Abstract
Postpericardiotomy syndrome (PPS), a potential complication of open heart surgery, has a variable clinical course and severity. This study evaluated the effectiveness of acetylsalicylic acid (ASA) prophylaxis in preventing PPS after surgical closure of atrial septal defects (ASDs) in pediatric patients. A retrospective review was performed for 177 patients who underwent uncomplicated ASD closure from 1986 to 2006. The study group received prophylactic ASA 20 to 50 mg/kg/day for 1 to 6 weeks after surgery, whereas the control group did not. The primary outcome was a diagnosis of PPS based on the presence of two or more of the following symptoms or signs occurring at least 72 h postoperatively: fever (temperature >38°C), pericardial or pleural rub, and worsening or recurring anterior pleuritic chest pain. Consequently, PPS developed in 5 (2.8%) of the 177 children: 2.8% (3/106) in the control group and 2.8% (2/71) in the study group (p = 1.00). The secondary outcomes were frequency of other postoperative complications. Postoperative pericardial effusions experienced by 26.7% of the patients were identified more frequently in the treatment group (p < 0.001). Postoperative prophylaxis ASA at a dose of 20 to 50 mg/kg/day for 1 to 6 weeks after surgical closure of ASD does not decrease the incidence of PPS in pediatric patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Postpericardiotomy syndrome (PPS) is a known complication after any open heart surgery or procedure involving entry into the pericardium [8, 11, 19, 21]. The diagnosis of PPS is by exclusion, requiring symptoms of fever, pericardial and/or pleural rub, and anterior pleuritic chest pain in the absence of other identifiable causes [12]. Elevation of inflammatory markers (erythrocyte sedimentation rate/C-reactive protein), leukocytosis, and findings of pericardial or pleural effusions may add to diagnostic certainty. The incidence cited in the literature varies widely, from 1% to 50% [1, 11, 14, 17, 20, 25, 26, 29, 31, 34].
Although PPS most often presents in the first few weeks postoperatively, it can occur months after surgery and has a variable clinical course. The severity of PPS is variable, and untreated PPS can lead to significant hemodynamic consequences such as pericardial tamponade [30].
According to one proposed theory, PPS is an immune-mediated inflammatory process involving the pericardium [24]. Acetylsalicylic acid (ASA) is used generally as first-line therapy for PPS, targeting the proposed inflammatory response. Indomethacin and steroids have been used for retractable cases [14, 36]. Colchicine, methotrexate, and intravenous immunoglobulin (IVIG) have been used for steroid-dependent patients with chronic-recurrent PPS, although their efficacy has not been definitively proven [16, 35, 37]. Pericardiocentesis, pericardectomy, or a surgical pericardial window is indicated in cases of hemodynamically significant pericardial effusions [30]. To date, there is no proven agent to prevent PPS [13].
Given the presumed mechanism of PPS, it has been proposed that prophylactic treatment with an antiinflammatory agent such as ASA may prevent its development. Previous PPS studies have failed to enroll a cohort of patients with the same cardiac lesion. Some authors have identified surgical closure of ASD lesions as posing a high risk for the development of PPS, although data in the literature are conflicting [33].
This retrospective study is the first to assess the effectiveness of ASA prophylaxis for a large cohort of patients with a single cardiac lesion. The primary outcome was the incidence of PPS after surgical closure of an uncomplicated atrial septal defect in patients with and without prophylactic ASA. Secondary outcomes were the incidence of postoperative complications and ASA therapy complications.
Materials and Methods
Between December 1986 and June 2006, 360 patients underwent surgical closure of uncomplicated ostium secundum or sinus venosus atrial septal defect (ASD) at the University of Alberta Stollery Children’s Hospital. Ethical approval for this retrospective review was obtained from our institutional Health Research Ethics Board. The surgical technique included the use of patch or suture. In- and outpatient charts were reviewed, and data were collected on patient demographics, cardiac and other noncardiac diagnoses, type of surgical closure performed, dose and duration of prophylactic ASA, echocardiography and chest X-ray findings, postoperative complications, and diagnosis of PPS.
Patients were included in the study if they met the following criteria: age between 0 and 18 years, secundum ASD closure, and a minimum of one documented follow-up visit at our institution within 1 year postoperatively. Patients were excluded if they required other cardiovascular surgery at the time of ASD closure apart from simple patent ductus arteriosus ligation. A total of 177 patients met the study criteria.
From December 1986 to September 1996, ASA was not used routinely in the postoperative period for asymptomatic patients. This population comprised the control group in our study. Treatment was prescribed at the discretion of the attending pediatric cardiologist for patients who presented with symptoms suggestive of PPS including fever, pleuritic chest pain, and pericardial or pleural rub. It should be noted that not all the patients who received treatment met the full diagnostic criteria for PPS.
In September 1996, an institutional policy was implemented that required all patients undergoing surgical ASD closure to receive ASA 30 mg/kg/day for 2 weeks postoperatively to prevent the development of PPS. These patients comprised the study arm of the study. Patients were included if they had received prophylaxis ASA for 1 to 6 weeks. Consideration for further treatment was warranted if patients were not responsive to the standard dosing or had more severe symptoms. This treatment may have included high-dose ASA (60 mg/kg/day), corticosteroids, or pericardiocentesis depending on clinical status. If complications arose due to ASA prophylaxis, ASA was discontinued or switched to alternative medication. Gastric protection medications were prescribed at the discretion of the attending pediatric cardiologist.
The patient’s clinical status was noted daily during postoperative hospitalization. The primary outcome was the incidence of PPS in each group. The diagnosis of PPS was based on two or more of the following symptoms or signs occurring at least 72 h postoperatively: fever (temperature >38°C), pericardial or pleural rub, and worsening or recurring anterior pleuritic chest pain. Echocardiography and chest X-ray radiographic imaging were noted if performed to determine presence of pericardial or pleural effusion. The clinical course was noted, particularly with respect to recurrence. Secondary outcomes included the incidence of postoperative complications. Those identified included pericardial effusion, pleural effusion, fever, pericardial rub, arrhythmia, infection, and pneumothorax.
The demographic, clinical, and perioperative variables were compared using the unpaired Student’s t test, the Mann–Whitney rank sum test, the chi-square test, and Fisher’s exact test. A p value less than 0.05 was considered significant. All statistical analyses were performed using SigmaStat Advisory Statistics for Scientists, Version 3.5 (Systat Software Inc., San Jose, CA, USA).
Results
Of the 177 children with surgical closure of uncomplicated ASD enrolled in this retrospective study, 71 (40.1%) were treated with prophylactic ASA. Table 1 summarizes the demographic and clinical variables of the patients in the two groups. Closure of ASD was performed at a mean age of 4.8 ± 3.8 years (median, 3.6 years; range, 0.6–17 years) and a mean weight of 19.5 ± 16.1 kg (median, 14.2 kg; range, 5.2–102 kg). There were no significant differences in terms of age or gender between the groups. The method of surgical closure differed between the control group (75.5% suture, 24.5% patch) and the study group (90.1% suture, 9.9% patch). Concurrent patent ductus arteriosus ligation was performed for 12 patients (6.8%).
The patients in the control group did not receive ASA prophylaxis. Initially, three patients (4.2%) in the control group met the diagnostic criteria for PPS, but only one (patient 1 in Table 2) was initially prescribed ASA, at a dose of 30 mg/kg/day for 2 weeks. Patient 2 in Table 2 received treatment with ASA when symptoms recurred. An additional six patients (8.5%) in the control group presented with symptoms resembling PPS during the immediate postoperative period. None of these patients met the diagnostic criteria for PPS. However, all six patients received ASA treatment in the hospital or when discharged at a dose of 40.1 ± 27.1 mg/kg/day (median, 39.4 mg/kg/day; range, 9.4–80.2 mg/kg/day) for 2.6 ± 2 weeks (median, 1.8 weeks; range, 1–6 weeks).
The study group (n = 71) received prophylactic ASA at 31.5 ± 14.7 mg/kg/day (median, 34.1 mg/kg/day; range, 1.4–87.1 mg/kg/day). Due to the wide range of doses, subgroup analysis was performed. The study group was separated into low-dose ASA (<20 mg/kg/day) and regular-dose ASA (20–50 mg/kg/day). The low-dose ASA study group (n = 13) received prophylactic ASA at 7.3 ± 4.9 mg/kg/day (median, 5.5 mg/kg/day; range, 1.4–17.9 mg/kg/day) for a duration of 1.9 ± 0.5 weeks (median, 2 weeks; range, 1–3 weeks). The regular-dose ASA study group (n = 55) received prophylactic ASA at 35.4 ± 6.3 mg/kg/day (median, 36.9 mg/kg/day; range, 20.9–47.1 mg/kg/day) for a duration of 2.7 ± 1.2 weeks (median, 2 weeks; range, 1–6 weeks). We assumed that a standard 2-week dose of ASA had been administered if documentation of the treatment duration was unclear.
It should be noted that three patients not included in the aforementioned subgroup analysis were prescribed ASA postoperatively for a presumptive diagnosis of PPS at respective doses of 53.8, 57.5, and 87.7 mg/kg/day. It was unclear whether these patients were receiving a lower dose of ASA before the dose was changed. These patients were included in the subsequent outcome analysis.
Gastric protection medications were prescribed for 15.5% (11/71) of the patients. Two of the patients (2.8%) received a proton pump inhibitor, and nine (12.7%) received a histamine H-2 antagonist. Complications related to ASA prophylaxis were experienced by 2.8% (2/71) of the patients, including gastritis and vomiting. For these patients, ASA was discontinued (n = 1) or replaced with an alternative medication (n = 1). There were no cases of Reye’s syndrome or clinically significant bleeding.
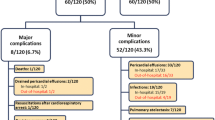
Of the 177 total patients, 5 (2.8%) met the diagnostic criteria for PPS (Table 2). There was no statistical difference in the incidence of PPS between the groups (p = 1.00). The incidence of PPS in the study group was 2.8% (2/71) compared with 2.8% (3/106) in the control group. There was no difference between the low-dose and regular-dose ASA groups (p = 0.33). The time between surgical ASD closure and diagnosis of PPS was 90.8. ± 159.3 days (median, 5 days; range, 3–371 days). The age at presentation ranged from 3.8 to 13.1 years (mean, 7.5 ± 3.7 years; median, 8 years). All patients presenting with PPS were females.
The clinical characteristics of the patients with a diagnosis of PPS are summarized in Table 2. Two cases of PPS (40%), identified as patients 2 and 4 in Table 2, experienced multiple recurrences. Patient 2, a subject in the control group, presented with recurrent episodes of pericarditis at 12, 15, and 30 months postoperatively. Each episode resolved with a short course ASA treatment. Patient 4, a subject in the treatment group, presented with PPS 3 days postoperatively. Despite various therapies, her symptoms continued to recur. At 11 months postoperatively, she underwent surgical management with bilateral pericardial windows and drains. A pericardial biopsy at this time showed chronic pericarditis.
The postoperative clinical findings are recorded in Table 3. Postoperative echocardiography was performed more frequently between 1996 and 2006 (98.6%) than between 1986 and 1996 (85.8%; p = 0.003). The percentage of patients whose postoperative echocardiography identifed pericardial effusions was greater in the study group (43.7%) than in the control group (17.6%; p < 0.001). There were significantly more incidences of pleural effusions (p < 0.001) and pneumothoraces (p = 0.014) in the study group than in the control group, as determined by chest X-ray and echocardiography. The incidence of fevers was less in the study group (2.8%) than in the control group (12.3%; p = 0.029). The subgroup analysis showed no difference in complications between the low-dose and regular-dose ASA groups.
Discussion
In 1953, PPS was first described by Janton et al. [17] as a constellation of fever and pleuritic chest pain after surgical treatment of patients with rheumatic mitral stenosis and described later by Dressler [7] after myocardial infarction. Since that time, identical symptoms have been reported after conditions or procedures causing irritation or disruption to the pericardium or underlying myocardium.
Much of the early work to establish the etiology of PPS was done by Engle et al. [9, 10], who looked at the relationship of antiheart antibodies and antiviral antibodies in patients with PPS. They proposed that a viral illness triggers an immune response, PPS [11]. Subsequent studies suggested that myocardial antigens, released from surgery or trauma, result in deposition of immune complex in the pericardial space [24]. However, others have demonstrated that immunosuppression does not block the inflammatory response that leads to PPS, challenging the concept of an antigen–antibody immune complex mechanism [3, 6, 26].
The diagnosis of PPS is based on clinical symptoms including fever not otherwise explained, pleuritic chest pain, and pericardial friction rub [5, 12–15, 20, 21, 26, 36]. It is supported by ancillary tests, but there is no single test available to confirm the diagnosis. Although the criteria required for a PPS diagnosis vary between studies, most investigators require the presence of two or more features.
For the purpose of this study, we defined PPS as involving two or more of the following symptoms or signs occurring at least 72 h postoperatively: fever (temperature >38°C), pericardial or pleural rub, and worsening or recurring anterior pleuritic chest pain. Pericardial effusion, an important complication of PPS, is not required for a diagnosis because only 10.3% to 11.5% of patients with a postoperative pericardial effusion experience PPS [1, 29].
A number of confounding factors make the diagnosis of PPS difficult. During the early postoperative period, a pericardial friction rub can be transiently heard. A postoperative fever is not uncommon after cardiopulmonary bypass [23]. Furthermore, the reliability of clinical symptoms in children presents a challenge because they often are too young to complain of chest pain, and differentiating it from incisional pain can be difficult [32].
The absence of universal criteria for the diagnosis of PPS and the heterogeneous study populations yield variable rates for the reported incidences of PPS in the literature. The incidence of PPS is traditionally reported to be 10% to 40%, with a slightly higher representation among children than among adults [11, 14, 17, 20, 26, 31]. However, other studies report lower incidence rates of 1% to 3% [1, 29, 34].
The natural history of PPS is highly variable, ranging from a brief self-limited episode to a complex condition with a protracted course and multiple recurrences. The reported recurrence rates for patients with the diagnosis of PPS are 21% to 23% [26, 27]. We found evidence of clinical variability in the natural history of PPS. One patient with a diagnosis of PPS in the control group had resolution of symptoms in the absence of antiinflammatory treatment. Variability in the clinical course may be related to the poor specificity of the PPS signs and symptoms.
The majority of studies pertaining to PPS include patients with a variety of cardiac lesions, making it difficult to establish that particular lesions are at increased risk. Mott et al. [26] reported that septal defects represented a large proportion of PPS diagnoses in their study group, with 49% of the patients having a ventricle septal defect, ASD, or both. An early case series by Timmis et al. [33] suggested an association between recurrent PPS and ASDs. Overall, the literature contains conflicting data, with the PPS incidence after surgical correction of ASD lesions reported to be 1% to 25% [1, 11, 18, 22, 26, 32, 34].
A number of factors contribute to the difficulty establishing the incidence of PPS in specific cardiac lesions such as the small study populations, the variety of lesions, and the inconsistent diagnostic criteria among studies. Additionally, advances in surgical techniques, therapy, and postoperative patient management may have caused differences in the incidence of PPS.
Our study is the largest to date investigating the incidence of PPS in a pediatric patient population with a single cardiac lesion. We report an overall PPS incidence after surgical ASD closure of 2.8% (5/187) and an overall incidence of complicated or recurrent PPS of 1.1% (2/177). This is a recurrence rate of 40% (2/5) for patients with a diagnosis of PPS, which compares with other studies [26, 27]. However, our small sample size limits our conclusions. Given the low incidence of both simple and recurrent PPS, our data do not support ASD as posing a high risk for either.
A number of studies have evaluated the role of prophylactic medications in the prevention of PPS. Prophylactic corticosteroids postoperatively have not been found to alter the natural history of PPS [6, 26]. Colchicine is being investigated for prevention of PPS [15] because a previous study indicated a trend of reduced incidence compared with placebo in adults [13].
Acetylsalicylic acid is used as first-line treatment for PPS. For most patients, ASA is effective at reducing symptoms and leads to resolution of the illness [5, 14, 36]. No studies have evaluated its role in prevention of PPS. Therefore, we hypothesized that prophylactic administration of ASA would prevent the development of PPS by preventing the inflammatory cascade.
Our data demonstrated no statistically significant difference (p = 1.00) in the incidence of PPS between the control group and the study group. This suggests that ASA prophylaxis for 1 to 6 weeks after surgical closure of uncomplicated ASDs does not prevent the development of PPS. Subgroup analysis confirms that this is true for both the low dose (<20 mg/kg/day) and the regular dose (20–50 mg/kg/day). However, our small sample of patients in each subgroup limits our ability to draw meaningful conclusions.
Postoperative pericardial effusions are found with relative frequency in patients undergoing cardiac surgery, reportedly in the range of 14% to 65% [1, 2, 4, 5, 28, 29]. The incidence of pericardial effusions in our study was found to be 26.6% (47/177), comparable with that in other studies. The size of the pericardial effusion was determined subjectively by echocardiography and ranged from trivial to moderate, with no cases of cardiac tamponade. The frequency of postoperative echocardiography has increased over the past 20 years, and many patients who experience a pericardial effusion often are asymptomatic [4]. Effusions, however, may cause symptoms such as fever, irritability, vomiting, and abdominal discomfort [4, 5], some of which may be symptoms of PPS development.
Beland et al. [2] found that 65% of patients experienced pericardial effusions postoperatively and that prophylactic ASA at a dose of 60 mg/kg/day for 7 days did not alter this incidence. Interestingly, in our study, we found a significantly increased incidence of pericardial effusions (p < 0.001) among patients prescribed ASA prophylaxis postoperatively (43.7% vs. 17.6%). This may be related to the increased frequency of echocardiography after cardiac surgery in the past 10 years. Patients in the 1986–1996 era may not have been imaged if they were asymptomatic. Our data reinforce the finding that ASA prophylaxis after cardiac surgery does not decrease the incidence of pericardial effusions.
Limitations of the Study
The retrospective nature of this study imposes inherent limitations. Detailed notation of ASA prophylaxis dose and duration was variable or poorly documented. Due to the wide range of dosing, an attempt was made to address this by subgroup analysis. Patient follow-up evaluation was inconsistent and incomplete due to the wide geographic distribution of patients over western Canada, necessitating the exclusion of many patients due to incomplete follow-up data.
Given the retrospective nature of the study, the duration and timing of postoperative signs and symptoms were difficult to determine. Postoperative patients who presented to their primary care physician or at a different center with symptoms of PPS or complications of treatment may have been missed. Because all radiographic data documented were before discharge, findings that may have developed in the later postoperative period but were absent at discharge may have been missed.
Conclusions
In conclusion, our study investigating the role of ASA prophylaxis did not find it effective at preventing the development of PPS. This could be more definitively answered by a prospective, double-blind, placebo-controlled randomized trial. Given that PPS can occur after many cardiac interventions, further research may have important implications for future clinical practice.
A significant challenge encountered when comparing studies is the lack of consistency in the diagnostic criteria for PPS. We propose a universal criterion for the diagnosis of PPS that all future studies should adopt, namely, two or more of the following symptoms or signs present at least 72 h postoperatively: fever (temperature >38°C), pericardial or pleural rub, and worsening or recurring anterior pleuritic chest pain. The presence or absence of pericardial effusion should not be required for the PPS diagnosis because it is a common postoperative finding. Until all future studies adopt a consistent approach to the diagnosis of PPS, meaningful comparisons and conclusions will be difficult to ascertain.
References
Baskett RJ, Tancock E, Ross DB (2003) The gold standard for atrial septal defect closure: current surgical results, with an emphasis on morbidity. Pediatr Cardiol 24:444–447
Beland MJ, Paquet M, Gibbons JE, Tchervenkov CI, Dobell AR (1990) Pericardial effusion after cardiac surgery in children and effects of aspirin for prevention. Am J Cardiol 65:1238–1241
Cabalka AK, Rosenblatt HM, Towbin JA, Price JK, Windsor NT, Martin AB, Louis PT, Frazier OH, Bricker JT (1995) Postpericardiotomy syndrome in pediatric heart transplant recipients: immunologic characteristics. Tex Heart Inst J 22:170–176
Cheung EW, Ho SA, Tang KK, Chau AK, Chiu CS, Cheung YF (2003) Pericardial effusion after open heart surgery for congenital heart disease. Heart 89:780–783
Clapp SK, Garson A Jr, Gutgesell HP, Cooley DA, McNamara DG (1980) Postoperative pericardial effusion and its relation to postpericardiotomy syndrome. Pediatrics 66:585–588
Dresdale DT, Ripstein CB, Guzman SV, Greene MA (1956) Postcardiotomy syndrome in patients with rheumatic heart disease: cortisone as a prophylactic and therapeutic agent. Am J Med 21:57–74
Dressler W (1956) A postmyocardial infarction syndrome: preliminary report of a complication resembling idiopathic, recurrent, benign pericarditis. J Am Med Assoc 160:1379–1383
Drusin LM, Engle MA, Hagstrom JW, Schwartz MS (1965) The postpericardiotomy syndrome: a six-year epidemiologic study. N Engl J Med 272:597–602
Engle MA, O’Loughlin JE (1987) Complications of cardiac surgery in children. Pediatr Rev 9:147–154
Engle MA, McCabe JC, Ebert PA, Zabriskie J (1974) The postpericardiotomy syndrome and antiheart antibodies. Circulation 49:401–406
Engle MA, Zabriskie JB, Senterfit LB, Tay DJ, Ebert PA (1975) Immunologic and virologic studies in the postpericardiotomy syndrome. J Pediatr 87:1103–1108
Engle MA, Zabriskie JB, Senterfit LB, Gay WA Jr, O’Loughlin JE Jr, Ehlers KH (1980) Viral illness and the postpericardiotomy syndrome: a prospective study in children. Circulation 62:1151–1158
Finkelstein Y, Shemesh J, Mahlab K, Abramov D, Bar-El Y, Sagie A, Sharoni E, Sahar G, Smolinsky AK, Schechter T, Vidne BA, Adler Y (2002) Colchicine for the prevention of postpericardiotomy syndrome. Herz 27:791–794
Horneffer PJ, Miller RH, Pearson TA, Rykiel MF, Reitz BA, Gardner TJ (1990) The effective treatment of postpericardiotomy syndrome after cardiac operations: a randomized placebo-controlled trial. J Thorac Cardiovasc Surg 100:292–296
Imazio M, Cecchi E, Demichelis B, Chinaglia A, Coda L, Ghisio A, Demarie D, Ierna S, Trinchero R (2007) Rationale and design of the COPPS trial: a randomised, placebo-controlled, multicentre study on the use of colchicine for the primary prevention of postpericardiotomy syndrome. J Cardiovasc Med (Hagerstown) 8:1044–1048
Imazio M, Cecchi E, Trinchero R (2007) Colchicine for the prevention of the postpericardiotomy syndrome: the COPPS trial. Int J Cardiol 121:198–199
Janton OH, Glover RP, O’Neill TJ (1953) Mitral commissurotomy in the older aged patient: an analysis of twenty patients over the age of fifty. Circulation 8:321–327
Just H, Mattingly TW (1968) Interatrial septal defect and pericardial disease: coincidence or causal relationship? Am Heart J 76:157–167
Khan AH (1992) The postcardiac injury syndromes. Clin Cardiol 15:67–72
Kirsh MM, McIntosh K, Kahn DR, Sloan H (1970) Postpericardiotomy syndromes. Ann Thorac Surg 9:158–179
Kronick-Mest C (1989) Postpericardiotomy syndrome: etiology, manifestations, and interventions. Heart Lung 18:192–198
Kroop IG, Carno I, Oshrain C (1961) Recurrent pleuropericarditis (postcardiotomy syndrome) after open heart repair of congenital cardiac defects. Circulation 24:976
Livelli FD Jr, Johnson RA, McEnany MT, Sherman E, Newell J, Block PC, DeSanctis RW (1978) Unexplained in-hospital fever following cardiac surgery: natural history, relationship to postpericardiotomy syndrome, and a prospective study of therapy with indomethacin versus placebo. Circulation 57:968–975
Maisch B, Berg PA, Kochsiek K (1979) Clinical significance of immunopathological findings in patients with post-pericardiotomy syndrome: I. Relevance of antibody pattern. Clin Exp Immunol 38:189–197
Miller RH, Horneffer PJ, Gardner TJ, Rykiel MF, Pearson TA (1988) The epidemiology of the postpericardiotomy syndrome: a common complication of cardiac surgery. Am Heart J 116:1323–1329
Mott AR, Fraser CD Jr, Kusnoor AV, Giesecke NM, Reul GJ Jr, Drescher KL, Watrin CH, Smith EO, Feltes TF (2001) The effect of short-term prophylactic methylprednisolone on the incidence and severity of postpericardiotomy syndrome in children undergoing cardiac surgery with cardiopulmonary bypass. J Am Coll Cardiol 37:1700–1706
Nishimura RA, Fuster V, Burgert SL, Puga FJ (1983) Clinical features and long-term natural history of the postpericardiotomy syndrome. Int J Cardiol 4:443–454
Pastorek JS, Allen HD, Davis JT (1994) Current outcomes of surgical closure of secundum atrial septal defect. Am J Cardiol 74:75–77
Prabhu AS, Ross RD, Heinert MR, Walters HLIII, Hakimi M (1996) Decreased incidence of postoperative pericardial effusions after cardiac surgery for congenital heart disease. Am J Cardiol 77:774–776
Scarfone RJ, Donoghue AJ, Alessandrini EA (2003) Cardiac tamponade complicating postpericardiotomy syndrome. Pediatr Emerg Care 19:268–271
Soloff LA, Zatuchni J, Janton OH, O’Neill TJ, Glover RP (1953) Reactivation of rheumatic fever following mitral commissurotomy. Circulation 8:481–497
Swan H, Kortz AB, Davies DH, Blount SG Jr (1959) Atrial septal defect, secundum: an analysis of one hundred patients undergoing open surgical repair. J Thorac Surg 37:52–80
Timmis GC, Gordon S, Ramos RG (1971) Recurrent postpericardiotomy syndrome: its protracted nature and association with atrial septal defects. Mich Med 70:539–542
Uricchio JF (1963) The postcommissurotomy (postcardiotomy) syndrome. Am J Cardiol 12:436–438
Wendelin G, Fandl A, Beitzke A (2008) High-dose intravenous immunoglobulin in recurrent postpericardiotomy syndrome. Pediatr Cardiol 29:463–464
Wilson NJ, Webber SA, Patterson MW, Sandor GG, Tipple M, LeBlanc J (1994) Double-blind placebo-controlled trial of corticosteroids in children with postpericardiotomy syndrome. Pediatr Cardiol 15:62–65
Zucker N, Levitas A, Zalzstein E (2003) Methotrexate in recurrent postpericardiotomy syndrome. Cardiol Young 13:206–208
Acknowledgment
Financial support ($3,000) was provided by the David and Beatrice Reidford Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gill, P.J., Forbes, K. & Coe, J.Y. The Effect of Short-Term Prophylactic Acetylsalicylic Acid on the Incidence of Postpericardiotomy Syndrome After Surgical Closure of Atrial Septal Defects. Pediatr Cardiol 30, 1061–1067 (2009). https://doi.org/10.1007/s00246-009-9495-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-009-9495-1