Abstract
Hodgkin lymphoma (HL), a disease of mostly young patients, also peaks in the elderly. Despite the profound improvement in the outcome of young patients, in the elderly, 5-year progression-free survival (PFS) rates are under 70%. Interim PET-CT (iPET) is known to be highly predictive for PFS in young HL patients, but it has not been sufficiently validated in the elderly patient population. In this multi-center collaboration, all consecutive elderly patients (age ≥ 60) diagnosed with HL between 1998 and 2016 were retrospectively reviewed. Baseline characteristics, outcome measures, and iPET results, classified according to the Deauville score, were recorded and analyzed. We identified 78 elderly HL patients (median age 69) who underwent iPET. ABVD was the treatment regimen in 52 (67%) patients. Eighty-three percent of patients had iPET scores of 1–3 while 17% had scores of 4–5. Patients with iPET scores of 1–3 had 5-year PFS and OS rates of 72% and 82% compared with 25% and 45%, respectively, in patients with scores of 4–5 (p < 0.001). Our findings show that iPET is highly predictive of outcome in elderly HL patients and provide evidence that iPET-guided therapy in this patient population may be key to achieving superior treatment outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hodgkin lymphoma (HL) is most commonly diagnosed in young adults, but has a bimodal age distribution curve, with up to a quarter of cases presenting in patients aged 60 and above [1,2,3]. The profound improvement in the clinical outcome of young HL patients in the last decades, with long-term failure-free survival rates in excess of 80% [4,5,6,7], has only partially extended to elderly patients aged ≥ 60 [8,9,10,11,12,13,14,15,16,17,18].
Positron emission tomography-computed tomography (PET-CT) with 18F-fluorodeoxyglucose (FDG) has gained widespread use and endorsement in the treatment of HL [19,20,21]. Response-adapted therapy, tailored to the results of interim PET-CT (iPET) that is usually performed after 2 chemotherapy cycles, has emerged as a tool for guiding therapeutic decisions in HL. This tool has been examined extensively in cohorts of young HL patients, where it was shown to be both a predictor of long-term outcome as well as an effective guide for tailoring treatment intensity in the individual patient [22,23,24,25,26,27,28,29]. Yet, the role of iPET has not been validated in cohorts of elderly HL patients, a population in which the potential use of this tool to predict clinical outcome is markedly prominent. Elderly patients with HL have been underrepresented in large prospective trials, with less than 10% of the patients in the German Hodgkin Study Group (GHSG) trials HD5–HD11 [30, 31] being older than 60 years; hence, data regarding the prognostic role of iPET in these patients are scant. Traditional prognostic markers in HL have been reported mostly in young patients and even the value of the commonly used International Prognostic Score (IPS) is questionable in elderly patients [18, 32]. Moreover, there is still an ongoing debate regarding the risk-benefit ratio of the various chemotherapy protocols in elderly patients with HL [14, 31, 33,34,35,36,37,38,39] as elderly patients suffer from increased rates of treatment-related toxicity [8, 9, 12, 13, 30, 40] and treatment is often administered with only partial dose intensity [10, 41]. Adoption of an iPET-driven therapeutic approach, similar to that employed in young HL patients, may improve outcome in elderly patients. For example, earlier cessation of an ineffective treatment has the potential to reduce treatment-related toxicity and perhaps improve tolerability to subsequent therapy. The objective of the present study was to evaluate the significance of iPET in an elderly HL patient population.
Patients and methods
Study design and patient population
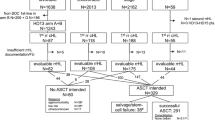
Data from all consecutive newly diagnosed classical HL patients aged 60 and above who were diagnosed and treated in five participating medical centers (Sourasky—Tel Aviv, Rambam, Hadassah—Jerusalem, Assaf Harofe, Ziv) between the years 1998 and 2016 were retrospectively reviewed. Biopsies were classified according to the WHO criteria [42, 43]. The study was approved by the local Institutional Review Boards. The inclusion criteria for this study were patients who had undergone iPET, defined as PET-CT completed after 2–3 treatment cycles and before completion of the planned treatment protocol. According to local treatment protocols in the participating sites, iPET was scheduled after 2 cycles of chemotherapy, 10–14 days after last chemotherapy administration.
Data collection—definitions
Baseline characteristics, treatment protocol, and outcome measures were recorded. PET-CT results at diagnosis, interim analysis (iPET), and end of treatment (EOT) were recorded and analyzed. Response to treatment was evaluated by iPET and EOT-PET-CT scans using the 5-point scale Deauville score (DS), according to the level of residual FDG uptake at involved sites [19, 21]. iPET DSs of 1–3 were considered negative, similar to studies that examined iPET in young HL patients [26, 27]. In cases which were treated before the incorporation of the DS into common practice, the imaging was reviewed locally, and the DS was used to evaluate response. Staging and response to treatment were assessed according to the 2014 Lugano classification [21]. Prognostic groups for early-stage and advanced-stage disease were defined according to the German Hodgkin Study Group (GHSG) [44, 45].
Statistical analysis
Statistical analysis was carried out with SPSS software (Chicago, IL). Demographics and baseline characteristics were summarized using descriptive statistics. t tests were used to compare means of normally distributed variables in two groups. Proportions across categories were compared using chi-squared tests. Progression-free survival (PFS) was defined as the time from diagnosis of HL to death or disease relapse/progression, including less than complete remission (CR) at the end of the treatment protocol. Overall survival (OS) was defined as the time from diagnosis of HL to death or last follow-up. The Kaplan-Meier method was used to assess survival patterns; two or more groups were compared using the log-rank test. Univariable associations between clinical/laboratory variables and outcome were derived using the Cox proportional hazards model. Variables with a p value < 0.05 in univariable analyses were entered into the multivariable Cox proportional hazards model in a stepwise fashion.
Results
Baseline characteristics
Ninety-five patients aged 60 and above, who were diagnosed with classical HL, were identified. Of these, 78 (82%) had undergone iPET and were included in the study. Patient characteristics are listed in Table 1. Forty (51%) patients were males and 38 (49%) were females. The median age was 68.7 (range 60–89) years. Forty-two percent of the patients had a clinically significant heart or lung disease.
Staging at diagnosis was performed by PET-CT in 68 (91%) patients and by CT in 7 (9%) patients. Twenty-two (28%) patients presented with early-stage disease (11 favorable, 11 unfavorable) and 56 (72%) patients presented with advanced-stage disease. Only one patient in this study group had a bulky mediastinal mass. The mean IPS for patients with advanced-stage disease was 3.4 ± 1.4.
Histological subtype was nodular sclerosis in 34 (44%) patients, mixed cellularity in 20 (26%) patients, lymphocyte rich in 2 (3%), and not specified in 22 (28%) patients.
Treatment
ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) was the most commonly used treatment regimen and was administered to 52 (67%) patients, baseline BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) was administered to 10 (13%) patients, and AVD (doxorubicin, vinblastine, dacarbazine) to 7 (9%) patients. Eight patients were treated with various combination chemotherapies such as COPP (cyclophosphamide, vincristine, procarbazine, prednisone) or MOPP (mustargen, vincristine, procarbazine, prednisone), administered alone or as part of ABVD hybrids (n = 6) and variations of BEACOPP protocols (n = 2). A single patient who declined chemotherapy and presented with impaired performance status was treated with brentuximab vedotin monotherapy. In 10 (13%) patients, chemotherapy was followed by involved field radiotherapy, 9 of which presented with an early-stage disease.
Response to treatment and outcomes
Analysis of iPET scan results showed a DS of 1 in 49 (63%) patients, a score of 2 in 7 (9%) patients, a score of 3 in 9 (12%) patients, a score of 4 in 8 (10%) patients, and a score of 5 in 5 (6%) patients. Two patients died during administration of the initial treatment protocol (an 87-year-old female with stage IV disease, IPS 4, who achieved an iPET score of 3 and completed 5 treatment cycles of ABVD and a 76-year-old female with stage III disease, IPS 3, who achieved an iPET score of 1 and completed 3 treatment cycles of ABVD; both succumbed to infectious complications). Of 76 evaluable patients, 73 completed EOT-PET: 62 (86%) patients achieved CR, 3 (4%) patients achieved partial response (PR), and 8 (11%) patients had evidence of progressive disease (PD); none had stable disease (SD) according to the Lugano classification [21].
With a median follow-up of 5 years (range 2 months–15 years), 10 out of 62 patients with EOT-CR, experienced relapse. Five-year PFS and OS rates for the entire group were 65% and 75%, respectively.
When evaluated according to prognostic groups, the 5-year PFS rates were 91%, 70%, and 57% for early favorable, early unfavorable, and advanced-stage disease, respectively, and the 5-year OS rates were 90%, 91%, and 69% for early favorable, early unfavorable, and advanced disease, respectively (Fig. 1). There were no statistically significant differences in outcome according to prognostic groups. However, patients’ outcome varied significantly according to the iPET DS, with patients having low scores faring better than those with higher scores. Patients with iPET scores of 1–3 had 5-year PFS and OS rates of 72% and 82% compared with 25% and 45% in patients with iPET scores of 4–5, respectively, p < 0.0001 (Fig. 1). The negative predictive value of iPET (scores 1–3) was 76% and the positive predictive value of iPET (scores 4–5) was 75%.
Patient outcomes (the Kaplan-Meyer curves). a Progression-free survival by prognostic group, early favorable (n = 11) vs. early unfavorable (n = 11) vs advanced disease (n = 53), p = 0.181, not significant. b Overall survival by prognostic group, early favorable (n = 11) vs. early unfavorable (n = 11) vs advanced disease (n = 56), p = 0.089, not significant. c Progression-free survival by interim PET-CT, DS 1–3 (n = 63) vs. DS 4–5 (n = 12), p < 0.0001. d Overall survival by interim PET-CT, DS 1–3 (n = 65) vs. DS 4–5 (n = 13), p < 0.0001. DS the Deauville score
Outcome of patients with advanced disease, treated with ABVD
Thirty-six patients with advanced HL were treated with ABVD. The mean IPS was 3 ± 1.4 (range 1–6).
Patients with advanced-stage HL that were treated with ABVD had 5-year PFS and OS rates of 58% and 80%, respectively. Twenty-six (72%) patients had an iPET score of 1, 1 (3%) patient had a score of 2, 2 (6%) patients had a score of 3, 4 (11%) patients had a score of 4, and 3 (8%) patients had a score of 5.
Of the 36 patients with advanced-stage disease that were treated with ABVD, 2 succumbed to infectious complications prior to completion of the treatment regimen. Two of the patients with an iPET score of 5 were switched to platinum-based protocols after 2 cycles of ABVD. The third patient with an iPET score of 5 and all 4 patients with an iPET score of 4 completed 6 cycles of ABVD (Table 2). Of the remaining 27 patients (25 with an iPET score of 1, 1 with a score of 2, and 1 with a score of 3), 23 completed 6 cycles of ABVD, and the remaining 4 received 4–5.5 treatment cycles.
Twenty-seven (82%) patients achieved CR at EOT, 2 (6%) achieved PR, 4 (12%) had evidence of PD, and none had SD. Of the 27 patients with EOT-CR, 5 relapsed within 11–28 months: a single relapse within less than a year (iPET DS of 4) and four later relapses with DSs of 1–3.
Among patients with advanced-stage disease that were treated with ABVD, patients who had low iPET DSs (1–3) fared better than those with higher scores. Five-year PFS and OS rates were 69% and 87% for patients with iPET scores 1–3 vs. 14% and 57% for patients with iPET scores 4–5, respectively (p < 0.0001 for PFS and p = 0.035 for OS, Fig. 2).
Outcome of patients with advanced-stage HL, treated with baseline BEACOPP
Ten patients, all diagnosed with advanced-stage HL, were treated with baseline BEACOPP. The mean IPS was 4.3 ± 1.3 (range 3–6). The average number of treatment cycles was 5.2 ± 1.6. None of these patients had bulky mediastinal disease and none received radiation therapy. These patients had 5-year PFS and OS rates of 65% and 75%, respectively. Four of the 10 patients had an iPET score of 1, 3 patients had a score of 2, and 3 patients had a score of 3. At EOT, nine patients achieved CR and a single patient had PR. None of the patients died during the treatment. Of the 9 patients with EOT-CR, 2 experienced relapse, within 12 and 22 months (both with iPET DSs of 1). The association between iPET score and outcome was not statistically significant, probably due to the small group size.
Outcome of ABVD-treated patients with early-stage HL
Sixteen patients with early-stage disease, 7 without unfavorable risk factors and 9 with unfavorable risk factors, were treated with ABVD. Six (38%) patients were treated with combined modality, having an additional radiotherapy, after two to four courses of ABVD.
Nine patients had an iPET score of 1, 3 patients had a score of 2, 2 patients had a score of 3, 1 patient had a score of 4, and 1 patient had a score of 5. At EOT, 14 patients achieved CR while 2 patients, one with an iPET score of 5 and one with an iPET score of 3, had PD at EOT. Of the 14 patients who achieved CR, only one patient relapsed. This was a patient with unfavorable risk who had an iPET score of 1 and relapsed 1.5 years after completing six courses of ABVD.
Early-stage patients treated with ABVD with low iPET scores fared better than patients with higher scores, with 5-year OS rates of 100% for scores 1–3 vs. 50% for score 4–5 (p < 0.01) (Fig. 3).
Univariable and multivariable analysis
Univariable analysis of common prognostic factors in HL [18, 31, 32, 36, 46] was performed in this population of elderly patients and results are summarized in Table 3. In univariable analysis, iPET was predictive of outcome (PFS and OS) for the entire study group, in advanced-stage patients and ABVD-treated patients. IPS was not predictive for PFS or OS in patients with advanced-stage disease. Stage and prognostic group were not predictive of outcome.
In multivariable regression analysis, iPET maintained its prognostic value for the entire cohort. For example, compared with patients with DSs of 1–3, patients with scores of 4–5 had a HR of 8.5 (95% CI 1.8–40.3, p = 0.007) for progression and 6.9 (95% CI 1.2–40.8, p = 0.031) for death. None of the variables maintained their prognostic significance in multivariable analysis of patient sub-populations.
Discussion
The prognosis of elderly patients with HL is inferior to that of their younger counterparts. The causes for poor outcome in older patients with HL are likely multifactorial, including different disease biology, the presence of comorbidities, lower performance status, and limited organ reserve, frequently resulting in the employment of less intensive regimens [17, 18]. Previous attempts to improve the outcome of elderly patients with HL were directed at better categorizing these patients according to prognostic markers and the employment of distinct treatment regimens in this patient group [14, 31, 33,34,35,36,37,38,39]. Yet, no strong and reproducible predictive prognostic factors have been identified in this age group and the optimal treatment protocol is still a matter of debate [17, 18]. PET-CT has gained a wide spread endorsement in the treatment of lymphoproliferative disease. The value of PET-CT has been demonstrated in initial disease staging, assessment of response to treatment and prediction of long-term outcome in HL, perhaps more than in any other lymphoproliferative disease [22,23,24,25,26,27,28,29]. Nonetheless, there is a paucity of data regarding its value in the treatment of HL in the elderly, despite the unmet clinical need in the growing population of elderly patients.
This cooperative retrospective, real-life study included HL patients aged 60 to 89. Almost a quarter of the patients in our cohort were older than 75 years. Patient and disease characteristics were comparable with previous reports of elderly HL patients, including significant comorbidities typical of this age group, an increased frequency of the mixed cellularity subtype and B symptoms, and a decreased incidence of bulky mediastinal disease [18, 30].
iPET scores were predictive of outcome in this cohort and specifically in the sub-group of ABVD-treated patients. Thirty-six patients in this trial had advanced-stage HL and were treated with ABVD. This group represents a common challenge for physicians, as treating elderly patients with 6 cycles of this regimen is often hampered by adverse events, preventing its completion. We were able to show that patients with advanced-stage disease, treated with ABVD, who had iPET scores of 1–3, fared better compared with patients with higher iPET scores. IPS was not predictive of PFS or OS in patients with advanced-stage disease. In this regard, iPET results have been previously shown to overshadow the value of traditional prognostic factors, such as IPS [24].
An early detection of insufficient response, strongly associated with a worse outcome, may serve as a platform for adopting a PET-CT-driven therapeutic approach. This treatment algorithm, though not explored yet in elderly HL patients, is highly relevant nowadays, considering the new therapeutic approaches available, including brentuximab vedotin that can substitute bleomycin [47]. Studies exploring this PET-CT-guided strategy are warranted, aiming to improve the outcome of elderly HL patients. PET-CT-guided strategy may save unnecessary toxicity of non-effective therapy and promote earlier induction of second line regimens, considering these elderly patients’ ineligibility for high dose therapy and autograft.
Treatment with baseline BEACOPP was chosen by the physicians for 10 patients, representing almost 20% of advanced disease patients in this cohort. All patients achieved DS ≤ 3 and none died during treatment. This protocol may serve as a feasible option in selected elderly patients with HL, especially with a carful iPET-guided response-adapted therapy plan.
This trial has several limitations. Relative dose intensity [35] and complete geriatric assessment [18], previously suggested as prognostic tools in elderly HL patients, were not available to us; rather, performance status was used. The DS, now commonly used to direct response-adapted therapy in patients with HL [19, 21, 26], was not used in all patients in the original interpretation of their iPET, as 29 patients were diagnosed before the original publication in 2010 [19]. At the time when part of our patients were treated, visual assessment was considered adequate for determining PET scan results, and the use of standardized uptake values (SUV) was not deemed necessary in the response criteria published in 2007 [48]. Accordingly, of the 9 patients who had iPET scores of 4 and above, treatment was upgraded to platinum-based salvage protocols in only 2 patients whereas the other 7, who all had residual uptake in iPET with no new lesions, continued the original treatment regimen (Table 2). Thus, iPET results did not serve to guide further treatment, in most cases, in this cohort. The adherence to the original treatment protocol, regardless of iPET scores, could reflect the spirit of that era, an uncertainty regarding optimal treatment approaches for HL evident by the early termination of treatment arms in several prospective trials such as HD13 and H10 which evaluated drug omission [49] and response-adapted therapy [50].
In summary, there is an unmet clinical need for defining the optimal therapeutic regimen in elderly patients with HL. To the best of our knowledge, this study is the first to specifically address the role of iPET in the management of elderly patients with HL. Patients with low iPET DS fared better than patients with evidence of disease on iPET and had improved 5-year PFS and OS. Those elderly patients with a positive iPET had worse prognosis and should be considered for additional treatment or incorporation of novel agents into their treatment plan. iPET was predictive of outcome and surpassed the predictive value of traditional prognostic factors such as prognostic group and IPS. Safe incorporation of iPET into the management of elderly patients with HL may allow for necessary chemotherapy dose reductions and perhaps even for very careful but crucial treatment escalations. Proper utilization of this means is an integral part in the transition from pre-treatment analysis–based therapy to response-adapted therapy for elderly patients with HL.
References
Halbsguth TV, Böll B, Borchmann P, Diehl V (2011) The unique characteristics and management of patients over 60 years of age with classic Hodgkin lymphoma. Curr Hematol Malig Rep 6:164–171. https://doi.org/10.1007/s11899-011-0089-7
Proctor SJ, Wilkinson J, Sieniawski M (2009) Hodgkin lymphoma in the elderly: a clinical review of treatment and outcome, past, present and future. Crit Rev Oncol Hematol 71:222–232. https://doi.org/10.1016/j.critrevonc.2008.12.007
Ries LA, Kosary CL, Hankey BF (1997) SEER cancer statistics review, 1973-1994, National Cancer Institute. NIH Pub. No. 97-2789. . Bethesda
Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, Shenkier TN, Slack GW, Skinnider B, Gascoyne RD, Connors JM, Sehn LH (2012) International prognostic score in advanced-stage Hodgkin’s lymphoma: altered utility in the modern era. J Clin Oncol 30:3383–3388. https://doi.org/10.1200/JCO.2011.41.0910
Klimm B, Goergen H, Fuchs M, von Tresckow B, Böll B, Meissner J, Glunz A, Diehl V, Eich HT, Engert A, Borchmann P (2013) Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: an analysis of international staging definitions. Ann Oncol 24:3070–3076. https://doi.org/10.1093/annonc/mdt413
von Tresckow B, Plütschow A, Fuchs M, Klimm B, Markova J, Lohri A, Kral Z, Greil R, Topp MS, Meissner J, Zijlstra JM, Soekler M, Stein H, Eich HT, Mueller RP, Diehl V, Borchmann P, Engert A (2012) Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 30:907–913. https://doi.org/10.1200/JCO.2011.38.5807
Engert A, Diehl V, Franklin J, Lohri A, Dörken B, Ludwig WD, Koch P, Hänel M, Pfreundschuh M, Wilhelm M, Trümper L, Aulitzky WE, Bentz M, Rummel M, Sezer O, Müller-Hermelink HK, Hasenclever D, Löffler M (2009) Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol 27:4548–4554. https://doi.org/10.1200/JCO.2008.19.8820
Austin-Seymour MM (1984) Hodgkin’s disease in patients over sixty years old. Ann Intern Med 100:13. https://doi.org/10.7326/0003-4819-100-1-13
Peterson BA, Pajak TF, Cooper MR, Nissen NI, Glidewell OJ, Holland JF, Bloomfield CD, Gottlieb AJ (1982) Effect of age on therapeutic response and survival in advanced Hodgkin’s disease. Cancer Treat Rep 66:889–898
Bosi A, Ponticelli P, Casini C, Messori A, Bellesi G, Biti G, Cionini L, Longo G, Mungai V, Rossi Ferrini P (1989) Clinical data and therapeutic approach in elderly patients with Hodgkin’s disease. Haematologica 74:463–473
Erdkamp FL, Breed WP, Bosch LJ et al (1992) Hodgkin disease in the elderly. A registry-based analysis. In: Cancer
Enblad G, Glimelius B, Sundström C (1991) Original article: treatment outcome in Hodgkin’s disease in patients above the age of 60: a population-based study. Ann Oncol 2:297–302. https://doi.org/10.1093/oxfordjournals.annonc.a057939
Levis A, Depaoli L, Urgesi A, Bertini M, Orsucci L, Vitolo U, Buchi G, Gallamini A, Gavarotti P, Novarino A, Rota Scalabrini D, Mazza U, Pileri A, Sannazzari GL, Resegotti L (1994) Probability of cure in elderly Hodgkin’s disease patients. Haematologica 79:46–54
Weekes CD, Vose JM, Lynch JC, Weisenburger DD, Bierman PJ, Greiner T, Bociek G, Enke C, Bast M, Chan WC, Armitage JO, Nebraska Lymphoma Study Group (2002) Hodgkin’s disease in the elderly: improved treatment outcome with a doxorubicin-containing regimen. J Clin Oncol 20:1087–1093. https://doi.org/10.1200/JCO.2002.20.4.1087
Macpherson N, Klasa RJ, Gascoyne R, O’Reilly SE, Voss N, Connors JM (2002) Treatment of elderly Hodgkin’s lymphoma patients with a novel 5-drug regimen (ODBEP): a phase II study. Leuk Lymphoma 43:1395–1402. https://doi.org/10.1080/10428190290033332
Kim HK, Silver B, Li S, Neuberg D, Mauch P (2003) Hodgkin’s disease in elderly patients (> or =60): clinical outcome and treatment strategies. Int J Radiat Oncol Biol Phys 56:556–560
Evens AM, Hong F (2013) How can outcomes be improved for older patients with Hodgkin lymphoma? J Clin Oncol 31:1502–1505. https://doi.org/10.1200/JCO.2012.47.3058
Evens AM, Helenowski I, Ramsdale E, Nabhan C, Karmali R, Hanson B, Parsons B, Smith S, Larsen A, McKoy JM, Jovanovic B, Gregory S, Gordon LI, Smith SM (2012) A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood 119:692–695. https://doi.org/10.1182/blood-2011-09-378414
Barrington SF, Qian W, Somer EJ, Franceschetto A, Bagni B, Brun E, Almquist H, Loft A, Højgaard L, Federico M, Gallamini A, Smith P, Johnson P, Radford J, O’Doherty MJ (2010) Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37:1824–1833. https://doi.org/10.1007/s00259-010-1490-5
Follows GA, Ardeshna KM, Barrington SF, Culligan DJ, Hoskin PJ, Linch D, Sadullah S, Williams MV, Wimperis JZ, The British Committee for Standards in Haematology (2014) Guidelines for the first line management of classical Hodgkin lymphoma. Br J Haematol 166:34–49. https://doi.org/10.1111/bjh.12878
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance, Australasian Leukaemia and Lymphoma Group, Eastern Cooperative Oncology Group, European Mantle Cell Lymphoma Consortium, Italian Lymphoma Foundation, European Organisation for Research, Treatment of Cancer/Dutch Hemato-Oncology Group, Grupo Español de Médula Ósea, German High-Grade Lymphoma Study Group, German Hodgkin’s Study Group, Japanese Lymphorra Study Group, Lymphoma Study Association, NCIC Clinical Trials Group, Nordic Lymphoma Study Group, Southwest Oncology Group, United Kingdom National Cancer Research Institute (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068. https://doi.org/10.1200/JCO.2013.54.8800
Raemaekers JMM, André MPE, Federico M, Girinsky T, Oumedaly R, Brusamolino E, Brice P, Fermé C, van der Maazen R, Gotti M, Bouabdallah R, Sebban CJ, Lievens Y, Re A, Stamatoullas A, Morschhauser F, Lugtenburg PJ, Abruzzese E, Olivier P, Casasnovas RO, van Imhoff G, Raveloarivahy T, Bellei M, van der Borght T, Bardet S, Versari A, Hutchings M, Meignan M, Fortpied C (2014) Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32:1188–1194. https://doi.org/10.1200/JCO.2013.51.9298
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, Wimperis J, Culligan D, Popova B, Smith P, McMillan A, Brownell A, Kruger A, Lister A, Hoskin P, O’Doherty M, Barrington S (2015) Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med 372:1598–1607. https://doi.org/10.1056/NEJMoa1408648
Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, di Raimondo F, D’Amore F, Biggi A, Vitolo U, Stelitano C, Sancetta R, Trentin L, Luminari S, Iannitto E, Viviani S, Pierri I, Levis A (2007) Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 25:3746–3752. https://doi.org/10.1200/JCO.2007.11.6525
Advani R, Maeda L, Lavori P, Quon A, Hoppe R, Breslin S, Rosenberg SA, Horning SJ (2007) Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol 25:3902–3907. https://doi.org/10.1200/JCO.2007.11.9867
Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, d’Amore F, Enblad G, Franceschetto A, Fulham M, Luminari S, O’Doherty M, Patrick P, Roberts T, Sidra G, Stevens L, Smith P, Trotman J, Viney Z, Radford J, Barrington S (2016) Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374:2419–2429. https://doi.org/10.1056/NEJMoa1510093
Press OW, Li H, Schöder H, Straus DJ, Moskowitz CH, LeBlanc M, Rimsza LM, Bartlett NL, Evens AM, Mittra ES, LaCasce AS, Sweetenham JW, Barr PM, Fanale MA, Knopp MV, Noy A, Hsi ED, Cook JR, Lechowicz MJ, Gascoyne RD, Leonard JP, Kahl BS, Cheson BD, Fisher RI, Friedberg JW (2016) US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim Fluorodeoxyglucose-positron emission tomography imaging: southwest oncology group S0816. J Clin Oncol 34:2020–2027. https://doi.org/10.1200/JCO.2015.63.1119
Dann EJ, Bar-Shalom R, Tamir A, Haim N, Ben-Shachar M, Avivi I, Zuckerman T, Kirschbaum M, Goor O, Libster D, Rowe JM, Epelbaum R (2007) Risk-adapted BEACOPP regimen can reduce the cumulative dose of chemotherapy for standard and high-risk Hodgkin lymphoma with no impairment of outcome. Blood 109:905–909. https://doi.org/10.1182/blood-2006-04-019901
Dann EJ, Bairey O, Bar-Shalom R, Mashiach T, Barzilai E, Kornberg A, Akria L, Tadmor T, Filanovsky K, Abadi U, Kagna O, Ruchlemer R, Abdah-Bortnyak R, Goldschmidt N, Epelbaum R, Horowitz NA, Lavie D, Ben-Yehuda D, Shpilberg O, Paltiel O (2017) Modification of initial therapy in early and advanced Hodgkin lymphoma, based on interim PET/CT is beneficial: a prospective multicentre trial of 355 patients. Br J Haematol 178:709–718. https://doi.org/10.1111/bjh.14734
Engert A, Ballova V, Haverkamp H, Pfistner B, Josting A, Dühmke E, Müller-Hermelink K, Diehl V (2005) Hodgkin’s lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin’s Study Group. J Clin Oncol 23:5052–5060. https://doi.org/10.1200/JCO.2005.11.080
Böll B, Görgen H, Fuchs M, Pluetschow A, Eich HT, Bargetzi MJ, Weidmann E, Junghanß C, Greil R, Scherpe A, Schmalz O, Eichenauer DA, von Tresckow B, Rothe A, Diehl V, Engert A, Borchmann P (2013) ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol 31:1522–1529. https://doi.org/10.1200/JCO.2012.45.4181
Hasenclever D, Diehl V (1998) A prognostic score for advanced Hodgkin’s disease. International prognostic factors project on advanced Hodgkin’s disease. N Engl J Med 339:1506–1514. https://doi.org/10.1056/NEJM199811193392104
Evens AM, Hong F, Gordon LI, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Gospodarowicz M, Cheson BD, Stiff PJ, Advani R, Miller TP, Hoppe RT, Kahl BS, Horning SJ (2013) The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol 161:76–86. https://doi.org/10.1111/bjh.12222
Azambuja E, Fleck JF, Batista RG, Menna Barreto SS (2005) Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther 18:363–366. https://doi.org/10.1016/j.pupt.2005.01.007
Landgren O, Algernon C, Axdorph U, Nilsson B, Wedelin C, Porwit-MacDonald A, Grimfors G, Björkholm M (2003) Hodgkin’s lymphoma in the elderly with special reference to type and intensity of chemotherapy in relation to prognosis. Haematologica 88:438–444
Ballova V, Rüffer JU, Haverkamp H, Pfistner B, Müller-Hermelink HK, Dühmke E, Worst P, Wilhelmy M, Naumann R, Hentrich M, Eich HT, Josting A, Löffler M, Diehl V, Engert A (2005) A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly). Ann Oncol 16:124–131. https://doi.org/10.1093/annonc/mdi023
Böll B, Goergen H, Behringer K et al (2016) Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood 127:2189–2192. https://doi.org/10.1182/blood-2015-11-681064
Levis A, Anselmo AP, Ambrosetti A, Adamo F, Bertini M, Cavalieri E, Gavarotti P, Genua A, Liberati M, Pavone V, Pietrasanta D, Ricetti MM, Scalabrini DR, Salvi F, Vitolo U, Angelucci E, Boccadoro M, Gallo E, Mandelli F, Intergruppo Italiano Linfomi (IIL) (2004) VEPEMB in elderly Hodgkin’s lymphoma patients. Results from an Intergruppo Italiano Linfomi (IIL) study. Ann Oncol 15:123–128
Proctor SJ, Wilkinson J, Jones G, Watson GC, Lucraft HH, Mainou-Fowler T, Culligan D, Galloway MJ, Wood KM, McNally RJQ, James PW, Goodlad JR (2012) Evaluation of treatment outcome in 175 patients with Hodgkin lymphoma aged 60 years or over: the SHIELD study. Blood 119:6005–6015. https://doi.org/10.1182/blood-2011-12-396556
Levis A, Depaoli L, Bertini M et al (1996) Results of a low aggressivity chemotherapy regimen (CVP/CEB) in elderly Hodgkin’s disease patients. Haematologica 81:450–456
Diaz-Pavon JR, Cabanillas F, Majlis A, Hagemeister FB (1995) Outcome of Hodgkin’s disease in elderly patients. Hematol Oncol 13:19–27
Swerdlow S, Campo E, Harris N (2008) WHO classification of hematopoietic and lymphoidtissues. In: IARC Press. Lyon, France
Jaffe ES, Harris NL, Stein H (2001) World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. In: IARC Press. Lyon, France
Eich HT, Diehl V, Görgen H, Pabst T, Markova J, Debus J, Ho A, Dörken B, Rank A, Grosu AL, Wiegel T, Karstens JH, Greil R, Willich N, Schmidberger H, Döhner H, Borchmann P, Müller-Hermelink HK, Müller RP, Engert A (2010) Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 28:4199–4206. https://doi.org/10.1200/JCO.2010.29.8018
Eichenauer DA, Plütschow A, Kreissl S, Sökler M, Hellmuth JC, Meissner J, Mathas S, Topp MS, Behringer K, Klapper W, Kuhnert G, Dietlein M, Kobe C, Fuchs M, Diehl V, Engert A, Borchmann P (2017) Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin study group. Lancet Oncol 18:1680–1687. https://doi.org/10.1016/S1470-2045(17)30696-4
Wedelin C, Björkholm M, Biberfeld P, Holm G, Johansson B, Mellstedt H (1984) Prognostic factors in Hodgkin’s disease with special reference to age. Cancer 53:1202–1208
Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illés Á, Picardi M, Lech-Maranda E, Oki Y, Feldman T, Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R, Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu R, Jolin HA, Huebner D, Radford J, ECHELON-1 Study Group (2018) Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med 378:331–344. https://doi.org/10.1056/NEJMoa1708984
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586. https://doi.org/10.1200/JCO.2006.09.2403
Behringer K, Goergen H, Hitz F, Zijlstra JM, Greil R, Markova J, Sasse S, Fuchs M, Topp MS, Soekler M, Mathas S, Meissner J, Wilhelm M, Koch P, Lindemann HW, Schalk E, Semrau R, Kriz J, Vieler T, Bentz M, Lange E, Mahlberg R, Hassler A, Vogelhuber M, Hahn D, Mezger J, Krause SW, Skoetz N, Böll B, von Tresckow B, Diehl V, Hallek M, Borchmann P, Stein H, Eich H, Engert A (2015) Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet 385:1418–1427. https://doi.org/10.1016/S0140-6736(14)61469-0
André MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, Casasnovas O, Brice P, van der Maazen R, Re A, Edeline V, Fermé C, van Imhoff G, Merli F, Bouabdallah R, Sebban C, Specht L, Stamatoullas A, Delarue R, Fiaccadori V, Bellei M, Raveloarivahy T, Versari A, Hutchings M, Meignan M, Raemaekers J (2017) Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 35:1786–1794. https://doi.org/10.1200/JCO.2016.68.6394
Author information
Authors and Affiliations
Contributions
OSB and EP analyzed the data and performed the statistical analysis; IA and CP designed the research study; OSB wrote the paper; all authors managed the patients and collected the data, reviewed the manuscript, and provided input.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bentur, O.S., Dann, E.J., Paran, E. et al. Interim PET-CT–guided therapy in elderly patients with Hodgkin lymphoma—a retrospective national multi-center study. Ann Hematol 98, 1665–1674 (2019). https://doi.org/10.1007/s00277-019-03686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03686-y