Abstract
Background
Few studies have been conducted on the relations between T1-weighted signal intensity changes in the pediatric brain following gadolinium-based contrast agent (GBCA) exposure.
Objective
The purpose of this study is to investigate the effect of multiple administrations of a macrocyclic GBCA on signal intensity in the globus pallidus and dentate nucleus of the pediatric brain on unenhanced T1-weighted MR images.
Materials and methods
This retrospective study included 50 patients, mean age: 8 years (standard deviation: 4.8 years), with normal renal function exposed to ≥6 administrations of the same macrocyclic GBCA (gadoterate meglumine) and a control group of 59 age-matched GBCA-naïve patients. The globus pallidus-to-thalamus signal intensity ratio and dentate nucleus-to-pons signal intensity ratio were calculated from unenhanced T1-weighted images for both patients and controls. A mixed linear model was used to evaluate the effects on signal intensity ratios of the number of GBCA administrations, the time interval between administrations, age, radiotherapy and chemotherapy. T-test analyses were performed to compare signal intensity ratio differences between successive administrations and baseline MR signal intensity ratios in patients compared to controls. P-values were considered significant if <0.05.
Results
A significant effect of the number of GBCA administrations on relative signal intensities globus pallidus-to-thalamus (F[8]=3.09; P=0.002) and dentate nucleus-to-pons (F[8]=2.36; P=0.021) was found. The relative signal intensities were higher at last MR examination than at baseline (P<0.001).
Conclusion
Quantitative analysis evaluation of globus pallidus:thalamus and dentate nucleus:pons of the pediatric brain demonstrated an increase after serial administrations of macrocyclic GBCA. Further research is necessary to fully understand GBCA pharmacokinetic in children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last 2 years, several published studies have demonstrated the association between an increased signal intensity (on unenhanced T1-weighted MR images in specific deep gray matter regions and previous exposure to gadolinium-based contrast agent (GBCA) administrations in patients with normal renal function [1, 2]. These observations were subsequently repeated by a postmortem study that used inductively coupled plasma mass spectrometry on brain tissue from patients exposed to previous linear GBCA administrations, confirming the presence of abundant gadolinium deposits in the neural tissue interstitium and within the endothelial wall [3]. So far, the published data have reported some differences among GBCAs with different chemical structures: A T1-weighted signal intensity increase was mostly found in patients exposed to serial administrations of linear GBCAs compared to macrocyclic ones and the difference has been attributed to the aforementioned different kinetic stabilities compared to their linear counterparts [4,5,6,7].
Recently, three retrospective studies have determined the presence of abnormal T1 hyperintensity in the dentate nucleus and globus pallidus of children following repeated administrations of the linear GBCA gadopentetate dimeglumine, thus confirming the results of adult studies. Therefore, it has been recommended to use more stable macrocyclic GBCAs in children who are considered at risk because of potential age-related immature renal function [8,9,10].
The purpose of the present study was to investigate whether repeated exposure to multiple administrations of the macrocyclic GBCA gadoterate meglumine (Dotarem; Guerbet, Villepinte, France) could be associated with an increase in signal intensity on T1-weighted sequences in specific brain regions in a pediatric population.
Materials and methods
Patients
This study was conducted in accordance with the Declaration of Helsinki and approved by the local institutional review board. As the whole study was based exclusively on medical records, the requirement to obtain informed consent from patients’ parents was waived.
All MR examinations with contrast media administration acquired from June 2011 to June 2016 were reviewed. Data were extracted from 256 consecutive children with at least 6 brain MR scans during the study period. The threshold number of MR scans was chosen on the basis of previous findings [11].
As gadoterate meglumine is the only GBCA used at our institution for all contrast-enhanced MR studies, irrespective of a patient’s renal function, all patients included in the present study were exclusively administered this GBCA.
Inclusion criteria were the presence of at least 6 consecutive contrast-enhanced MR scans with exclusive administration of gadoterate meglumine alone, all MR scans were performed with the same 3-T scanner at our institution and the patients’ ages ranged from 2 years to 18 years. The limit of 2 years of age was chosen to exclude younger children with incomplete myelination [12].
The number of patients not meeting inclusion criteria was 127.
Exclusion criteria were the presence of edema, hemorrhage, tumors or other lesions involving both globus pallidus or dentate nucleus, thalami or pons (n=8); the diagnosis of meningoencephalitis (n=5), neurofibromatosis type 1 (n=32) or multiple sclerosis (n=12); missing or unsatisfactory unenhanced T1-weighted sequences (n=20), and the presence of altered hepatic (n=1) or renal functions (n=1).
No patients had Langerhans cells histiocytosis or a history of parenteral nutrition and none was affected by metabolic disorders or malformations of the central nervous system.
The control group consisted of GBCA-naïve subjects selected from our electronic database. They all had one normal non-contrast MR brain examination performed during the same period of time (from June 2011 to June 2016) with the same 3-T MR scanner as the patient group. Each control subject was matched to a patient for age (± ≤1 year) at both first and last MR examination within the age range of 2 years to 18 years. The presence of a normal MR examination was considered the only inclusion criterion whereas the history of GBCA exposure and presence of altered hepatic or renal functions were exclusion criteria.
Abnormal liver function was defined by abnormal serum concentrations of aspartate aminotransferase, alanine aminotransferase, total bilirubin or γ-glutamyl transpeptidase and it was checked in medical records. Renal function was assessed before each MR examination to screen for renal failure, which was evaluated by calculating the estimated glomerular filtration rate from a blood sample. Estimated glomerular filtration rate calculation was performed using revised Schwartz formula [13]. Renal function was classified as normal (≥90 ml/min/m2) or abnormal (<60 ml/min//m2). According to institutional policy, GBCA is administered only when appropriate for the imaging study and is withheld from any patient with abnormal estimated glomerular filtration rate, as defined above, unless strictly necessary for diagnostic purposes.
For all patients, we ascertained their age, gender, number and date of previous GBCA administrations, diagnosis and clinical reason for brain MR, and their history of chemotherapy and radiotherapy. Radiotherapy was defined as history of radiation therapy to the brain.
Magnetic resonance imaging protocol
All brain MR examinations were acquired using a 3-T MRI system (Skyra; Siemens, Erlangen, Germany), configured with a 20-element head matrix coil. The standard brain MR protocols with contrast administration varied according to clinical indications, but all protocols included a pre-contrast axial turbo spin echo T1-weighted sequence with the following parameters: repetition time/echo time: 500–600 ms/9.9–6.4 ms; section thickness: 3 mm; spacing: 0.3 mm; field of view: 220 mm; matrix size: 256 × 256, flip angle: 138° and echotrain: 42. Moreover, all brain protocols included axial diffusion-weighted imaging sequences (repetition time/echo time: 9,000 ms/98 ms; section thickness: 3 mm; spacing: 0.6 mm, field of view: 220 mm and matrix size: 192 × 192) and axial T2-weighted sequences (repetition time/echo time: 8,600 ms/122 ms; section thickness: 3 mm; spacing: 0.6 mm, field of view: 220 mm and matrix size: 384 × 324). These sequences were used to localize regions of interest (ROIs) as explained thereafter.
Our institution’s MR safety policy for the reported risk for gadolinium-related nephrogenic systemic sclerosis requires the use of gadoterate meglumine at the standard dose of 0.1 mmol/kg of body weight in all patients, administered intravenously.
Image and data analysis
Quantitative analysis was performed for all MR examinations. All MR images were reviewed on Carestream PACS, version 11.0 (Carestream Health, Inc.; Rochester, NY U.S.A.).
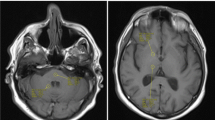
Signal intensity measurements were obtained by applying a circular ROI of 4-mm diameter within the globus pallidus, the dentate nucleus, the thalamus and the pons to the unenhanced T1-weighted images. ROI placement was agreed upon by consensus of two readers (M.C.R.E. and L.F.-T. with 7 years and 11 years of experience in neuroradiology, respectively) blinded to clinical data and to the serial number of the MR scan. In each subject, ROI measurement was conducted once and always placed on the right side. If the right side could not be assessed, then the left side was used (n=4 patients for globus pallidus:thalamus measurement). B0 images from axial diffusion-weighted and axial T2-weighted sequences were always used to guide the correct ROI placement (Fig. 1).
Region of interest (ROI) analysis at axial MRI in a 8 years old boy. a-b For quantitative analysis of globus pallidus-to-thalamus signal intensity ratio, ROIs were drawn in the globus pallidus (white circle) and thalamus (black circle) on unenhanced T1-weighted images (a) using B0 images from diffusion-weighted imaging (b) as reference (arrow in Fig. 1b indicates the globus pallidus). c-d For quantitative analysis of dentate nucleus-to-pons signal intensity ratio, ROIs were drawn on T1-weighted images (c) in the dentate nucleus (white circle) and pons (black circle) using B0 images from diffusion-weighted imaging (d) as reference (arrow in Fig. 1d indicates the dentate nucleus)
Mean ROI values from the dentate nucleus, globus pallidus, thalamus and pons were collected and globus pallidus-to-thalamus signal intensity ratio and dentate nucleus-to-pons signal intensity ratio were calculated.
Visual inspection of MR images was always done by the two readers to check for T1 hyperintensity in the aforementioned brain regions.
Statistical analysis
Statistical analysis was performed using software PASW Statistics, version 21.0 (SPSS, Chicago, Ill.)
The data were tested for normality by using a Lilliefors test.
A mixed linear model was used to verify the effect of the number of administrations on signal intensity ratios over time. The variables included in the model were defined as follows: signal intensity ratio (globus pallidus:thalamus or dentate nucleus:pons) ≈ number of administrations + age at the first MR + chemotherapy (yes/no) + radiotherapy (yes/no) + time interval between administrations (days) + intercept. The number of administrations, age at first MR, radiotherapy (yes/no), chemotherapy (yes/no) and the time interval between examinations (days) were selected as fixed factors with intercept as a random factor. Multiple paired t-tests with Bonferroni correction were performed to compare signal intensity ratio values at each administration point versus the first administration (baseline).
The number of administrations for the mixed linear model was limited to 9, as the number of patients with assessable globus pallidus-to-talamus and dentate nucleus-to-pons and more than 9 administrations was inadequate for statistical comparison. Missing administration points were excluded by case. For the mixed model, the output was presented with F (degree of freedom = number of administrations) along with the P-value associated.
In order to compare signal intensity ratios of first and last MR examination as well as to compare baseline signal intensity ratios in the patient group versus control group, a two-sample t-test analysis was performed.
One sample t-test was used to assess whether the slope of the globus pallidus:thalamus and dentate nucleus:pons increase, fitted with first order polynomial function, was different from zero.
P-values were set at 0.05.
Results
The population included 50 patients, mean age: 8 years (standard deviation [SD]: 4.8 years, range: 2 years–18 years) and 59 controls, mean age 8.4 years (SD: 4.2 years, range: 2 years–18 years).
Table 1 summarizes the characteristics of patients and controls.
In patients who underwent posterior fossa surgery, dentate nucleus:pons data were not assessed. Therefore, dentate nucleus:pons evaluation was performed in 26/50 patients.
The mixed linear model analysis showed a significant effect of number of GBCA administrations both on globus pallidus:thalamus (F[8]=3.09; P=0.002) and on dentate nucleus:pons (F[8]=2.36; P=0.021) (Fig. 2).
Age, the interval between administrations, radiotherapy and chemotherapy did not have a significant effect on either globus pallidus:thalamus and dentate nucleus:pons over time.
A paired t-test between the imaging at the ninth administration and the baseline imaging in the patient group showed significant differences in both globus pallidus:thalamus (P=0.04), evaluated in 35 patients, and dentate nucleus:pons (P=0.001), evaluated in 20 patients.
A paired t-test between imaging following the fifth administration and the baseline imaging in the patient group showed significant differences in the dentate nucleus:pons (P=0.012), evaluated in 26 patients, but not in the globus pallidus:thalamus (P=0.57), evaluated in 50 patients.
A significant difference between the first and last administration in both the globus pallidus:thalamus (P=0.004) and the dentate nucleus:pons (P=0.001) was observed, demonstrating higher values in the last administration. Mean globus pallidus:thalamus values at the first and the last MR examinations were: 1.06±0.04 (SD) and 1.09±0.05. Mean dentate nucleus:pons values at the first and last MR examinations were: 0.95 ± 0.06 and 1.02±0.06. No significant differences in baseline MR globus pallidus:thalamus and dentate nucleus:pons between patients and controls were observed (respectively, P = 0.16 and P = 0.15) and mean signal intensity ratios in the control group were 1.07 ± 0.05 for the globus pallidus:thalamus and 0.96±0.05 for the dentate nucleus:pons (Fig. 3).
The mean globus pallidus:thalamus (0.0042±0.0084) and dentate nucleus:pons (0.020±0.048) slopes were significantly higher than zero with P<0.001 and P<0.02, respectively.
At visual inspection, no significant hyperintense signal on unenhanced T1-weighted images was observed in the patient group after serial administrations of gadoterate meglumine.
Discussion
Our study showed increased globus pallidus:thalamus and dentate nucleus:pons on unenhanced T1-weighted images in children treated with more than six administrations of the macrocyclic GBCA gadoterate meglumine, and we demonstrated a significant effect of the number of GBCA injections on signal intensity ratios. Both the globus pallidus:thalamus and dentate nucleus:pons were not influenced by age at first administration, radiotherapy and chemotherapy.
So far, only a few studies have been conducted in children exposed to multiple administrations of the ionic linear GBCA gadopentetate dimeglumine [8,9,10]. In these studies, authors have demonstrated a significant increase in the dentate nucleus:pons or globus pallidus:thalamus in the last MR examinations compared to the first ones. Even though lacking in histological confirmation, these findings support the hypothesis of gadolinium deposition in specific brain regions after multiple administrations of linear GBCA and replicate previous findings in adults [5, 7, 14, 15]. Conversely, serial administrations of macrocyclic GBCA in adults have not been associated with a significant increase in signal intensity ratios on unenhanced T1-weighted MR images in the above-mentioned brain regions compared to linear GBCAs of the previous studies [5,6,7, 14]. These results corroborated the hypothesis that, due to their high kinetic and thermodynamic stability, macrocyclic GBCAs are probably less prone to dechelation and transmetallation, which may ultimately contribute to gadolinium deposition in tissues [16,17,18]. The only exception to these findings was the study of Stojanov et al. [19] who observed significant differences in both the dentate nucleus:pons and globus pallidus:thalamus in patients affected by relapsing remittent multiple sclerosis (MS) after serial administrations of the macrocyclic GBCA gadobutrol. However, Radbruch et al. [6] did not confirm these results and several criticisms have been raised about Stojanov’s study, especially concerning potentially confounding factors such as possible patient exposure to linear GBCAs and the absence of a control group without MS [6, 20].
Our results are not in line with the majority of findings in adults. There are several possible explanations for these differences: compared to adult studies, we retrospectively selected a population with a higher mean number of GBCA administrations (mean: 10) and we excluded all those patients with known previous exposure to other GBCAs. Possible differences in the physiological T1 signal of gray matter structures between the pediatric and adult brain have also to be considered. During the first months of life, gray matter signal intensity increases over time on T1-weighted MR images because of the physiological myelination process, which is considered completed in the second year of life [12]. To avoid this possible confounding factor, in our study we did not included patients younger than 2 years old. The average age of our patient population was 8 years. Moreover, in the control group, we did not observe any evident T1 signal intensity increase related to the age range (2–17 years; Fig. 3). The possible residual effect of age on signal intensity in the patient group was considered in the statistical model and no significant effect was found. Therefore, we assume that age didn’t affect our findings.
More recently, another study was conducted in children exposed to multiple administrations of the macrocyclic GBCA gadoterate meglumine [21]. The authors did not find any significant signal intensity increase in the population after multiple administrations, thus confirming previous findings in the majority of adult studies. However, that study has several methodological differences from our study. First, the mean number of administrations is higher in our series (mean: 10 doses ± 2.8). Second, since part of the patient cohort was exposed to different GBCAs prior to the actual starting point (study baseline) of the study, the real number of patients with exclusive exposure to gadoterate meglumine and without hyperintensities on T1-weighted images was lower (n=16) than the present study. Moreover, the absence of an age-matched control population never exposed to GBCA administration does not allow testing for individual baseline signal intensity differences. Lastly, in our series, we excluded all the disorders that could potentially be associated with an increase in T1 signal intensity such as Langerhans cell histiocytosis [22].
Even though we never observed a clearly visible hyperintense signal on precontrast T1-weighted images, our study suggests a relationship between the increase of signal intensity in both the dentate nucleus and globus pallidus on unenhanced T1-weighted images and the number of previous GBCA injections. A possible reason for this discordance between qualitative and quantitative evaluation might be the possibility of a deposition of other macromolecules related to the GBCA administrations instead of free gadolinium in the tissue. The increase in T1 signal intensity becomes detectable at visual inspection because of the effective relaxivity of the chemical form of gadolinium that accumulates in the brain. We hypothesized that high doses of macrocyclic GBCA in children might lead to a small deposit of macromolecules in different forms compared to those that deposit after linear GBCA administrations.
The process by which gadolinium is deposited in the brain is not known so far. The ICP-MS analysis used in the majority of autopsy brain studies does not allow the characterization of its chemical form in the tissue [23]. Therefore, we may speculate that only a consistent number of macrocyclic GBCA administrations might favor the accumulation of substances that have still not been characterized in the pediatric brain tissue as shown by the small but significant signal increase seen in our study. So far, only a few pathological-proven studies demonstrating gadolinium deposition in cerebral tissues have been published [24,25,26]. Among those, only Murata et al. [26] demonstrated the presence of gadolinium retention in different brain regions of patients previously exposed to macrocyclic GBCA. However, the authors reported very low concentrations compared to those found in patients exposed to linear agents and to gadolinium concentrations of the bone tissue [26]. Therefore, if on one hand these findings support the hypothesis that gadolinium retention in the brain after macrocyclic GBCA administration is possible, on the other hand, they only provide limited evidences in consideration of the small population included and do not define the molecular speciation of the gadolinium deposited. Recently, in an ex vivo animal study, Frenzel et al. [27] reported the presence of low gadolinium concentrations in the brain of rats exposed to multiple administrations of macrocyclic GBCA mainly consisting of soluble fractions that has been hypothesized to represent the intact GBCA molecule.
The possible deposition of the intact molecule in macrocyclic GBCA compared to other forms of deposition in linear GBCA may account for the discrepancy between quantitative and qualitative results obtained. In fact, this difference between macrocyclic and linear GBCA deposition may justify the discrepancy in their signal intensity on T1-weighted MR images after multiple administrations: The chemical form after macrocyclic GBCA exposures might influence T1 relaxivity (r1) thus leading to visibly undetectable effect on the images, as we showed [28].
Our study has several limitations. First of all, this is a small single-centre retrospective study, which included only oncological patients. Generalization of results is therefore limited for the presence of possible confounding factors such as radiation therapy, chemotherapy and different treatment protocols. However, in the statistical analysis, these clinical variables have been included as fixed factors and did not show any significant effect on signal intensity ratios over time. Moreover, Adin et al. [29] did not show any effect of radiotherapy on signal intensity ratios increased over time. Secondly, similarly to the majority of previous studies on this topic, we used a ROI-based method, which may be affected by limitations due to the fact that ROI placing can be quite subjective and not reproducible.
Moreover, we did not include a population exposed to multiple linear GBCA administrations since gadoterate meglumine is the only GBCA used for MR examinations during the last 5 years at our institution. Finally, our results lack postmortem analysis demonstrating gadolinium retention in neural tissues. So far, contrary to adult studies, gadolinium deposition has never been histologically ascertained in the pediatric brain and further studies are necessary to fill this gap.
To overcome these limitations and to favor possible comparison between different studies, it would be necessary to conduct prospective longitudinal multicentre studies, possibly using automatic or semiautomatic methods for regional measurements or T1-mapping techniques and considering the entire anatomical structures of interest.
Conclusion
This study shows that dentate nucleus and globus pallidus signal intensity increases in children exposed to multiple administrations of macrocyclic GBCA on unenhanced T1-weighted images suggesting possible accumulation of GBCA-related molecules. The clinical relevance of the results provided in this field in both adult and pediatric populations remains unclear and further studies are needed to expand the knowledge of possible effects of prolonged gadolinium exposure.
Change history
18 August 2017
An erratum to this article has been published.
References
Ramalho J, Semelka RC, Ramalho M et al (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37:1192–1198
Beomonte Zobel B, Quattrocchi CC, Errante Y et al (2015) Gadolinium-based contrast agents: did we miss something in the last 25 years? Radiol Med 121:478–841
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Radbruch A, Weberling LD, Kieslich PJ et al (2015) High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Investig Radiol 50:805–810
Weberling LD, Kieslich PJ, Kickingereder P et al (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol 50:743–748
Flood TF, Stence NV, Maloney JA et al (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282:222–228
Hu HH, Pokorney A, Towbin RB et al (2016) Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46:1590–1598
Roberts DR, Chatterjee AR, Yazdani M et al (2016) Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol 37:2340–2347
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Branson HM (2004) Normal myelination: a practical pictorial review. Neuroimaging Clin N Am 23:183–195
Schwartz GJ, Muñoz A, Schneider MF et al (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Ramalho J, Castillo M, AlObaidy M et al (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276:836–844
Frenzel T, Lengsfeld P, Schirmer H et al (2008) Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Investig Radiol 43:817–828
Aime S, Caravan P (2009) Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30:1259–1267
Gibby WA, Gibby KA, Gibby WA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investig Radiol 39:138–142
Stojanov DA, Aracki-Trenkic A, Vojinovic S et al (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast age. Eur Radiol 26:807–815
Agris J, Pietsch H, Balzer T (2016) What evidence is there that gadobutrol causes increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W MRI in patients with RRMS? Eur Radiol 26:816–817
Radbruch A, Bickelhaupt S, Paech D et al (2017) Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology 8:162980
Martin-Duverneuil N, Idbaih A, Hoang-Xuan K et al (2006) MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol 16:2074–2082
Kanal E, Tweedle MF (2015) Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 275:630–634
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 0:150025
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non–group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue. Investig Radiol 51:447–453
Frenzel T, Apte C, Jost G et al (2017) Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents comparative study in rats. Investig Radiol. doi:10.1097/RLI.0000000000000352
Hagberg GE, Scheffler K (2013) Effect of r1 and r2 relaxivity of gadolinium-based contrast agents on the T1-weighted MR signal at increasing magnetic field strengths. Contrast Media Mol Imaging 8:456–465
Adin ME, Kleinberg L, Vaidya D et al (2015) Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 36:1859–1865
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
An erratum to this article is available at https://doi.org/10.1007/s00247-017-3950-6.
Rights and permissions
About this article
Cite this article
Rossi Espagnet, M.C., Bernardi, B., Pasquini, L. et al. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol 47, 1345–1352 (2017). https://doi.org/10.1007/s00247-017-3874-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3874-1