on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol 50:743–748
Roberts D, Holden K (2016) (2015) Progressive increase of T1 SI intensity in the GP and GP on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Radbruch A, Weberling L, Kieslich P et al (2015) High SI intensity in the GP and GP on unenhanced T1-weighted images. Investig Radiol 50:805–810
Cao Y, Huang DQ, Shih G, Prince MR (2016) SI change in the GP on T1-weighted MR images after multiple administrations of gadopenetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206:414–419
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 51(7):447–453
Robert P, Lehericy S, Grand S, Violas X, Fratellier N, Idee JM, Ballet S, Corot C (2015) T1-weighted hyperSI in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats. Investig Radiol 50:473–480
Robert P, Violas X, Grand S et al (2016) Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol 51:73–82
Jost G, Lenhard DC, Sieber MA et al (2016) SI increase on unenhanced T1-weighted images in the Rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Investig Radiol 51:83–89
Smith AP, Marino M, Roberts J et al. (2016) Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology
Stojanov D, Aracki-Trenkic A, Stojanov DB (2016) Gadolinium deposition within the GP and GP after repeated administrations of gadolinium-based contrast agents—current status. Neuroradiology 58:43–441
Van Beers BE, Pastor CM, Hussain HK (2012) Primovist, Eovist: what to expect? J Hepatol 57:421–429
Kahn J, Posch H, Steffen IG et al (2017) Is there long-term signal intensity increase in the central nervous system on T1-weighted images after MR imaging with the hepatospecific contrast agent gadoxetic acid? a cross-sectional study in 91 patients. Radiology. doi:10.1148/radiol.2016162535
Balch CM, Gershenwald JE, Soong SJ et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206
Eggermont AM, Chiaron-Sileni V, Grob JJ et al (2015) Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 16:522–530
Petralia G, Padhani A, Summers P et al (2013) Whole-body diffusion-weighted imaging: is it all we need for detecting metastases in melanoma patients? Eur Radiol 23:3466–3467
Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ (2008) Stability of gadolinium based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol 43(12):817–828
Ramalho J, Ramalho M, AlObaidy M, Nunes RH, Castillo M, Semelka RC. (2016) T1 signal-intensity increase in the dentate nucleus after multiple exposures to gadodiamide: intraindividual comparison between 2 commonly used sequences. AJNR Am J Neuroradiol
Cao Y, Zhang Y, Shih G, et al. (2016) Effect of renal function on gadolinium-related signal increases on unenhanced T1-weighted brain magnetic resonance imaging. Invest Radiol
Roccatagliata L, Vuolo L, Bonzano L et al (2009) Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted images is associated with the secondary progressive subtype. Radiology 251:503–510
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Giorgio Conte.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
Dr. Giorgio Conte and Sara Raimondi kindly provided statistical advice for this manuscript.
Informed consent
Patients signed a written informed consent.
Ethical approval
Institutional Review Board approval was obtained
Methodology
• observational study
• retrospective
• performed at one institution
Rights and permissions
About this article
Cite this article
Conte, G., Preda, L., Cocorocchio, E. et al. Signal intensity change on unenhanced T1-weighted images in dentate nucleus and globus pallidus after multiple administrations of gadoxetate disodium: an intraindividual comparative study. Eur Radiol 27, 4372–4378 (2017). https://doi.org/10.1007/s00330-017-4810-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4810-3