Abstract
Background
Interim 18F-FDG PET helps predict outcome and tailor treatment in adults with Hodgkin disease (HD).
Objective
The purpose of this study was to assess predictive values of interim 18F-FDG PET/CT in children with HD and to define the potential added value to interim PET of low-dose CT.
Materials and methods
Children were prospectively enrolled August 2002–April 2007. PET/low-dose CT was performed at staging, after 2 cycles, at the end of treatment and during follow-up (mean 45 months). Treatment was unchanged regardless of interim results. PET and low-dose CT were read independently.
Results
Of 34 enrolled children (ages 3–17 years), 27 achieved complete response, 4 had progressive disease and 3 had relapse. Interim PET alone had positive and negative predictive values of 67% and 89%, respectively. Interim low-dose CT alone had positive and negative predictive values of 35% and 100%, respectively. Interim PET/CT had positive and negative predictive values of 75% and 96%, respectively.
Conclusions
Early interim PET/CT was a good predictor of outcome. Integrated PET and low-dose CT improved the predictive value in children with HD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
18F-FDG imaging has been used for management of patients with lymphoma for more than two decades with PET/CT being at present the main diagnostic tool for staging and monitoring treatment response. The criteria defining treatment response in lymphoma, recently revised by Cheson et al. [1], have resulted in new guidelines that take into consideration the results of both the PET and CT components of the hybrid imaging study [2].
Up to 80% of patients with Hodgkin disease (HD) treated with chemoradiation will be cured. However, a significant number will suffer from treatment-related morbidity and mortality. The occurrence, incidence and severity of long-term sequalae of chemoradiation are of particular significance in children with HD. Only a small fraction of the tumor mass in HD is composed of malignant cells with the rest being reactive infiltrative cells. Since antineoplastic therapy affects mainly the tumor cells, shrinkage of the mass demonstrated by CT is delayed as compared to the metabolic changes measured by the FDG-PET component.
Interim 18F-FDG imaging aiming at prediction of outcome and tailoring individual treatment regimens demonstrates early response to therapy in adults with HD [3–10]. 18F-FDG PET/CT performed after one to three treatment courses has a high positive and negative predictive value (PPV, NPV) in predicting outcome and can be therefore potentially used for treatment tailoring in the individual patient. These results have been recently reproduced in a pediatric population with HD confirming the high NPV of early negative 18F-FDG PET/CT studies [11]. An additional main goal of interim 18F-FDG PET/CT studies is to define non-responders in whom inefficient treatment should be discontinued early and second-line, more aggressive therapy needs to be instituted as soon as possible. Previous studies assessing the value of 18F-FDG studies in children with malignancies have reported a relatively high incidence of foci of increased tracer uptake unrelated to cancer that may account for a higher rate of false-positive studies and subsequently lower PPV [12].
The aims of present study were to assess the PPV and NPV of early interim 18F-FDG PET/CT performed after the second cycle of treatment in children with HD and to define whether the low-dose CT (LDCT) component may have an incremental role as a refinement tool to 18F-FDG PET resulting in better performance indices in order to predict early progressive disease or recurrence.
Materials and methods
Children diagnosed with HD in a single institution between August 2002 and April 2007 were prospectively enrolled in the study group. There were a total of 34 patients, ages 3.5–18 years (mean 13 years). Patient characteristics are detailed in Table 1.
18F-FDG PET/CT was performed at presentation, prior to institution of treatment, after the second treatment cycle, at the end of treatment and periodically every 6 months for a follow-up period of up to 5 years. The baseline study was performed within a few days of the diagnosis, and the interim 18F-FDG PET/CT study just prior to the third cycle of treatment, at an interval of at least 14 days from the previous administration of chemotherapy. The study was approved by the Institutional Ethics Committee and the legal guardian of the child gave a written informed consent for performing the study and for retrospective evaluation of their files.
Treatment regimen
All of the children received chemotherapy based on their staging and risk stratification: low risk were treated by protocols AHOD0431 and EURONET-PHL-C1, intermediate risk according to protocols AHOD0031 and ABVD, and advanced disease by protocols AHOD0831 and ESC.BEACOPP [13–17]. Involved field radiotherapy (IFRT) was further added in children with bulky disease. Second-line protocols were tailored.
18F-FDG PET/CT acquisition and processing
The children were instructed to fast, except for glucose-free oral hydration, for 4 h before the injection of a weight-adjusted dose of 5.3 MBq/kg up to a total dose of 296–444 MBq (8–12 mCi) of 18F-FDG. Blood glucose levels were measured before injection. Oral contrast was administered to the patients during the uptake time. No intravenous contrast material was injected. For children younger than 5 years, sedation was given by an anesthesiologist. All of the children underwent head to mid-thigh scans. Lower-limb scanning was added when clinically indicated. PET and CT images were acquired consecutively 90 min after the injection of 18F-FDG, using a PET/CT system (Discovery LS; GE Healthcare, Waukesha, MI, USA) combining a third-generation multislice spiral CT scanner with a dedicated full-ring PET scanner with bismuth germanate crystals, which are mechanically aligned and share a common table. CT data were used for low-noise attenuation correction of PET emission data and for fusion with attenuation-corrected PET images. The parameters for the localization CT component of the PET/CT study were set according to manufacturer’s recommendations including “Smart mA” with mA varying between minimum 50 mA and maximum 100 mA with noise level 20, 100–120 kV, 0.7 s/rotation, pitch 1.375:1. PET, CT, and fused PET/CT images were available for review and were displayed in axial, coronal and sagittal planes.
18F-FDG PET/CT image interpretation and analysis
Interpretation of the PET by nuclear medicine physicians using the CT part for anatomical localization was done blinded to the interpretation done by radiologists. The PET and LDCT components were evaluated on a site-based analysis for all involved lymph nodes and extra nodal lesions.
Analysis of the PET component was performed using the CT component for anatomical localization of foci of increased FDG activity, such as to exclude sites of physiological or benign uptake (e.g., bowel, brown fat, etc.). The interim PET study was assessed for the presence or absence of abnormal FDG uptake in sites of known disease detected on the baseline study. Studies with no areas of abnormal FDG foci were defined as indicating a complete response (CR). Studies with foci of abnormal FDG uptake were analyzed and compared with findings on the baseline study including assessment of intensity as compared to physiological FDG activity in liver and mediastinum, size of FDG abnormality and number of residual foci of abnormal FDG uptake as compared to baseline. Positive interim studies were defined as partial response (PR) in the presence of a decrease in the intensity or size of the lesion showing tracer uptake, or in the number of 18F-FDG avid sites, or as progressive disease (PD) in studies showing an increase in the intensity and size of the abnormal FDG uptake, or the presence of additional new FDG-avid sites.
As the study aims to evaluate the predictive value of interim studies, the end point for evaluation of the child is the last follow-up study. Complete remission was defined as the disappearance of all disease manifestations at the end of therapy. Primary progressive disease was defined as persistence of disease or progression during treatment. Relapse was defined as the appearance of new clinical or imaging findings, further proven by biopsy to be foci of disease, in children who had remained in complete remission for more than 3 months after the completion of therapy. For all components of the study, a true-positive interim study was defined as a study with evidence of disease at interim imaging followed by progressive disease; a true-negative interim study was a study with no evidence of disease and complete remission up until the end of follow-up; a false-positive interim study was a study with evidence of disease in a child who achieved and remained in complete remission, and a false-negative interim study was a study without pathological findings, showing evidence of disease later on up to the end of follow-up. The positive predictive value (PPV) was defined as the number of truly positive patients divided by the number of positive interim studies (for PET, CT and PET/CT results). The negative predictive value (NPV) was calculated as the number of truly negative patients divided by the number of patients with negative interim studies (for PET, CT and PET/CT results).
Low-dose CT images were interpreted in accordance with the standardized international working group criteria for assessment of response, and classified as complete response (CR), complete response unconfirmed CR (CRu), partial response (PR), radiologic stable disease (radiologic SD), and progressive disease (PD) [18]. The LDCT component was defined as positive in the presence of PR, radiologic SD or PD, and negative when concluded as CR and CRu.
A PET/CT study was defined as positive in the presence of a positive PET (both PR and PD) and/or a LDCT study defined as SD. The SD limb of the positive PET/CT study was added based on the rationale that a non-shrinking mass on CT is a strong sign of non responsiveness and was thus read as PET/CT+ regardless of the PET stand-alone reading. The hybrid PET/CT was positive in all PET+ and in all LDCTs with SD. PET/CT was negative in PET− studies except for SD on CT.
Statistical analysis
Performance indices of PET, CT stand-alone and PET/CT studies were compared using the Fisher exact test, an adaptation of the chi square test for small patient numbers.
Results
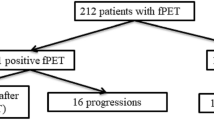
The 34 children included in the present study population were followed up for a mean of 58 months (range 32–94 months). Of the 34 children, 27 achieved complete response, 4 showed progressive disease during treatment and 3 patients had recurrent disease (RD) at 7, 12 and 14 months after completion of chemotherapy (Table 2).
Interim FDG PET results
The PET component was negative in 28 children including 25 true negative studies in patients who achieved complete response and three false-negative studies in children who developed recurrent disease. The PET component was positive in six children including four true-positive studies in children who showed progressive disease and two FP studies in patients who had no further evidence of disease. Of the positive interim studies, visual analysis indicated the presence of a single site of abnormal FDG uptake in all six children. In five of these children, intensity of uptake decreased as compared to the baseline study although it was still above the intensity of activity in both the liver and mediastinum. The size of the abnormal FDG uptake also decreased significantly in all five children and in three of them additional sites of disease became negative. These studies were defined as partial response. In one additional child, the intensity and size of the lesion of abnormal uptake increased between the baseline and interim PET study and it was defined as progressive disease. Performance indices of the PET component were defined including both the visual and quantitative results since they did not differ and categorized the same children as partial response or progressive disease. FDG PET had a PPV of 67% and a NPV of 89%. Both FP studies became negative at 27 and 35 months after completion of treatment respectively, and the two children remain in continuous complete remission (CCR) after a follow-up period of 52 and 48 months, respectively.
Interim CT results
The LDCT component after two cycles of chemotherapy was negative in 14 children, all of whom achieved complete response and were defined as true-negative. The LDCT component was positive in 20 children (16 with partial response and 4 with stable disease) including seven true-positives (3 with partial response and 4 with stable disease) with children showing further evidence of active HD (4 had progressive disease and 3 relapsed) and 13 false-positives (all with partial response) in children who had no further evidence of active disease. Interim LDCT had a PPV of 35% and NPV of 100%. Of the 13 false-positive studies, three became negative after the fourth cycle of treatment, five at the end of chemotherapy, and five during follow-up at 6–13 months after completion of treatment.
Interim PET/CT results
FDG PET/CT after two cycles of treatment was negative in children, including 26 true-negative studies in children who achieved complete response and one false-negative study in a child who developed recurrent disease. Interim PET/CT was positive in eight children, including six true-positive studies in children with further evidence of active disease (four with progressive disease, two with recurrent disease) and two false-positive studies in children who showed no further evidence of disease. Interim FDG PET/CT had a PPV of 75% and NPV of 96% (Table 2). Both false-positive studies became negative during follow-up at 27 and 35 months after completion of treatment, and the children remain in continuous complete remission after a clinical follow-up period of 52 and 48 months, respectively. There was no statistically significant difference between the PPV and NPV of PET, CT and PET/CT.
Discussion
It has been previously demonstrated that FDG imaging performed early during treatment has a high prognostic value in adults with HD. Most patients, further defined as early responders, will become FDG-negative after two to three cycles of standard chemotherapy. The few retrospective studies performed in children with HD, most of them evaluating FDG imaging at the end of treatment, report a NPV of 100%, associated with a poor and variable PPV ranging between 11% and 75% [19, 20]. Only two previous studies have assessed interim PET/CT in children with HD. A retrospective study of 31 children indicated that mid-treatment FDG PET had a PPV of 100% and NPV of 96%. The surprisingly high PPV could be related to the small number of only three positive interim PET studies as well as to a relatively short follow-up period [21]. On the other hand, an additional recent prospective study reported that interim FDG PET/CT had a very low PPV of 14% but excellent NPV of 100% in a pediatric patient population with HD, in which 38 of 40 children (95%) reached complete remission [11].
As the majority of children with HD will be cured and have a long life expectancy, treatment-related morbidity is highly significant in this population as compared to that in adults. The first aim of this study was to assess the prognostic value of FDG PET/CT in children with HD. Present results indicate that when both components of the study are analyzed, interim FDG PET/CT had a good PPV of 75% and high NPV of 96%. These results are similar to those reported in adults with HD, including a PPV ranging between 44% and 85% and NPV of 87–97% [6, 7, 12, 22, 23]. Furthermore, present results underscore the long-term predictive value of PET/CT as the children were followed for a period of up to 7.8 years using the late outcome rather than early complete response as the end point.
Whether these results justify to safely shorten or modify current treatment protocols based on results of interim FDG PET/CT is still debatable, both in adults and more so in children with HD. As for now, there are no guidelines regarding therapeutic modulation based on interim PET/CT results. More data in large multicenter studies are needed.
In the approach taken in the present study, the LDCT component was not only used as an anatomical guide for the FDG PET part of the study but was also interpreted as an independent examination by a pediatric radiologist. We followed the ALARA and Image Gently concepts that even LDCT without intravenous contrast should be read, as it was already acquired as part of the PET/CT. In the LDCT component, most children (81% of cases) showing either some shrinkage of the mass after two treatment cycles (consistent with PR 47%) or disappearance of the mass (consistent with complete response 41%) achieved complete response. The group of 20% of children with SD who showed no change in the size of the mass on CT had a different, poor outcome, with subsequent recurrent disease or PD in all children (Fig. 1), unrelated to the fact that the interim FDG PET component was negative in 2/6 children in this group. Adding the results of the LDCT component to the adjusted criteria for a positive and negative PET/CT study shifted all children with anatomical stable disease into the PET/CT+ group, including those with a PET− study. As a result, the PPV and NPV of PET/CT were better than PET or CT stand-alone. This information would have been lost if the LDCT component would have been used as an anatomical guide for the PET part only.
FDG PET/CT in a 19-year-old young woman with stage IIA Hodgkin disease. a Staging study: the blue arrows indicate a supradiaphragmatic lymph node on the left, with FDG uptake shown on the FDG and fused images. The yellow arrowhead indicates physiological uptakes in liver and renal pelvis. b Interim study: the blue arrow indicates a supradiaphragmatic lymph node on the left, without FDG uptake shown on the FDG and fused images. The yellow arrowhead indicates physiological uptakes in liver. c Follow-up study: the blue arrow indicates the same supradiaphragmatic lymph node on the left, with new FDG uptake shown on the FDG and fused images
Mac Manus et al. [22] have previously suggested that PET/CT studies performed at the end of therapy for HD structural imaging data may add useful information to the metabolic component, stating that at this point during the course of disease the tumor mass has had enough time to shrink [22]. In the present study, dynamic changes such as the presence or absence of tumor shrinkage on CT early during treatment proved to be a clinically significant add-on criterion for defining response to treatment and patient outcome in children with HD. In addition, the presence of stable disease on CT with or without the presence of a FDG-positive metabolically active residual tumor on the interim PET/CT study facilitates reclassification of children with HD into early good responders. Present data suggest that by including the data from the LDCT component, the predictive value of PET/CT for early prediction of outcome increases in the pediatric population with HD.
The strength of this study is the homogeneity of the study population (all HD pediatric patients), the use of the same experienced reader team in all cases and the long follow-up period. The heterogeneity with respect to staging and risk stratification reflects the variety of children referred for treatment.
The limitations of the present study are the moderate-sized study population with only four cases of stable disease on CT, a number too small to reach statistical significance. We share this weakness with most of the other studies, which may be overcome by a future meta-analysis.
Another limitation is the lack of pathology as a golden standard, though it is impossible to biopsy all residual masses in clinical practice. The relative long follow-up overcomes this limitation.
At present, interim FDG PET/CT in children with HD is used as a tool for changes in treatment protocols only in several ongoing trials of early FDG PET/CT response-adapted therapies [24]. As these studies will reach final results, provided that the preliminary good performance of FDG PET/CT in predicting response in children with HD will be kept, a shift from a theoretical prognostic value of interim PET/CT to clinical recommendations may be expected.
Conclusion
Early interim PET/CT showed good positive predictive value (PPV) and high negative predictive value (NPV) for patient outcome. Present data suggest that including data from the LDCT component could increase the predictive value of PET/CT for early prediction of outcome in children with Hodgkin disease (HD).
References
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol 25:571–578
Hoekstra OS, Ossenkoppele GJ, Golding R et al (1993) Early treatment response in malignant lymphoma, as determined by planar fluorine-18-fluorodeoxyglucose scintigraphy. J Nucl Med 34:1706–1710
Kostakoglu L, Coleman M, Leonard JP et al (2002) PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J Nucl Med 43:1018–1027
Torizuka T, Nakamura F, Kanno T et al (2004) Early therapy monitoring with FDG-PET in aggressive non-Hodgkin’s lymphoma and Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging 31:22–28
Hutchings M, Mikhaeel NG, Fields PA et al (2005) Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 16:1160–1168
Hutchings M, Loft A, Hansen M et al (2006) FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 107:52–59
Zinzani PL, Tani M, Fanti S et al (2006) Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin’s disease patients. Ann Oncol 17:1296–1300
Gallamini A, Rigacci L, Merli F et al (2006) The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 91:475–481
Gallamini A, Hutchings M, Rigacci L et al (2007) Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian–Danish study. J Clin Oncol 25:3746–3752
Furth C, Steffen IG, Amthauer H et al (2009) Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol 27:4385–4391
Bar-Sever Z, Keidar Z, Ben-Barak A et al (2007) The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging 34:630–637
Keller FG, Castellino SM, Nachman JB (2009) What is the best treatment for children with limited-stage Hodgkin lymphoma? Curr Hematol Malig Rep 4:129–135
Mauz-Körholz C, Hasenclever D, Dörffel W et al (2010) Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol 28:3680–3686
Borchmann P, Diehl V, Engert A (2011) ABVD versus BEACOPP for Hodgkin’s lymphoma. N Engl J Med 365:1545–1546
Schwartz CL, Constine LS, Villaluna D et al (2009) A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high risk Hodgkin lymphoma: the results of P9425. Blood 114:2051–2059
Diehl V, Franklin J, Pfreundschuh M et al (2003) Standard and increased dose BEACOPP chemotherapy compared with COPP–ABVD for advanced Hodgkin’s disease. N Engl J Med 348:2386–2395
Cheson BD, Pfistner B, Juweid ME (1999). Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphoma. NCI Sponsored International Workshop Group. J Clin Oncol 1244–1253
Meany HJ, Gidvani VK, Minniti CP (2007) Utility of PET scans to predict disease relapse in pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer 48:399–402
Levine JM, Weiner M, Kelly KM (2006) Routine use of PET scans after completion of therapy in pediatric Hodgkin disease results in a high false positive rate. J Pediatr Hematol Oncol 28:711–714
Miller E, Metser U, Avrahami G et al (2006) Role of 18F-FDG PET/CT in staging and follow-up of lymphoma in pediatric and young adult patients. J Comput Assist Tomogr 30:689–694
MacManus MP, Seymour JF, Hicks RJ (2007) Overview of early response assessment in lymphoma with FDG-PET. Cancer Imaging 7:10–18
Haioun C, Itti E, Rahmouni A et al (2005) FDG-PET in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 106:1376–1381
Hutchings M, Barrington SF (2009) PET/CT for therapy response assessment in Lymphoma. J Nucl Med 50:21s–30s
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilivitzki, A., Radan, L., Ben-Arush, M. et al. Early interim FDG PET/CT prediction of treatment response and prognosis in pediatric Hodgkin disease—added value of low-dose CT. Pediatr Radiol 43, 86–92 (2013). https://doi.org/10.1007/s00247-012-2517-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2517-9