Abstract
Up to 25% of patients with classical Hodgkin lymphoma (cHL) and a negative interim PET/CT will progress. Unfortunately, there are few published studies on the predictive value of PET/CT performed after finishing treatment. The objective of our study was to assess the role of the final PET/CT (fPET/CT) in predicting progression in a retrospective series of patients treated in the last 10 years with a homogeneous protocol (ABVD + / − radiotherapy). We reviewed a cohort of 227 patients with newly diagnosed cHL. fPET/CT was performed on 212 patients (93%). In patients with a positive fPET, progression-free survival at 60 months was 17% (94% if fPET was negative, p = 0.000). The positive and negative predictive values for the fPET were 76% and 94%, respectively (Fisher’s exact test, p = 0.000). In the subgroup of patients with advanced-stage cHL, progression-free survival at 60 months was 91% with negative fPET and 0% with positive fPET (p = 0.000). However, fPET was negative in 19 of the 29 patients with a positive interim PET/CT (only 2 showed progression). In conclusion, fPET is a useful tool to predict treatment failure in patients with newly diagnosed cHL, especially advanced-stage disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classical Hodgkin’s lymphoma (cHL) affects preferentially young people and first-line treatment achieves the cure of 75–80% of cases [1, 2]. Such therapeutic effectiveness, along with the aggressiveness of treatment and the early age of most patients, leads to the main cause of death being not the lymphoma itself, but the late toxicity of the treatment, which usually appears after the fifth year of follow-up, mostly second neoplasms (specially breast cancer, lung cancer, and leukemia) and major cardiovascular events (such as ischemic heart disease, cerebrovascular accident, and congestive heart failure) [3, 4].
Recent studies have shown that the intensity of treatment could be reduced in most patients, maintaining the effectiveness and, therefore, decreasing the toxicity, as shown the GATLA LH-05, RAPID, and HD16 trials. These studies have shown that an important group of patients (85–90%), regardless of stage, remain without progression at 3–5 years if after 2–3 cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) a complete metabolic remission (final PET) is obtained and no further treatment is administered [5,6,7]. In this context, tools that allow for the identification of patients with a better prognosis are needed so that they can receive the minimum required treatment. Furthermore, as the treatment does not succeed in 10–15% of early-stage cases and in 20–30% of advanced-stage cases, it is important to identify those patients with a high risk of progression to intensify their treatment early, because only 50% can be rescued with second or further line treatments [1, 2].
Since 2007, PET/CT (fluorodeoxyglucose positron emission tomography/computed tomography) has become the tool of choice for the assessment of the response in cHL. Interim PET/CT (iPET, PET performed after the first 2 cycles of chemotherapy) has been accepted as a tool for increasing or decreasing the intensity of the treatment, depending on the response obtained [8]. It offers a good negative predictive value (NPV) in the assessment of the response of cHL, allowing for a decrease of the intensity of the treatment and maintaining a high cure rate [9, 10]. However, the positive predictive value (PPV) of iPET is far from being of clinical utility, due to the fact that it is not sufficiently high (around 40–50% after treatment with ABVD) [11,12,13,14,15], with relapse rates of 15–60% [10, 13,14,15,16].
Roughly 50% of patients with a positive iPET will remain progression-free, and 20–25% of patients with a negative iPET will progress [17]. Due to such a rate of false positives and to more intensive regimens being linked to a higher cure rate but also to higher acute and long-term toxicity, there is need for a more reliable predictive tool for progression. Although some researchers have stated that final PET (fPET) may not be necessary in the event of a negative iPET result, several studies contradict this. Among them, Hindie et al. proved a lower sensitivity of iPET compared to fPET. Consequently, fPET should not be omitted even in cases of a negative iPET [18,19,20]. Similar results have been obtained with the new combination of nivolumab and AVD, where a positive iPET does not match the fPET results regarding progression rate and progression-free survival (PFS) [21].
The objective of our study was to assess the role of the fPET results as a predictive factor for progression in patients with cHL treated with a prospectively planned, uniform protocol, based on ABVD chemotherapy in real-life treated patients.
Materials and methods
This is a retrospective study in a cohort of 227 patients with newly diagnosed cHL in Málaga, Spain, from January 2008 to June 2018. All patients were treated with ABVD chemotherapy + / − radiotherapy (Table 1).
Patients were assigned to one of the following prognostic groups (according to the German Hodgkin Study Group criteria): early stage (favourable early stage), intermediate stage (unfavourable early stage), and advanced stage [22]. The International Prognostic Score (IPS) reported by Hasenclever and colleagues was used for the prognostic stratification of advanced stages [23].
In all cases, it was recommended to use PET/CT for staging and final assessment of the response to the treatment (fPET). The implementation of PET/CT after 2 cycles of ABVD (iPET) was optional. PET/CT examinations were performed on a General Electric Discovery STE4 hybrid PET/CT scanner, approximately 60 min after administration of FDG (4.75 MBq × kg of weight), and extended from the base of the skull to the proximal femurs. Low-dose CT (70–80 mA, 140 kVp) was used for attenuation correction and anatomical location. The acquisition time by BED was 2–5 min; 128 × 128 matrix. Data reconstruction was done with the iterative reconstruction algorithm (VUE Point HD, GE Healthcare) implemented in the scanner software, with attenuation correction and measurement of the variables to be studied done on an iMac with OsiriX 5.8.5 software. Patients were recommended to avoid intense exercise in the 24 h prior to the PET/CT study, minimum fasting of 6 h and abundant hydration (capillary blood glucose was < 200 mg/dL).
For assessment of the metabolic response with PET/CT, the five-point Deauville score (DS) was used; DS1: no uptake above the background; DS2: uptake equal or slightly increased compared to the mediastinum; DS3: uptake between mediastinum and liver; DS4: uptake moderately increased compared to the liver; DS5: uptake markedly increased compared to the liver or new lesions [17]. Cases with DS1–3 were considered as a complete metabolic response (negative PET), and cases with DS4–5 were considered as an incomplete metabolic response (positive PET). For the final assessment of the response, the 2007 Cheson criteria were used [24]; fPET was done 4–6 weeks after the last chemotherapy dose (8–12 weeks if radiotherapy was administered).
To assess the overall survival (OS) and PFS, the Kaplan–Meier method was used. All patients who progressed during or after the ABVD treatment, or those who did not obtain a complete metabolic response and required a second-line systemic treatment were considered to be in progression. The administration of limited radiotherapy on positive PET areas after ABVD was not considered progression. PFS was considered from the diagnosis of cHL up to the date of progression, exitus or last follow-up of the patient. OS was considered from diagnosis up to the date of death or last follow-up. The probability of relapse was assessed with Fisher’s exact test. A probability (p value) lower than 0.05 was considered statistically significant.
The Institutional Review Board (IRB) approved the conduct of this retrospective study and patient informed consent was waived based on the retrospective design.
Results
Two hundred and twenty-seven patients with cHL (128 males and 99 females) were assessed. Table 2 displays the clinicopathological characteristics of the global population, as well as the characteristics of the subgroups of patients who progressed and those who did not. The median age was 37 years (range 14–82 years) (42 years in the subgroup who experienced progression). Stages I–II were predominant (60%); however, in the subgroup with progression, stages III–IV prevailed (61%). The most frequent histological subtype in the 3 groups was nodular sclerosis, followed by mixed cellularity. As for other prognostic risk factors, we can highlight, in the global population, 26% extranodal involvement, 45% B symptoms, 22% bulky disease and 68% more than 2 affected node areas.
fPET was performed on 212 patients (93%). After a median follow-up time of 60 months, the PFS and OS rates were 86% and 94% for the global population, 94% and 100% for favourable early stages (n = 35), 90% and 98% for unfavourable early stages (n = 100), and 77% and 87% for advanced stages (n = 92).
Radiotherapy (RT) was administered to 76 patients (33%), with no significant differences in 5-year survival between the subgroup that received RT and the subgroup that did not (PFS: 90% with RT vs 84% without RT, p = 0.171; OS: 98% with RT vs 92% without RT, p = 0.443).
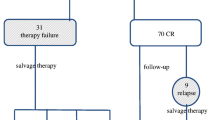
Figure 1 shows the evolution of the 212 patients who underwent fPET. Of the 21 patients with a positive fPET, 10 received localized consolidation RT (without receiving a later systemic treatment) and 5 of them showed no further progression. The rest of the patients with a positive fPET progressed (5 after receiving consolidation RT) and required second-line systemic treatment, with the exception of 1 case, due to a local relapse treated with IFRT.
Of the 16 patients who progressed after a positive fPET, 4 were in unfavourable early stages at the time of diagnosis, 3 of them treated with 4 cycles of ABVD plus RT and 1 case with 6 cycles of ABVD. The remaining 12 patients who progressed after a positive fPET were in advanced stages at the time of diagnosis (9 with IPS ≥ 3). All these progressions occurred during the first 2 years after diagnosis (median of 9.5 months, range 5–23 months).
Twelve patients with a negative fPET progressed after a median time of 12 months (range 6–67 months), 6 patients with early stages (5 unfavourable and 1 favourable), and 6 with advanced stages (4 with IPS ≤ 2).
iPET/CT was performed on 174 patients (77%) on day 25–28 of the second ABVD cycle. All 6 patients with a positive fPET after a negative iPET (145 negative of 174 patients with iPET) progressed and required second-line therapy. However, only 2 of 19 patients with a negative fPET after a positive iPET (29 positive of 174 patients with iPET) showed progression.
The rate of progression in patients with a positive fPET was very high, reaching 49% at 12 months and 83% at 24 months, with no progression observed after 2 years from diagnosis. The risk of progression was very low in patients with a negative fPET (PFS at 12 and 60 months was 98% and 94%, respectively; p = 0.000). Despite the high rate of progression of the disease in patients with a positive fPET, most of them could be rescued, 70% remaining alive after 60 months of follow-up. Figure 2 shows the Kaplan–Meier curves for PFS according to the fPET results for all stages. The sensitivity, specificity, PPV and NPV of the fPET were 57%, 97%, 76%, and 94%, respectively (Fisher’s exact test, p = 0.000).
In the subgroup of patients with advanced stages, highly relevant differences were observed between patients with positive fPET and negative fPET (PFS at 60 months: 0% with positive fPET vs 91% with negative fPET, p = 0.000; OS at 60 months: 65% with positive fPET vs 93% with negative fPET, p = 0.002). Figure 3 displays the Kaplan–Meier curves for PFS according to the response obtained on the fPET in the subgroup with advanced stage. The sensitivity, specificity, PPV and NPV in advanced-stage patients were 67%, 99%, 92%, and 92%, respectively (Fisher’s exact test, p = 0.000).
fPET was performed on 123 of 135 patients with early-stage disease (91%). In this subgroup, significant differences in survival according to the fPET were obtained as well. However, the differences were less impressive (PFS at 60 months: 41% with positive fPET vs 95% with negative fPET, p = 0.000; OS at 60 months: 80% with positive fPET vs 99% with negative fPET, p = 0.008).
Among patients with favourable early stages, no differences in progression according to the fPET results (n = 30) were observed, as only 1 patient progressed (with negative fPET). Nonetheless, in unfavourable early-stage patients, differences in survival were observed (PFS at 60 months: 95% with negative fPET vs 35% with positive fPET, p = 0.000; OS at 60 months: 99% with negative fPET vs 80% with positive fPET, p = 0.019). In unfavourable early-stage patients, the sensitivity, specificity, PPV, and NPV for fPET were 44%, 96%, 57%, and 94%, respectively (Fisher’s exact test, p = 0.001).
Discussion and conclusions
This study showed PFS and OS rates at 5 years comparable to the survival reported in recent clinical trials (94% and 100% for favourable early stage, 90% and 98% for unfavourable early stage, and 77% and 87% for advanced stages) [5, 25, 26]; and the results highlight the high predictive value of fPET to predict disease progression. Patients with a positive fPET showed a progression rate of 83% (all occurring in the first 2 years of follow-up), and only 6% experienced progressive disease after a negative fPET (PPV and NPV of 76% and 94%, respectively).
There are few published data on cHL about the role of fPET as a predictive factor for progression using the DS, as most studies focus on the role of iPET. A retrospective study of 128 HL patients with residual masses after the end of the treatment, using a tumour to background ratio > 3 to define PET positivity, reported a sensitivity, specificity, PPV, and NPV for fPET of 83%, 93%, 74%, and 93%, respectively [27]. Another retrospective study reported in 2006 also obtained high PPV and NPV (82% and 89% respectively), although only in 38 patients, without the use of the DS and without clearly defining the treatments administered [28]. In 2011, Barnes et al. concluded that iPET scans were not predictive of outcome, compared with scans carried out at completion of therapy. They studied a cohort of 96 patients with early-stage, non-bulky HL treated with ABVD + / − IFRT; no significant differences were found between the iPET results and PFS (87% vs 91%, p = 0.57), whereas it was found that fPET was highly predictive for survival, regardless of the iPET (PFS at 4 years: 94% with negative fPET vs 54% with positive fPET, p < 0.0001; OS at 4 years: 100% with negative fPET vs 84% with positive fPET, p < 0.0001) [29]. However, that study did not use the DS and performed most of the iPETs after the third cycle of ABVD. Finally, using DS, Mesguich et al. in a small retrospective study reported a sensitivity, specificity, PPV and NPV for iPET of 46.7%, 85.2%, 43.8%, and 86.7%, respectively. For fPET, the results were 80%, 93.4%, 75%, and 95%, respectively [20]. Our results confirm these data in a larger and more recent population of patients, including all stages and using the current DS, obtaining a higher PPV for fPET. We observed a recurrence rate in positive fPET patients of 100% for advanced-stage and 59% for early-stage patients.
The role of iPET to predict progression is more unclear, with very different results regarding PPV. The meta-analysis of Adams et al. studied the role of iPET in the assessment of response, including a total of 1389 patients from 10 studies, and reported a progression rate in patients with a positive iPET (true positive) from 0 to 86%; the reported false negative rate was 1.6–15.6% [25]. Furthermore, a recent retrospective study that included 246 early-stage patients treated with 4 cycles of ABVD plus IFRT showed that patients with a positive fPET presented a lower OS (69% vs 98%); in contrast, iPET predicted PFS but not OS [30]. These data, along with the results of our study, support the use of fPET, regardless of the iPET results. Although fPET cannot be used as a tool for the escalation or de-escalation of treatment, it could be used for design studies with a shorter treatment duration, with the objective of reducing acute and long-term toxicity in negative fPET patients without compromising PFS. In this scenario, new therapeutic strategies can be offered to positive-fPET patients, trying not to compromise Hodgkin lymphoma-specific survival.
The GATLA LH-05 trial, recruiting patients with cHL in all stages, revealed a 3-year PFS of 90% in those patients with a negative PET after only 3 cycles of ABVD (with no further RT or chemotherapy). A 3-year PFS of 65% was observed in patients with a positive PET after 3 cycles of ABVD (p < 0.0001), despite having received 3 more ABVD cycles and complementary RT [5]. The RAPID study reported a PFS and OS at 3 years of 90.8% and 99%, respectively, in those patients with early stages and a negative PET treated only with 3 cycles of ABVD [7]. Reducing the intensity of the treatment even more, the results of the HD16 study showed a PFS at 5 years of 86% in patients with favourable early stages and a negative PET after only 2 cycles of ABVD and no further treatment [6].
Our data support, in real life, the excellent predictive value of fPET in patients with advanced stages (PPV 92%, NPV 92%) and unfavourable early stages (PPV 57%, NPV 94%). However, it is not the case for favourable early stages (PPV 0%, NPV 97%), because only 1 patient with fPET and favourable early stage progressed (with negative fPET). Prospective clinical trials are needed to assess the real role of fPET as a tool for the development of treatment reduction strategies.
A strategy based on a short treatment with ABVD (2–4 cycles, depending on the prognostic group) followed by fPET would also allow us to plan an early escalation of the treatment for patients who will not achieve a complete metabolic remission on fPET. For that matter, some evidence shows that the use of the escalated BEACOPP scheme in patients with advanced-stage disease and a positive PET after 2 cycles of ABVD could improve the PFS of these patients [31,32,33].
The low PPV of iPET in all stages and the low PPV of fPET in early stages requires the search for new factors to replace or improve the capacity of PET to predict progression. At this point, the genotyping of circulating tumour DNA (ctDNA) could have a significant role. Spina et al. conducted a retrospective study to assess the mutational status of ctDNA in patients with cHL. A 100-fold or 2-log drop in ctDNA after 2 cycles of ABVD was confirmed as the best cut-off to predict progression and cure in this cohort. Quantification of ctDNA complemented the results of early PET/CT for the outline of the residual disease. Indeed, cured patients who were assessed as having a positive iPET experienced a greater than 2-log drop in ctDNA, whereas patients with a negative iPET and later relapse experienced a less than 2-log drop in ctDNA [34].
Among the main limitations of our study, we can highlight its retrospective nature (even though the treatment protocol was designed prospectively), and that the iPET was done in just over three-quarters of the patients (n = 174, 77%). Nevertheless, it is a large cohort of patients treated with a uniform protocol in real life with very relevant results for fPET as a predictive tool for progression. In conclusion, our study highlights the high PPV and NPV of fPET in real life, with a very high progression rate in the subgroup of patients with positive results, allowing for the selection of patients who require a closer follow-up (for at least 2 years) and those with a negative fPET who could have their follow-up reduced. New prospective studies and/or clinical trials should be designed to evaluate the role of fPET (associated or not with biological factors, such as ctDNA) to help identify patients who could receive shorter therapeutic regimens.
References
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41.
Donaldson SS, Hancock SL, Hoppe RT. The Janeway lecture. Hodgkin’s disease–finding the balance between cure and late effects. Cancer J Sci Am. 1999;5(6):325–33.
Canellos GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 2014;32(3):163–8.
Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399–408.
Pavlovsky A, Fernandez I, Kurgansky N, Prates V, Zoppegno L, Negri P, et al. PET-adapted therapy after three cycles of ABVD for all stages of Hodgkin lymphoma: results of the GATLA LH-05 trial. Br J Haematol. 2019;185(5):865–73.
Fuchs M, Goergen H, Kobe C, Kuhnert G, Lohri A, Greil R, et al. Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: final results of the International, Randomized Phase III HD16 Trial by the German Hodgkin Study Group. JCO. 2019;37:2835.
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598–607.
André MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786–94.
Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–9.
Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–94.
Fernández KS, Schwartz CL, Chen L, Constine LS, Chauvenet A, de Alarcón PA. Outcome of adolescents and young adults compared to children with Hodgkin lymphoma treated with response-based chemotherapy on pediatric protocols: a Children’s Oncology Group report. Pediatr Blood Cancer. 2017;64(12):e26681.
Henderson TO, Parsons SK, Wroblewski KE, Chen L, Hong F, Smith SM, et al. Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: An adult intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) comparative analysis: outcomes of AYAs With Hodgkin Lymphoma. Cancer. 2018;124(1):136–44.
de Wit M, Bohuslavizki KH, Buchert R, Bumann D, Clausen M, Hossfeld DK. 18FDG-PET following treatment as valid predictor for disease-free survival in Hodgkin’s lymphoma. Ann Oncol. 2001;12(1):29–37.
Spaepen K, Stroobants S, Dupont P, Thomas J, Vandenberghe P, Balzarini J, et al. Can positron emission tomography with [(18)F]-fluorodeoxyglucose after first-line treatment distinguish Hodgkin’s disease patients who need additional therapy from others in whom additional therapy would mean avoidable toxicity? Br J Haematol. 2001;115(2):272–8.
Weihrauch MR, Re D, Scheidhauer K, Ansén S, Dietlein M, Bischoff S, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood. 2001;98(10):2930–4.
Schaefer NG, Taverna C, Strobel K, Wastl C, Kurrer M, Hany TF. Hodgkin disease: diagnostic value of FDG PET/CT after first-line therapy—Is biopsy of FDG-avid lesions still needed? Radiology. 2007;244(1):257–62.
Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma. 2009;50(8):1257–60.
Hindié E, Mesguich C, Bouabdallah K, Milpied N. Advanced Hodgkin’s lymphoma: end-of-treatment FDG-PET should be maintained. Eur J Nucl Med Mol Imaging. 2017;44(8):1254–7.
Zinzani PL, Rigacci L, Stefoni V, Broccoli A, Puccini B, Castagnoli A, et al. Early interim 18F-FDG PET in Hodgkin’s lymphoma: evaluation on 304 patients. Eur J Nucl Med Mol Imaging. 2012;39(1):4–12.
Mesguich C, Cazeau A-L, Bouabdallah K, Soubeyran P, Guyot M, Milpied N, et al. Hodgkin lymphoma: a negative interim-PET cannot circumvent the need for end-of-treatment-PET evaluation. Br J Haematol. 2016;175(4):652–60.
Domingo-Domenech E, Ramchandren R, Rueda A, Trněný M, Feldman T, Lee HJ, et al. Nivolumab más doxorrubicina, vinblastina y dacarbazina para linfoma de Hodgkin clásico avanzado recién diagnosticado: seguimiento ampliado de dos años de la cohorte del estudio fase 2 CHECKMATE 205. Spanish Society Oncology Medical Congress 2019. P-Dest 51. 22–25 Oct.
Sieber M, Engert A, Diehl V. Treatment of Hodgkin’s disease: results and current concepts of the German Hodgkin’s Lymphoma Study Group. Ann Oncol. 2000;11(Suppl 1):81–5.
Hasenclever D, Diehl V, Armitage JO, Assouline D, Björkholm M, Brusamolino E, et al. A prognostic score for advanced Hodgkin’s disease. N Engl J Med. 1998;339(21):1506–14.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86.
Adams HJA, Nievelstein RAJ, Kwee TC. Prognostic value of interim FDG-PET in Hodgkin lymphoma: systematic review and meta-analysis. Br J Haematol. 2015;170(3):356–66.
Casasnovas R-O, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019;20(2):202–15.
Molnar Z, Simon Z, Borbenyi Z, Deak B, Galuska L, Keresztes K, et al. Prognostic value of FDG-PET in Hodgkin lymphoma for posttreatment evaluation. Long term follow-up results. Neoplasma. 2010;57(4):349–54.
Pracchia LF, Chaves AAR, Cerci JJ, Soares Junior J, Meneghetti JC, Buccheri V. Metabolic test with fluorine-18-fluorodeoxyglucose in staging and detection of residual tumor or recurrence in Hodgkin lymphoma. Clinics (Sao Paulo). 2007;62(2):121–6.
Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin’s lymphoma. Ann Oncol. 2011;22(4):910–5.
Rigacci L, Puccini B, Zinzani PL, Biggi A, Castagnoli A, Merli F, et al. The prognostic value of positron emission tomography performed after two courses (INTERIM-PET) of standard therapy on treatment outcome in early stage Hodgkin lymphoma: a multicentric study by the fondazione italiana linfomi (FIL): Interim-FDG-PET and early stage Hodgkin’s disease. Am J Hematol. 2015;90(6):499–503.
Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419–29.
Stephens DM, Li H, Schöder H, Straus DJ, Moskowitz CH, Leblanc M, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach for stage III/IV Hodgkin lymphoma. Blood. 2019;134:1238.
Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mulé A, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 Trial. JCO. 2018;36(5):454–62.
Spina V, Bruscaggin A, Cuccaro A, Martini M, Di Trani M, Forestieri G, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131(22):2413–25.
Acknowledgements
None declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest, financial or otherwise. Lectures and advisory fees from Takeda and BMS. Investigational fee from Celgene.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Galvez-Carvajal, L., Quero, C., Casanova, M. et al. Ability of final PET/CT to predict response to first-line treatment in real patients with classical Hodgkin lymphoma. Int J Hematol 115, 54–60 (2022). https://doi.org/10.1007/s12185-021-03223-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03223-6