Abstract
Surgical repair for atrial septal defects (ASD) generally occurs during childhood. Post-pericardiotomy syndrome (PPS) after cardiac surgery has a reported incidence of 1–40 %. We focused exclusively on secundum ASD repair to evaluate the incidence of PPS. The purpose of this study is to determine the incidence of PPS after surgical repair of secundum ASD and investigate what risk factors may be predictive of its development. A retrospective study was performed, and 97 patients who underwent surgical closure of a secundum ASD were identified. 27 (28 %) were diagnosed with PPS within the first postoperative year. Diagnosis was made if they had evidence of new or worsening pericardial effusion and the presence of ≥2 of the following criteria: fever >72 h postoperatively, irritability, pleuritic chest pain, or pericardial friction rub. Closure of secundum ASDs was performed at a median age of 3.8 years (Interquartile Range (IQR): 2.2–6.0 years) and a median weight of 14.3 kilograms (IQR: 10.9–19.3 kilograms). The median time for development of PPS was 8 days post-op (IQR: 5–14). Significantly, 19 (27 %) of 70 patients in the non-PPS group had a small pericardial effusion on their discharge echocardiogram, while of the 27 patients who developed PPS, 17 (63 %) had a small pericardial effusion on their discharge echocardiogram (p = 0.001). PPS is relatively common following surgical closure of secundum ASDs. A small pericardial effusion on discharge echocardiogram is predictive of development of PPS postoperatively. In patients who develop PPS, there is a good response to therapy with a benign course.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrial septal defect (ASD) has an incidence of 0.3–0.9 cases per 1,000 live births [1–3] and accounts for approximately 10 % of all congenital heart disease [2, 3]. In children, symptoms are often not present and diagnosis is made during routine heart murmur evaluation [4]. Surgical repair generally occurs during childhood, to prevent symptoms of chronic volume overload of the right atrium and ventricle, pulmonary hypertension, atrial arrhythmias, and heart failure [5, 6], and has a very low mortality rate [7, 8]. Post-pericardiotomy syndrome (PPS) is a known complication of any cardiac surgery, with reported incidence varying widely from 1 to 40 % [9–12]. While data in the literature are conflicting, some authors have noted that the surgical closure of ASD poses a higher risk for the development of PPS than other operations [13]. We are not aware of any studies that have analyzed secundum ASDs exclusively to determine the true incidence of PPS. The purpose of this study is to determine the incidence of PPS after surgical secundum ASD closure and investigate whether there are any risk factors that may be predictive of its development.
Methods
This retrospective review was approved by our institutional review board. We reviewed the surgical database for all patients with ostium secundum ASD who underwent surgical repair between April 2008 and June 2013. Inclusion criteria for the study were patients less than 21 years of age who underwent surgical closure of a secundum ASD and had a minimum of one documented follow-up visit within 1 year postoperatively, as well as electrocardiograms and echocardiograms available for review. Patients were excluded if they had a pacemaker, prior cardiac surgery, or required additional cardiac surgery at the time of ASD closure aside from patent ductus arteriosus ligation.
The surgical techniques for closure of the defects included primary closure or use of patch material. Both inpatient and outpatient charts were reviewed, and data collected included patient demographics, pre-operative electrocardiogram, surgeon, type of surgical closure performed, bypass and cross-clamp times, postoperative troponin levels, postoperative complications, hospital length of stay, mediastinal tube drainage duration and output, echocardiography findings, and diagnosis and treatment of PPS. Postoperative electrocardiograms within 4 h after arrival in intensive care unit and on postoperative days 1–3 were analyzed for PR interval, QRS interval and axis, as well as ST segment elevation.
According to published clinical trials, the following diagnostic criteria for PPS were adopted [10, 14, 15]: onset of fever >72 h postoperatively, patient irritability or pleuritic chest pain, pericardial friction rub, and evidence of new or worsening pericardial effusion. The diagnosis of PPS was based on the presence of two or more criteria.
The subjects were divided into two groups based on whether they developed PPS in the postoperative period. Group 1 included patients without PPS and group 2 patients with PPS.
Continuous variables are expressed as mean ± standard deviation for normally distributed data and median and interquartile range for non-normally distributed data, and categorical variables as numbers and percentage. Student’s t test or Mann–Whitney test is used for comparison of continuous variables. Chi squared testing or Fisher’s exact test is used for categorical variables or dichotomous variables. A p value <0.05 is considered statistically significant.
Results
We identified 114 patients who underwent surgical closure of a secundum ASD and a total of 97 patients were included in this study according to inclusion and exclusion criteria. Of those, 27 (28 %) were diagnosed with PPS within the first postoperative year. Table 1 summarizes the demographic and surgical characteristics of the patients in the two groups. Closure of secundum ASDs was performed at a median age of 3.8 years (IQR: 2.2–6.0 years) and a median weight of 14.3 kilograms (IQR: 10.9–19.3 kilograms). There were no significant differences between the two groups with regards to age, gender, surgeon, surgical method, patch material used, postoperative troponin levels, hospital length of stay, chest tube drainage duration, or chest tube output. Analysis of pre- and post-operative ECGs showed there was no significant difference between the groups in HR, PR interval, QRS interval or axis, or in the incidence of ST segment elevation or ST segment elevation height. Significantly, 19 (27 %) of 70 patients in group 1 had a small pericardial effusion on their discharge echocardiogram, while of the 27 patients in group 2, 17 (63 %) had a small pericardial effusion on their discharge echocardiogram (p = 0.001). The positive predictive value (PPV) of a small pericardial effusion on discharge echocardiogram was 47 %.
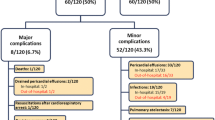
Of the 27 patients with PPS, irritability was present in 18 (67 %), fever in 12 (44 %), chest pain in 8 (28 %), and a pericardial friction rub in 8 (26 %). In the patients who did not develop PPS only 15 (21 %) complained of irritability at their follow-up visit. All but one had a pericardial effusion on echocardiogram ranging in size from moderate to large. The median time postoperatively to diagnosis and development of PPS was 8 days (IQR: 5–14 days). Fifteen (55 %) of the 27 patients with PPS had lab tests performed at the time of diagnosis. The white blood cell count median was 10.5 × 109/L (IQR: 7.5–13.1 × 109/L), 12 had a C-reactive protein tested with a median level of 33.8 mg/L (IQR: 7.0–91.3 mg/L), and ten had an erythrocyte sedimentation rate sent with a median of 31 mm/h (IQR: 8–46 mm/h).
During the postoperative period, the majority of the patients (n = 85, 88 %) were treated with ketorolac for pain control. There was no difference between the groups in number of patients treated or doses received. Twenty patients (74 %) in group 2 were treated with ibuprofen, a non-steroidal anti-inflammatory drug (NSAID), and two (7.4 %) required pericardiocentesis and NSAIDs due to the finding of a large pericardial effusion on echocardiogram. Neither of the two patients who required pericardiocentesis displayed symptoms of hemodynamic compromise or had been treated medically initially. Five were monitored by the cardiologists as an outpatient and were not treated. In all 27 patients in group 2, the PPS resolved in a median time of 25 days (IQR: 12–32 days). The median time to initial follow-up was 7 days (IQR: 5–18 days). However, there were ten patients who did not present for initial follow-up until greater than 1 month postoperatively.
Discussion
It is well established that surgical closure of secundum atrial septal defects is safe with a low morbidity and mortality. [5, 9, 16] PPS is an inflammatory process that occurs in patients following surgical interventions that involves the pleura, pericardium or both [17]. Initially described as “postcommisurotomy syndrome” following the treatment of patients with rheumatic mitral disease [18], in 1958, it was first described and termed as post-pericardiotomy syndrome following repair of congenital heart defects [19]. In their original paper, Ito et al. describe 13 patients who developed PPS, only one of whom underwent ASD closure. The reported incidence of PPS varies widely from 1 to 40 % [9–12]. While data in the literature are conflicting, some authors have noted that the surgical closure of ASD poses a higher risk for the development of PPS than other operations [13]. We report the incidence of PPS in a pediatric population who underwent surgical repair of only a secundum ASD. We report a relatively high incidence of 28 % developing PPS following surgical ASD closure.
Diagnosis of PPS is made in the presence of clinical symptoms that include fever without alternate causes, pleuritic chest pain, pericardial friction rub, evidence of pericardial effusion, and evidence of pleural effusion. [10, 11, 15, 20] We also included patient irritability in our criteria as our patient population of children presented a challenge in that they may have been too young to complain of chest pain. While exact criteria differ between the various studies, most investigators require at least two of the above-mentioned features. In addition, we found that of the labs that were sent, both CRP and ESR were elevated above normal values; however, WBC was not elevated. In our population, all patients, except one, had at least a moderate size pericardial effusion. As has been noted in prior studies, there is a lack of consistency with regards to diagnostic criteria for PPS. Based on the fact that almost all (except one) of our patients had a pericardial effusion on echocardiogram, we propose universal criterion for pediatric patients for the diagnosis of PPS, specifically, the presence of a pericardial effusion on echocardiogram, along with two or more of the following symptoms: fever >38 °C more than 72 h postoperatively, pericardial friction rub, patient irritability, or pleuritic chest pain. In addition, elevation of inflammatory markers could be considered as a supplementary sign of PPS.
We found that the presence of a small pericardial effusion upon discharge from the hospital following surgery was associated with the development of PPS in 47 % of the patients who had a small pericardial effusion on discharge echocardiogram. While only 27 % of the patients in the group that did not develop PPS had a small pericardial effusion, 63 % of the patients in the group who did develop PPS had a pericardial effusion at discharge. ST segment elevation on postoperative ECG was common in this patient population (52 %); however, it was not predictive of the development of PPS. Similarly, other variables tested were not associated with the development of PPS.
Several studies have been done to assess the role of prophylactic medications in the prevention of PPS. Mott et al. compared intravenous methylprednisolone and placebo administration pre- and post-cardiopulmonary bypass in pediatric patients undergoing surgery for congenital heart disease and found no difference in the incidence of PPS [21]. Similarly, Gil et al. compared pediatric patients undergoing ASD repair who received aspirin prophylaxis with those who did not, and they found no difference between the two groups in the incidence of PPS [10]. In the largest clinical trial in adults, the COPPS trial, colchicine significantly reduced the incidence of PPS at 12 months compared with placebo [14]. We found in our population that using an NSAID for pain control immediately after surgery did not impact PPS development.
PPS can range from being a self-limited episode to a complex condition requiring multiple treatment courses and interventions. In our group, five patients did not require treatment and had a self-limited course, while the remainder were treated with medication alone or pericardiocentesis followed by a course of medication. In our population, 74 % of the patients with PPS were treated with ibuprofen, with complete resolution of their symptoms [22]. Given the high incidence of PPS, it was reassuring to see that only two patients required pericardiocentesis and all resolved within a median of 25 days with ibuprofen alone. One patient had recurrent PPS that was successfully treated with ibuprofen. No patient in our study received steroids for treatment of their PPS. The median time to follow up for our patient population was 7 days; however, there were ten patients who did not follow up until more than a month after discharge. In this setting, there is the possibility that some of these patients may have had a self-limited course of PPS that was not brought to medical attention.
While Imazio et al. found that in adults, patients with PPS had longer length of stay in the hospital following cardiac surgery [11], in our population there was no difference between the groups in their hospital length of stay following surgery.
In conclusion, our study demonstrated a relatively high incidence of PPS following surgical repair of secundum ASD in a pediatric population. A small pericardial effusion on discharge echocardiogram following surgery was predictive of later development of PPS and should prompt closer follow-up of these patients after discharge.
References
Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Neill CA, Perry LW, Hepner SI, Downing JW (1985) Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol 121(1):31–36
Carlgren LE (1959) The incidence of congenital heart disease in children born in Gothenburg 1941-1950. Br heart J 21(1):40–50
Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39(12):1890–1900
Moss AJ, Adams FH, Emmanouilides GC (1995) Moss and Adams heart disease in infants, children, and adolescents : including the fetus and young adult, 5th edn. Williams & Wilkins, Baltimore
Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter CJ, Ilstrup DM, McGoon DC, Puga FJ, Kirklin JW, Danielson GK (1990) Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. The New England journal of medicine 323(24):1645–1650. doi:10.1056/NEJM199012133232401
Roos-Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, van Rijen EH, Utens EM, Bogers AJ, Simoons ML (2003) Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21–33 years. Eur heart J 24(2):190–197
Zea Du (2002) Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults. J Am Coll Cardiol 39(11):1836–1844
Bichell DP, Geva T, Bacha EA, Mayer JE, Jonas RA, del Nido PJ (2000) Minimal access approach for the repair of atrial septal defect: the initial 135 patients. Ann Thorac Surg 70(1):115–118
Baskett RJF, Tancock E, Ross DB (2003) The gold standard for atrial septal defect closure. Pediatr cardiol 24(5):444–447. doi:10.1007/s00246-002-0131-6
Gill PJ, Forbes K, Coe JY (2009) The effect of short-term prophylactic acetylsalicylic acid on the incidence of post-pericardiotomy syndrome after surgical closure of atrial septal defects. Pediatr cardiol 30(8):1061–1067. doi:10.1007/s00246-009-9495-1
Imazio M, Brucato A, Rovere ME, Gandino A, Cemin R, Ferrua S, Maestroni S, Barosi A, Simon C, Ferrazzi P, Belli R, Trinchero R, Spodick D, Adler Y (2011) Contemporary features, risk factors, and prognosis of the post-pericardiotomy syndrome. Am J cardiol 108(8):1183–1187. doi:10.1016/j.amjcard.2011.06.025
Imazio M, Brucato A, Adler Y (2012) Is possible to prevent the Post-Pericardiotomy Syndrome? Int J Cardiol 159(1):1–4. doi:10.1016/j.ijcard.2012.01.034
Timmis GC, Gordon S, Ramos RG (1971) Recurrent post-pericardiotomy syndrome. Its protracted nature and association with atrial septal defects. Mich Med 70(13):539–542
Imazio M, Trinchero R, Brucato A, Rovere ME, Gandino A, Cemin R, Ferrua S, Maestroni S, Zingarelli E, Barosi A, Simon C, Sansone F, Patrini D, Vitali E, Ferrazzi P, Spodick DH, Adler Y, Investigators C (2010) COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur Heart J 31(22):2749–2754. doi:10.1093/eurheartj/ehq319
Imazio M, Brucato A, Ferrazzi P, Spodick DH, Adler Y (2013) Post-pericardiotomy syndrome: a proposal for diagnostic criteria. J Cardiovasc Med 14(5):351–353. doi:10.2459/JCM.0b013e328353807d
Kutty S, Hazeem AA, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA (2012) Long-term (5- to 20-year) outcomes after transcatheter or surgical treatment of hemodynamically significant isolated secundum atrial septal defect. Am J Cardiol 109(9):1348–1352. doi:10.1016/j.amjcard.2011.12.031
Imazio M (2012) The post-pericardiotomy syndrome. Curr Opin Pulm Med 18(4):366–374. doi:10.1097/MCP.0b013e32835311a2
Janton OH, Glover RP, Oneill TJE, Gregory JE, Froio GF (1952) Results of the Surgical Treatment for Mitral Stenosis - Analysis of 100 Consecutive Cases. Circulation 6(3):321–333
Ito T, Engle MA, Goldberg HP (1958) Post-pericardiotomy syndrome following surgery for nonrheumatic heart disease. Circulation 17(4, Part 1):549–556
Imazio M, Brucato A, Markel G, Cemin R, Trinchero R, Spodick DH, Adler Y (2011) Meta-analysis of randomized trials focusing on prevention of the post-pericardiotomy syndrome. Am J Cardiol 108(4):575–579. doi:10.1016/j.amjcard.2011.03.087
Mott AR, Fraser CD Jr, Kusnoor AV, Giesecke NM, Reul GJ Jr, Drescher KL, Watrin CH, Smith EO, Feltes TF (2001) The effect of short-term prophylactic methylprednisolone on the incidence and severity of post-pericardiotomy syndrome in children undergoing cardiac surgery with cardiopulmonary bypass. J Am Coll Cardiol 37(6):1700–1706
Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB (1999) Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med 340(18):1377–1382. doi:10.1056/NEJM199905063401801
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heching, H.J., Bacha, E.A. & Liberman, L. Post-Pericardiotomy Syndrome in Pediatric Patients Following Surgical Closure of Secundum Atrial Septal Defects: Incidence and Risk Factors. Pediatr Cardiol 36, 498–502 (2015). https://doi.org/10.1007/s00246-014-1039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-014-1039-7